Role of wall teichoic acid glycosylations in Gram-Positive
pathogens resistance to antimicrobial peptides and surface
protein anchoring
Master Degree in Clinical Laboratory Biology
Diana Isabela Macedo Meireles
Doutor Didier Cabanes Professora Doutora Ana Sampaio
Role of wall teichoic acid glycosylations in Gram-Positive
pathogens resistance to antimicrobial peptides and surface
protein anchoring
Master Degree in Clinical Laboratory Biology
Diana Isabela Macedo Meireles
Doutor Didier Cabanes Professora Doutora Ana Sampaio
Composição do júri:
________________________________________________________
________________________________________________________
________________________________________________________
As doutrinas apresentadas neste trabalho são da exclusiva responsabilidade do autor. É autorizada a reprodução parcial desta tese apenas para efeitos de investigação, mediante declaração escrita do interessado, que a tal se compromete.
Universidade de Trás-os-Montes e Alto Douro O autor:
Diana Meireles
Orientador:
_______________________________________ Doutor Didier Cabanes
(i3S-IBMC, Universidade do Porto)
Co-orientador:
_______________________________________ Professora Doutora Ana Cristina Sampaio
“Persistence is the smallest path of success.”
Agradecimentos
A realização deste trabalho, bem como o culminar de dois anos de crescimento e aprendizagem, não teriam sido possíveis sem a instrução, ajuda e apoio de diversas pessoas. Desta forma, gostaria de deixar um agradecimento especial a todos aqueles que, direta ou indiretamente, contribuíram para que os meus objetivos fossem concretizados:
À Professora Ana Sampaio: por toda a ajuda, disponibilidade, paciência, orientação e apoio manifestados não só na qualidade de Coordenadora do Mestrado de Biologia Clínica Laboratorial mas também na qualidade de Docente sempre atenta e dedicada, trabalho esse que nem sempre foi fácil mas que sempre exerceu de forma admirável e excecional.
Ao meu orientador, Doutor Didier Cabanes: um imenso obrigada! Não só pela oportunidade que me concedeu em desenvolver a minha tese de mestrado no seu laboratório de Microbiologia Molecular mas também por todos os conhecimentos cruciais que me facultou ao longo do tempo. Deixo ainda o meu agradecimento especial por toda a orientação, paciência e disponibilidade oferecidas.
À Super Rita Pombinho: não existem palavras que consigam expressar toda a minha gratidão pela disponibilidade (temporal bem como intelectual) prestada para que este trabalho fosse possível. Ainda assim, deixo o meu mais sincero obrigada pelo exemplo, ajuda, partilha de conhecimentos, dedicação e orientação assim como por toda a amizade, palavras de motivação e paciência (muita paciência). Obrigada de coração!
À Doutora Sandra Sousa: por todos os conhecimentos transmitidos e ajuda disponibilizada bem como por toda a paciência, tolerância e chamadas de atenção que indiscutivelmente, foram importantes para a minha formação, um muito e sincero obrigada.
A todos os meus colegas de laboratório (Ana, Joana, Déborah, Ricardo, Cláudia, Sara,
Alexandra e Ana Costa): por todo o companheirismo, ambiente memorável e momentos
divertidos proporcionados, que sem dúvida tornaram o trabalho mais agradável, assim como por todas as palavras de motivação e interajuda facultadas.
À minha Família (Pais e Ricardo): o meu eterno obrigada, por todo o amor e apoio incondicionais, compreensão e sacrifício que permitiram a realização deste sonho! Obrigada.
A todos os meus amigos: deixo a minha palavra de apreço por toda a força, motivação e conforto que sempre me ofereceram, bem como por todo o apoio incondicional.
Resumo
As bactérias Gram-positivas (Gram+) têm vindo a destacar-se no mundo moderno entre os agentes patogénicos mais comuns diretamente associados ao aumento de infeções clínicas. A aquisição de novos fatores de virulência por parte da bactéria bem como o desenvolvimento de resistência antimicrobiana destacam-se entre as causas mais frequentes desta realidade.
Listeria monocytogenes e Staphylococcus aureus são exemplos de Gram+ patogénicos
importantes, associados a elevadas taxas de mortalidade nos países desenvolvidos. A sua patogenicidade é conferida por várias proteínas, encontrando-se grande parte delas associadas à parede celular. Sabe-se que a parede celular das bactérias Gram+ é densamente decorada com glicopolímeros, entre os quais os ácidos teicóicos da parede (ATs), que desempenham importantes funções na fisiologia bacteriana, nomeadamente na defesa contra a atividade de substâncias antimicrobianas como os péptidos antimicrobianos (PAMs) e os antibióticos. Estudos recentes apontam para o importante papel das substituições dos ATs com açúcares não essenciais, na proteção bacteriana.
No presente trabalho, demonstrámos que as modificações dos ATs, tanto em L.
monocytogenes como em S. aureus, são cruciais para a resistência destas bactérias contra
PAMs e antibióticos. Adicionalmente, revelámos que a ausência de mais que uma glicosilação dos ATs potencia a susceptibilidade bacteriana a substâncias antimicrobianas. Assim, os nossos resultados sugerem que as glicosilações dos ATs retardam a entrada de agentes antimicrobianos diminuindo a permeabilidade da parede celular bacteriana aos mesmos.
Por fim, o nosso estudo confirmou que algumas modificações dos ATs são cruciais para manter os níveis proteicos de proteínas associadas à superfície celular, particularmente InlB e Ami, duas proteínas importantes ancoradas à parede celular através de uma região especifica composta por repetições de glicina-triptofano (domínio GW), inteiramente relacionadas com a virulência de L. monocytogenes. Concomitantemente, destacamos ainda o importante papel das glicosilações dos ATs em processos biológicos relacionados com a fisiologia bacteriana e a patogenicidade, sugerindo assim que os substituintes dos ATs são alvos promissores para fármacos direcionados para mecanismos de anti-virulência.
Keywords: ATs, Listeria monocytogenes, Staphylococcus aureus, PAMs, antibióticos, fatores de virulência
Abstract
Gram-positive (Gram+) bacteria have become the most common human pathogens associated with increasing clinical infections due to the acquisition of new virulence properties and/or antimicrobial resistance. Listeria monocytogenes and Staphylococcus aureus are two important Gram+ pathogens accounting for a huge number of deaths among developed countries and their pathogenicity is provided by several virulence proteins, most of them associated with the cell wall. Gram+ cell wall is densely decorated with glycopolymers such as wall teichoic acids (WTAs), with key roles in bacterial physiology including protection against the activity of antimicrobial peptides (AMPs) and antibiotics. The WTAs display different non-essential sugar substituents, whose role is directly related with bacterial protection.
In this work, we showed that the L. monocytogenes and S. aureus WTA glycosylations are required for resistance to AMPs and antibiotics. Moreover, we also found that in the absence of more than one WTA glycosylation the bacterial susceptibility is even more pronounced. Our data suggest that WTA-glycosylations promote a delay in the crossing of the bacteria cell wall by antimicrobial agents, possibly by decreasing the bacterial cell wall permeability.
Additionally, we demonstrated that some bacterial WTA-glycosylations are crucial to maintain the levels of cell surface associated InlB and Ami, two important GW proteins previously related with bacteria virulence. Altogether, our data highlight the important role of WTA glycosylations in biological processes related with bacterial physiology and pathogenesis, thus suggesting that WTA substituents hold promise for anti-virulence drug targeting.
Keywords: WTA glycosylations, Listeria monocytogenes, Staphylococcus aureus, AMPs, antibiotics, virulence factors
Contents
Agradecimentos ... i Resumo ... iii Abstract ... v Figure Index ... ix Table Index ... xiList of abbreviations ... xiii
Chapter I – Introduction ... 1
A. Gram-positive bacteria in infectious disease ... 3
A.1Listeria monocytogenes ... 3
A.1.1. History ... 3
A.1.2. Taxonomy and classification ... 4
A.1.3. General features and listeriosis ... 4
A.1.4. Cellular infection cycle ... 6
A.1.5. Major virulence factors ... 8
A.2 Staphylococcus aureus ... 12
A.2.1. History ... 12
A.2.2. Taxonomy and classification ... 13
A.2.3. General features ... 13
A.2.4. Staphylococcus aureus bacteremia/infection ... 14
A.2.5. Major virulence factors of S. aureus ... 14
B. Gram-positive cell envelope ... 16
B.1 Wall teichoic acids ... 17
Wall teichoic acids glycosylations in L. monocytogenes and S. aureus ... 18
B.2 Surface proteins and anchoring mechanisms ... 20
Non-covalently cell surface-associated proteins: GW proteins ... 21
C. Antibiotics ... 22
D. Antimicrobial peptides (AMPs) ... 24
Chapter II – Project presentation ... 29
Chapter IV – Results ... 43
Chapter V – Discussion ... 69
Chapter VI – Conclusion ... 77
Figure Index
Figure 1. Successive steps of human listeriosis and major organs affected by the disease ... 6
Figure 2. Schematic representation of the intracellular infection cycle of L. monocytogenes and the major virulence factors involved in adhesion (Ami) and bacteria internalization (InlA and InlB) ... 7
Figure 3. Schematic representation of (A) autolysin Ami and (B) internalin B ... 9
Figure 4. Entry of L. monocytogenes into host non-phagocytic cells ... 11
Figure 5. Schematic representation of cell wall structure of Gram+ bacteria ... 16
Figure 6. Structural components of WTA ... 17
Figure 7. Schematic representation of S. aureus wall teichoic acid biosynthesis pathway .... 18
Figure 8. Schematic representation of the wall teichoic acid structure of (A) L. monocytogenes serotypes 1/2a and 4b and (B) S. aureus ... 19
Figure 9. Major groups of L. monocytogenes surface proteins ... 21
Figure 10. Mechanisms of antibiotics resistance and their main targets ... 23
Figure 11. Schematic representation of proposed mechanisms of action for AMPs against bacteria ... 27
Figure 12. Construction of lmo1079 deletion mutant strain ... 45
Figure 13. Construction of lmo1079 deletion mutant strain ... 46
Figure 14. Growth behaviour of the different mutant strains and their respective WT in standard culture conditions in vitro (BHI at 37 ºC, with agitation) through optical density measurement ... 48
Figure 15. WTA glycosylation with GlcNAc in serotype 1/2a promotes L. monocytogenes resistance against AMPs ... 50
Figure 16. WTAs glycosylations with GlcNAc and L-rhamnose in serotype 1/2a promotes L. monocytogenes resistance against AMPs ... 52
Figure 17. WTAs glycosylations with both galactose and glucose in serotype 4b promotes L. monocytogenes resistance against AMPs ... 54
Figure 18. WTA-gly with α-O-GlcNAc of S. aureus promote a slight resistance against AMPs ... 56 Figure 19. E-test for gentamicin, ampicillin, benzylpenicillin and cefotaxime of L.
monocytogenes serotype 1/2a ... 58
Figure 20. E-test for gentamicin, ampicillin, benzylpenicillin and cefotaxime of L.
monocytogenes serotype 4b ... 60
Figure 21. E-test for vancomycin, rifampicin, clindamycin, benzylpenicillin, cefotaxime and oxacillin of S. aureus ... 62 Figure 22. WTA-glycosylations promote GW proteins association to L. monocytogenes 1/2a surface ... 64 Figure 23. WTA-galactosylation promotes GW proteins association to L. monocytogenes 4b surface ... 66 Figure 24. WTA-glycosylations are dispensable for GW proteins association to S. aureus surface ... 67
Table Index
Table 1. Bacterial strains and plasmid used in this study ... 35-36 Table 2. Primers used to amplify the flanking regions ... 37 Table 3. Range of antibiotic concentrations tested against L. monocytogenes and S. aureus. 39 Table 4. WTA-related function of the genes analysed in this work ... 47-48 Table 5. MICs ranges of L. monocytogenes serotype 1/2a ... 59 Table 6. MICs ranges of L. monocytogenes serotype 4b ... 61 Table 7. MICs ranges of S. aureus strains ... 63
List of abbreviations
ActA Actin assembly-inducing protein
Amp Ampicillin
AMPs Antimicrobial peptides
Arp2/3 Actin-related proteins 2 and 3
BHI Brain and heart infusion
bp Base pairs
CDCs Cholesterol-dependent cytolysins
CFUs Colony-forming units
Clf A Clumping factor A
Clf B Clumping factor B
Cna Collagen-binding protein
CNS Central nervous system
CRAMP Cathelicidin-related antimicrobial peptide
C-Met Tyrosine-protein kinase (receptor)
C-terminal Carboxy-terminal
DOC Sodium deoxycholate
DNA Deoxyribonucleic acid
DW Downstream region
E-cadherin Epithelial cadherin
Ery Erythromycin
ECM Extracellular matrix
EU/EEA European Union and European Economic Area
E-test Epsilometer test
FnBPA Fibronectin-binding protein A
FnBPB Fibronectin-binding protein B
GAGs Glycosaminoglycans
GC Guanine-cytosine content
GroP Glycerol 3-phosphate
GlcNAc N-acetylglucosamine
GPI Glycosylphosphatidylinositol
Gram+ Gram-positive
GW Glycine-tryptophan dipeptide
HGF Hepatocyte growth factor
InlA Internalin A InlB Internalin B IR Inter-repeat kDa Kilodalton LB Lysogeny Broth LCP LytR-CpsA-Psr protein LLO Listeriolysin O
LPXTG Leucine-Proline-any amino acid-Threonine-Glycine
LRR Leucine-rich repeats
LTAs Lipoteichoic acids
MCS Multiple cloning site
MH Mueller Hinton agar
MIC Minimum inhibitory concentration
MRSA Methicillin-resistant S. aureus
MSCRAMM Microbial surface components recognizing adhesive matrix molecules
MurNAc N-acetylmuramic acid
nm Nanometres
N-terminal Amino-terminal
PBPs Penicillin binding proteins
PBS Phosphate buffer solution
PCR Polymerase Chain Reaction
PGN Peptidoglycan
PI3K Phosphoinositide-3 kinase
PlcA Phosphatidyl-inositolphospholipase
PlcB Phosphatidyl-choline-phospholipase C
RboP Ribitol 5-phosphate
rRNA Ribosomal ribonucleic acid
ROS Reactive oxygen species
SDS Sodium dodecyl sulphate
SpA Staphylococcal protein A
Tar Teichoic acid ribitol
TAs Teichoic acids
TCA Trichloroacetic acid
TESS The European Surveillance System
UDP Uridine diphosphate
UP Upstream region
WASP Wiskott-Aldrich syndrome protein
WT Wild-type
WTA Wall teichoic acid
A. Gram-positive bacteria in infectious disease
Infectious diseases are still one of the major causes of mortality worldwide. Gram-positive (Gram+) bacteria have become the most common human pathogens associated with increasing clinical infections, ranging from mild skin infections to sepsis. Although a number of drugs are already available to treat these conditions, the emergence of new infections and the acquisition of new virulence properties or antimicrobial resistance by known bacteria is currently an alarming public health concern and a great challenge in medicine. The overall problem is exacerbated by the globalization being mass migration, urban areas congestion, global travel and the environmental changes, important players in this process. Therefore, it is crucial to develop innovative antivirulence strategies to disarm pathogens, rendering it harmless and allowing the natural host defences to fight infection.
The elucidation of the bacterial molecular mechanisms that cause infection has been of major scientific interest and provides crucial insights to develop new therapeutic strategies.
Listeria monocytogenes (L. monocytogenes) and Staphylococcus aureus (S. aureus) are two
important Gram+ pathogens and will be the focus of this study.
A.1
Listeria monocytogenes
A.1.1. History
L. monocytogenes is an interesting model organism to study host-pathogen interactions
and cellular microbiology [1]. The discovery of this bacterium dates back to 1926 and was firstly reported by E. G. D. Murray and his colleagues upon an epidemic outbreak among rabbits and guinea pigs in their laboratory in Cambridge, England. Due to the increased numbers of monocytes observed in the infected rabbits bloodstream, Murray named the new specie Bacterium monocytogenes [2]. The following year, J. Harvey Pirie isolated the same species from the liver of rodents in South Africa and renamed it Listerella hepatolytic, honouring the father of antiseptic surgery, Lord Joseph Lister [3]. However, as the International Committee on Systematic Bacteriology did not approve the name Listerella for taxonomic reasons, in 1940, Pirie proposed the current name Listeria monocytogenes [4].
Listeriosis was largely considered as a zoonosis at that time despite the cases of bacteremia and/or meningitis reported in humans, but it was only in 1981, after a severe outbreak of the disease in Canada, that was recognized as a foodborne human disease since most of the cases were related with the consumption of Listeria contaminated food and led to a high mortality rate [5–7]. Currently, this pathogen is recognized as a cause of many clinical
diseases such as gastroenteritis, bacteremia, central nervous system (CNS) and perinatal infections.
A.1.2. Taxonomy and classification
The biodiversity of genus Listeria comprises 20 recognized species, being two of them pathogenic: L. monocytogenes (infects humans and animals) and L. ivanovii (infects animals). The remaining species are non-pathogenic and live as saprophytes in nature (L. innocua, L.
marthii, L. seeligeri, L. welshimeri, L. rocourtiae, L. grayi, L. fleichmannii, L. floridensis, L. aquatica, L. newyorkensis, L. cornellensis, L. weihenstephanensis, L. grandensis, L. riparia, L. booriae, L. costaricensis, L. goaensis and L. thailandensis) [8–11].
In 2001, an important step forward in Listeria research was the publication of the first complete genome sequences of L. monocytogenes EGD-e and the phylogenetically close but non-pathogenic L. innocua CLIP 11262 [12]. Few years later, the whole-genome sequence of other Listeria species became also available [13–15]. Sequence analysis of 16S and 23S rRNA revealed not only a robust conservation but also a high stability in the genome organization among the different species of Listeria. However, the non-pathogenic L. innocua appears to have evolved from a L. monocytogenes ancestor but losing its virulence gene cluster [12].
Serotyping of Listeria strains became an important diagnosis tool for epidemiological purposes. The classification based on somatic (O) and flagellar (H) antigens allows the identification of 13 distinct serotypes in L. monocytogenes (1/2a, 1/2b, 1/2c, 3a, 3b, 3c, 4a, 4ab, 4b, 4c, 4d, 4e and 7). However, this classification is not very specific due to the high antigenic overlap between serotypes [16,17].
Thus, based on genetic-based subtyping methodology, L. monocytogenes serotypes were grouped into four evolutionary lineages: lineage I (1/2b, 3b, 4b, 4d and 4e), lineage II (1/2a, 1/2c, 3a and 3c), lineage III (4a and 4c) and lineage IV (7). The lineage I and the lineage II are commonly associated with human clinical cases of listeriosis. On the other hand, the lineages III and IV are rare and predominantly isolated from animal sources, namely ruminants [17].
A.1.3. General features and listeriosis
L. monocytogenes is a Gram+, facultative anaerobic and facultative intracellular
foodborne disease in susceptible hosts named listeriosis, through the ingestion of contaminated food products [18]. This small bacillus (0,5 x 1-2 m) is a low GC content bacteria unable to form capsule and spores [19].Its optimal growth occurs between 30 ºC and 37 ºC, although it is capable to grow in a broad range of temperature (<0 to 45 ºC). At temperatures up to 25 ºC, L. monocytogenes can survive by expressing a flagella which confers it motility [8,19,20]. L. monocytogenes is also able to resist to extreme pH (4.3 to 9, being optimal at 7) and salt conditions (up to 10% NaCl) [10,21]. This makes L.
monocytogenes particularly prone among foodborne pathogens to growth in refrigerated foods
with high salt content and low humidity being difficult to eliminate by some common food decontamination processes [22].
Phenotypically, L. monocytogenes is distinguished from the other Listeria species through biochemical tests that show its hemolytic activity, the positive catalase reaction, the incapacity to reduce nitrate to nitrite and positive reaction in the Voges-Proskauer test [10]. In addition, L. monocytogenes is also capable of fermenting D-arabitol, L-rhamnose, α-methyl D-glucoside, sucrose, maltose and lactose [10,23–25].
In the environment, L. monocytogenes is usually found in soil, water, vegetation, sewage and faeces, but it is also found in a variety of processed food such as cheese, meat, vegetables and dairy products [26]. Human contamination with L. monocytogenes occurs mainly following the ingestion of contaminated food products once Listeria has the advantage of overcoming harsh gastric conditions to reach the intestinal lumen. The transgression of the intestinal barrier by this pathogen is host-specific and mainly mediated by the interaction of
Listeria Internalin A and host E-cadherin exposed on goblet cells and on epithelial folds and
enterocytes from the villi [21]. In immunocompetent individuals, listeriosis may manifests itself as a febrile gastroenteritis. Once inside the host, it has the capacity to cross the intestinal epithelial barrier, disseminates through the mesenteric lymph nodes and blood, and ends up in the liver and spleen, which are the major target organs for bacterial colonization (Figure 1) [21,27,28]. Importantly, circulating bacteria may also gain access to the CNS by transgressing the blood-brain barrier or to the uterus by crossing the placental barrier (Figure 1) [21,29].
Figure 1. Successive steps of human listeriosis and major organs affected by the disease. Adapted
from Radoshevich and Cossart, 2017 [30].
The ability to cause disease depends on the infectious dose, on bacterial pathogenic potential and also on the immunological status of the host. Incidence of listeriosis has been increasing in the European Union and European Economic Area (EU/EEA) countries since 2008. During 2012–2016, between 1754 and 2555 cases of L. monocytogenes infections were reported to The European Surveillance System (TESS) in 30 EU/EEA countries [31,32]. In 2017, according to Surveillance Atlas of Infectious Diseases, 42 cases of listeriosis were reported in Portugal, with 7 of them resulted in deaths (fatality rate of 17%). The most affected age group is 65 or older, and the second most affected group comprised ages between 45 and 64 years old [33]. In Portugal, listeriosis has been notified since April 2014, but there is no active surveillance program for the disease [34].
Due to its high case-fatality rate, listeriosis is considered the first most frequent cause of foodborne infection-related deaths in Europe [35]. The therapy usually used to treat patients with invasive listeriosis involves the administration of β-lactams antibiotics (penicillin or ampicillin) combined with an aminoglycoside (gentamicin), unless in the cases of patients allergic to β-lactams, where the treatment with trimethoprim-sulphamethoxazole or erythromycin is considered [29].
A.1.4. Cellular infection cycle
The potential of L. monocytogenes to establish infection within different host tissues results from its capacity to invade and replicate in both phagocytic (macrophages, neutrophils and dendritic cells) and non-phagocytic cells (hepatocytes, enterocytes, epithelial and
endothelial cells) [36]. The first step of L. monocytogenes cellular infection cycle requires bacterial adhesion to the host cell membrane through the expression of surface proteins called adhesins. Afterwards, Listeria triggers its own internalization in non-phagocytic cells through the interaction of the bacterial surface proteins, InlA and InlB, and their respective eukaryotic membrane receptors, E-cadherin and Met, leading to a host cell cytoskeleton reshaping around the bacterium-cell interaction site [26,30,37]. Once inside the cell, L. monocytogenes is incorporated into a phagocytic vacuole, which is lysed by the bacterium through the expression of the pore-forming cytolytic toxin, listeriolysin O (LLO) and two phospholipases (phosphatidyl-inositolphospholipase (PlcA) and phosphatidyl-choline-phospholipase C (PlcB), thus releasing the bacteria free in the cytosol. Shortly after, cytosolic L.
monocytogenes exploits the host cells to uptake the required nutrients for its intracellular
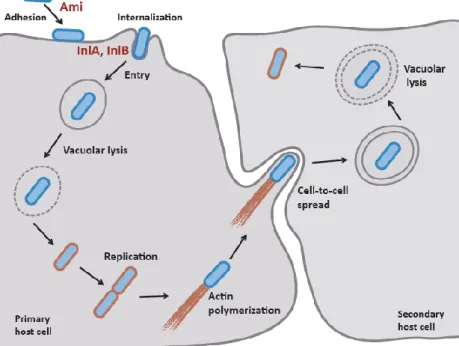
multiplication and deploys an actin assembly-inducing protein (ActA) to harness the actin polymerization machinery to allow bacteria spread from cell-to-cell. Through the formation of plasma membrane protrusions in the secondary host cell, L. monocytogenes disseminates to neighbouring cells without being exposed to the extracellular medium. Once inside the double-membrane vacuole of neighbouring cells, L. monocytogenes has the ability to escape from this secondary vacuole and restart its infection cycle, spreading throughout the infected tissues while keeping itself protected from the extracellular immune surveillance (Figure 2) [37,38].
Figure 2. Schematic representation of the intracellular infection cycle of L. monocytogenes and the
A.1.5. Major virulence factors
It is important to notice that the success of each step of L. monocytogenes infection is tightly controlled by the proper expression of multiple virulence factors. The positive regulatory factor A (PrfA) is the major transcriptional regulator responsible for the correct expression of most virulence factors that mediate L. monocytogenes intracellular life cycle. The self-regulation of prfA expression and protein activity involves complex transcriptional, post-transcriptional and post-translational mechanisms [39]. PrfA is able to activate gene transcription in response to a variety of environmental signals including temperature and nutrient availability [40,41]. Meanwhile, other Listeria virulence regulators have been described, such as Sigma B and VirR, whose regulated genes are involved in stress tolerance, carbohydrate metabolism, transport and cell envelope processes [42,43]. In this section, the most representative virulence factors will be briefly described.
Adhesion
The first contact with a eukaryotic cell surface is a crucial step for L. monocytogenes cellular infection cycle and involves a number of surface adhesion factors. The autolysin Ami is directly implicated in L. monocytogenes pathogenicity once it is required for efficient bacterial adhesion in eukaryotic cells and virulence [45]. The surface protein Ami (~99 kDa) is an autolytic amidase exhibiting N-acetylmuramyl-L-alanine amidase activity [46]. This protein possesses a (i) N-terminal catalytic domain responsible for the cleavage of the amine bond between N-acetylmuramic and L-alanine in the cell wall peptidoglycan (PGN), and (ii) a C-terminal cell wall-anchoring domain containing eight glycine-tryptophan (GW) repeated domains, by which Ami associates to the bacterial surface possibly interacting with lipoteichoic acids (LTAs) (Figure 3A) [47]. The Ami protein is found exclusively in association with the bacterial surface and its cell wall-anchoring domain is sufficient to mediate bacterial adhesion to epithelial cells [48]. Higher is the number of GW domains of Ami, higher efficient is the anchoring of this protein to the bacterial cell surface [45,47,48]. Besides its role in adhesion to eukaryotic cells, Ami also has a subtle role in motility due to a reduction on swarming [46].
Figure 3. Schematic representation of (A) autolysin Ami and (B) internalin B. LTA- Lipoteichoic
acids; GW- Glycine-Tryptophan repeated modules.
Internalization
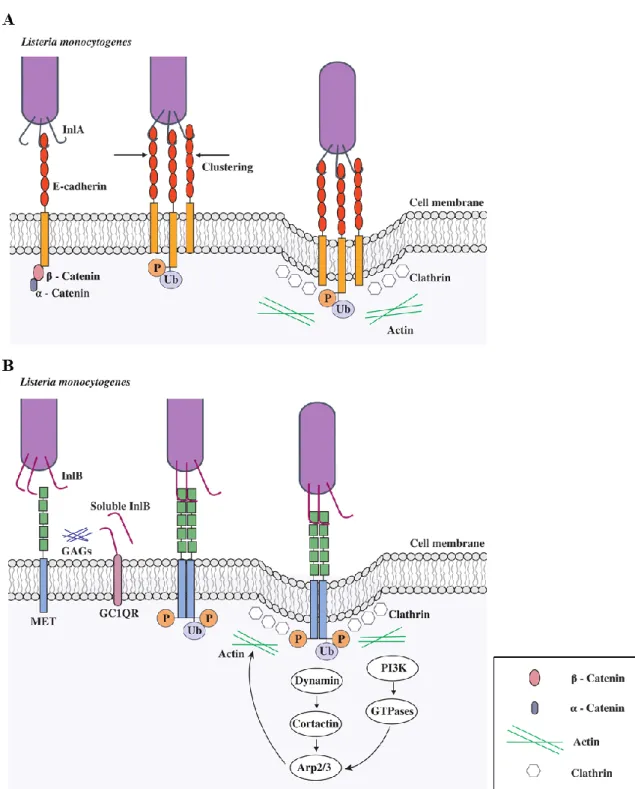
Bacterium internalization by phagocytic cells is mostly driven by the cell itself. However, internalization of L. monocytogenes into non-phagocytic cells, requires several invasins. The bacterium displays surface proteins (InlA and InlB) that interact with host cell-surface receptors, activating signalling cascades that lead to its internalization within host cells. The InlA and InlB proteins, both regulated by PrfA, are among the 27 proteins of the internalin family, composed by a signal peptide at the amino-terminal, with leucine-rich repeats (LRR) which are involved in protein-protein interactions with host-cell ligands [38,49].
InlA is a 800 amino-acid protein composed by 15 LRRs and also by an inter-repeat (IR) region (downstream the LRR region), responsible for binding the LRR repeats domain to E-cadherin [50]. The C-terminal domain of this protein display a LPXTG domain that promotes the covalent attachment of InlA to the cell wall [51]. The InlA receptor is the transmembrane glycoprotein E-cadherin that mediates adherens junctions formation and plays an important role in the maintenance of structural and functional tissue stability [52]. The engagement of E-cadherin by InlA triggers complex signalling pathways involving actin cytoskeleton rearrangements and clathrin-mediated endocytosis, which further culminates with bacteria internalization into the host cell (Figure 4A) [53]. The extracellular domain of E-cadherin is sufficient to bind InlA, whilst the intracellular one binds to catenins and promote
internalization [38,54]. The interaction between InlA and E-cadherin induces the phosphorylation of the receptor E-cadherin and consequently its ubiquitination, promoting the internalization of the bacterium [38].
InlB is encoded on the same operon of inlA [51]. It is a 630 amino-acid protein with a cell wall anchoring C-terminal domain similar to the Ami protein: the C-terminal displays three conserved GW repeated modules (Figure 3B), which mediate the association of the protein to the bacterial membrane through non-covalent interactions with LTAs [48,55]. Unlike InlA, InlB promotes bacterial invasion by interacting with different partners at the host cell surface. C-Met, which binds hepatocyte growth factor (HGF) was identified as a major InlB receptor [56]. This tyrosine kinase receptor is also capable to control cell migration, embryogenesis and metastasis of cancer cells [56,57]. InlB also binds to the cellular glycosaminoglycans (GAGs) and to the glycosylphosphatidylinositol (GPI) complement receptor gC1qR, which are not sufficient to allow internalization but are crucial to cooperate with HGF receptor (figure 4B). The interaction between InlB and c-Met promotes the receptor autophosphorylation, which induces the recruitment of some adaptor proteins that lead to the activation of phosphoinositide (PI)-3 kinase (PI3K), triggering host cell cytoskeletal rearrangements and clathrin-mediated endocytosis until the bacteria being engulfed by host cells [21,58]. The actin polymerization is initially coordinated by dynamin and cortactin of the Arp2/3 complex and then by a signalling cascade which involves, depending on the cell type, small GTPases Rac1 and Cdc42, abil, Wave and N-WASP. Upon bacterial internalization, depolymerization of actin is triggered by a LIM-Kinase and a cofilin protein [38].
The surface associated autolysin Auto (~62 kDa) is also involved on the internalization of L. monocytogenes into host cells. This autolysin contains a N-terminal autolysin domain followed by an amidase domain, and also a C-terminal cell wall-anchoring domain composed of four GW modules also implicated in bacterial adhesion [44].
Figure 4. Entry of L. monocytogenes into host non-phagocytic cells through (A) InlA and (B) InlB
signaling pathways. P- phosphorylation; Ub- ubiquitination; GAGs- glycosaminoglycans; GC1QR- complement component 1Q subcomponent-binding protein.
Vacuolar escape
To escape from the phagocytic vacuole, L. monocytogenes secretes a cholesterol-dependent pore-forming toxin, LLO, which is part of cholesterol cholesterol-dependent pore-forming cytolysins (CDCs) family and whose expression is positively regulated by PrfA [59,60]. LLO
is highly involved in Listeria escape from primary and secondary vacuoles impairing the phagosome-lysosome fusion through the formation of small pores. The activity of LLO needs to be tightly regulated for a safe pore formation and is quite dependent on pH, being its activity optimal at acidic pH. Beyond this, LLO has several additional functions described so far as (i) activate and amplified different signalling host pathways, (ii) mediate host cell apoptosis, (iii) control ROS production in L. monocytogenes-containing phagosomes, (iv) induction of autophagy and mitochondrial fragmentation and (v) is able to promotes the degradation of several host proteins [61–65].
Both phospholipases, PlcA and PlcB, contribute to LLO-mediated phagosomal membrane disruption [59,66]. LLO is secreted for the extracellular medium by Listeria, inducing a temporary calcium and potassium efflux across the host plasma membrane potentiating LLO-dependent internalization [66].
Replication, intracellular motility and cell-to-cell spread
Once within the cytosol, L. monocytogenes uses an hexose phosphate transporter (Hpt), whose expression is also dependent on PrfA, to import hexose sugars available in the cytoplasm as a carbon energy source for its growth and replication [67–69]. L. monocytogenes possess the actin-based motility that mediates bacterial propulsion within the cytosol and promotes the cell-to-cell spread, modulating the speed and direction of the bacterial movement. The surface-anchored protein ActA interacts with the host actin nucleator Arp2/3 complex, mediating actin polymerization and thus the formation of actin filaments [70–73]. Besides its role in bacterium motility, ActA is involved in L. monocytogenes attachment and internalization within several host cells. It also plays a role in escaping from autophagy, either by conferring actin-based movement to the bacteria or by actin-masking the bacteria that is no longer recognized by the autophagy machinery [21,72].
A.2 Staphylococcus aureus
A.2.1. History
Staphylococcus was first described in 1880 by a Scottish surgeon, Sir Alexander
Ogston. During a surgical procedure, he noticed groups of bacteria in pus, from surgical abscess in a knee joint [74]. Ogston inject pus from acute abscesses into guinea pigs and mice and demonstrated that the blood of the septic animals acquired micrococci arranged in
clumps. Take this into account, he proposed that acute abscesses were cause for micrococci and named the cluster micrococci by “staphylococci” [75]. In 1884, the German physician Friedrich J. Rosenbach isolate two strains of staphylococci and based on the color of the pigmented colonies he named them: Staphylococcus aureus (S. aureus), from the latin aurum which means gold, and Staphylococcus albus (currently called Staphylococcus epidermidis due its ubiquity on human skin), from the latin albus (white) [76].
A.2.2. Taxonomy and classification
The genus Staphylococcus comprises more than 30 different species of Gram+ catalase-positive cocci including commensals and pathogens of human and animal species. This genus is distinguished from the others genera (such as Micrococcus) due to their pigment and their ability to produce acid from glucose aerobically [77]. The sequence analysis of 16S rRNA revealed a highly conserved genome organization between the species, being itself insufficient to differentiate between species [78]. Recent phylogenetic analysis allowed to organize staphylococcal species into six groups composed of a 15 refined cluster groups [79]. However, lateral gene transfer compromises this phylogenetic analysis [80].
S. aureus is the most common and important staphylococcal species causing human
disease, even though other staphylococcal species have also been implicated in human infections, among them S. intermedius, S. delphini, S. schleiferi, S. lutrae, S. epidermidis, S.
haemolyticus, S. lugdenensis and some strains of S. hyicus [81,82].
A.2.3. General features
Staphylococcus aureus is a facultative anaerobe and a non-spore forming bacterium that
causes a wide range of clinical infections. It colonizes human body, either acting as a commensal bacterium or often causing superficial skin infections such as abscesses and impetigo or serious invasive infections such as septic arthritis, pneumonia, osteomyelitis, endocarditis and atherosclerosis [83–86].
S. aureus possesses a spherical shape - the cocci form of about 0.5-1 µm in diameter -
and a DNA with low GC content. It has also the ability to form clumps that grow in a wide range of temperatures (7-48.5 ºC; being optimal at 37 ºC), pH (4.2 to 9.3; being optimal at 7– 7.5) and sodium chloride concentration (up to 15 %) [84,87]. In addition, this pathogen can change its osmotic pressure and survive to host bactericidal factors and environmental stresses [86]. Moreover, this bacterium is characterized as an organism tolerant to desiccation, being
capable to survive in potentially dry and stressful environments, such as the human nose and skin but also in fomites like clothing and surfaces, being easily dispersed through the air or carried on materials [88]. These characteristics are also a competitive advantage for S. aureus growth in many food products [89].
A.2.4. Staphylococcus aureus bacteremia/infection
The infections caused by S. aureus have been reaching globally epidemic proportions often associated with high mortality rates. Treatment of these infections with classical antibiotics has become increasingly difficult overtime due to the emergence of multidrug-resistant strains, such as methicillin-multidrug-resistant S. aureus (MRSA) strains [84,90].
S. aureus infection comprises essentially five important stages: (1) colonization, (2)
local infection, (3) systemic dissemination and/or sepsis, (4) metastatic infection and (5) toxinosis [91]. The most important biological property of S. aureus is the ability to asymptomatically colonize healthy individuals [92]. Therefore, healthy individuals carrying S.
aureus as a part of the commensal flora and may contaminate immunocompromised
individuals by direct contact skin-to-skin, but also through contaminated objects and surfaces [84,92]. Localized infection occurs when the pathogen is inoculated into the skin from a site of carriage, leading to the formation of local skin abscesses. The infection may spread locally and give rise to different clinical manifestations such as carbuncle, cellulitis, impetigo and wound infection, or it can penetrates the skin or subcutaneous tissues and gain access to the blood, infect any organ and cause septic shock [84,91]. The infection dissemination may also result in specific staphylococcal conditions, such as endocarditis and osteomyelitis. Even if this pathogen does not access into the bloodstream, it has the capacity of cause specific syndromes (e.g. shock syndrome, foodborne gastroenteritis and scalded skin syndrome) through the secretion of specific toxin [91].
A.2.5. Major virulence factors of S. aureus
A successful staphylococcal infection depends on the expression of several virulence factors, such as toxins, immune-modulatory factors and exoenzymes, under stressful stimuli (e.g. host immune response or antibacterial agents) [93,94]. The most representative virulence factors of S. aureus are briefly discussed below.
Adherence factors
The colonization process is initiated by the attachment of S. aureus to the host cells surface. This process is mediated by several adhesins responsible to adhere to the host extracellular matrix (ECM). The specific bacterial surface adhesins belong to the class of MSCRAMM (microbial surface components recognizing adhesive matrix molecules) and are covalently attached to the peptidoglycan by the LPXTG sequence located in their C-terminal portion [95–97]. The typical members of the MSCRAMM family are staphylococcal protein A (SpA), fibronectin-binding proteins A and B (FnBPA and FnBPB, respectively), collagen-binding protein (Cna) and clumping factor A and B (Clf A and Clf B) [84,95]. Moreover, Atl is a major autolysin peptidoglycan hydrolase, previously reported to mediate staphylococcal attachment to several host cellular components during infection such as fibronectin, gelatin, vitronectin and heparin. This autolysin was also found to activate platelets and induce the formation of aggregates in whole blood. It is involved not only in cell separation during the cell division, but also in bacterial cell wall degradation, by the cleavage of the bond between N-acetylmuramic and L-alanine in cell wall PGN [98,99].
Exoproteins
For invasion of tissues, S. aureus has the ability to secret exotoxins with cytolytic activity (including the α-, β- and γ- hemolysins) that may disrupt the skin barrier by forming pores in skin cell membranes, and several enzymes (including nucleases, proteases, lipases, collagenase and hyaluronidase) that destroy the tissues [84,100]. S. aureus is also able to evade the host immune response by secreting anti-opsonizing proteins as the chemotaxis inhibitory protein, which may prevent its own phagocytosis by neutrophils. For escaping the host immune system, S. aureus is able to secrete both leukocidins that lyse leukocytes, and enterotoxins that are superantigens with potent mitogenic activity on T cells and thus change the normal immune response. The staphylococcal protein A, located at the surface of S.
B. Gram-positive cell envelope
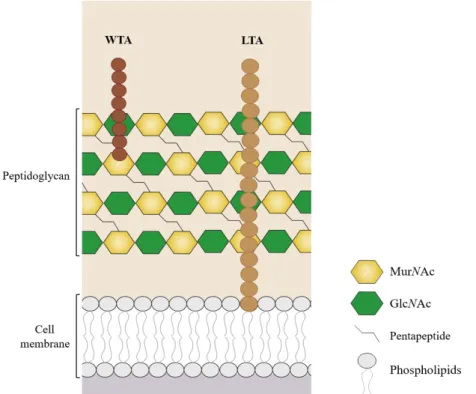
Gram+ bacteria possess a complex cell wall organization that provides a structural support for bacteria and plays a crucial role in protecting these organisms from their external and often harsh environments. While the cell envelope of Gram-negative bacteria is composed by an inner membrane, a peptidoglycan cell wall and an outer membrane, the one of Gram+ bacteria has a multi-layered architecture composed just by the inner membrane and a thicker PGN layer, which accounts for about 40% of the total cell wall mass and confers resistance to turgor pressure and protect them from external aggressions (Figure 5) [101–103].
Figure 5. Schematic representation of cell wall structure of Gram+ bacteria. WTA, wall teichoic
acids; LTA, lipoteichoic acids.
The PGN is a highly polymerized macromolecule, composed of linear, parallel glycan strands linked perpendicularly by short peptide bridges, whose chemical unit structure consists of a disaccharide composed of N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc) linked by β(1-4) glycosydic bonds (Figure 5) [104]. In addition, it serves as a scaffold for attaching proteins, polysaccharides and glycopolymers, such as lipoteichoic acids (LTA) and wall teichoic acids (WTA) with relevant physiological roles [105]. Both Gram+ bacteria, L. monocytogenes and S. aureus, possess this cell wall organization. We are particularly interested to understand the specifications of their WTAs, respective glycosylations and the main surface proteins associated, which will be the focus of this section.
B.1 Wall teichoic acids
The Gram+ PGN is densely decorated with glycopolymers such as LTAs, which are anchored to the head groups of membrane lipids by a diacylglycerol, and WTAs, which are covalently attached to peptidoglycan via phosphodiester linkage (Figure 5). Bacterial glycopolymers often display highly variable structures and have crucial roles in the protection of the bacteria as well as in bacterial adhesion to the host cells, inflammation and immune cell activation [101,102,106–108].
WTA are the most abundant PGN-linked polymers in several Gram+ organisms such as
L. monocytogenes and S. aureus [109]. These anionic glycopolymers present two essential
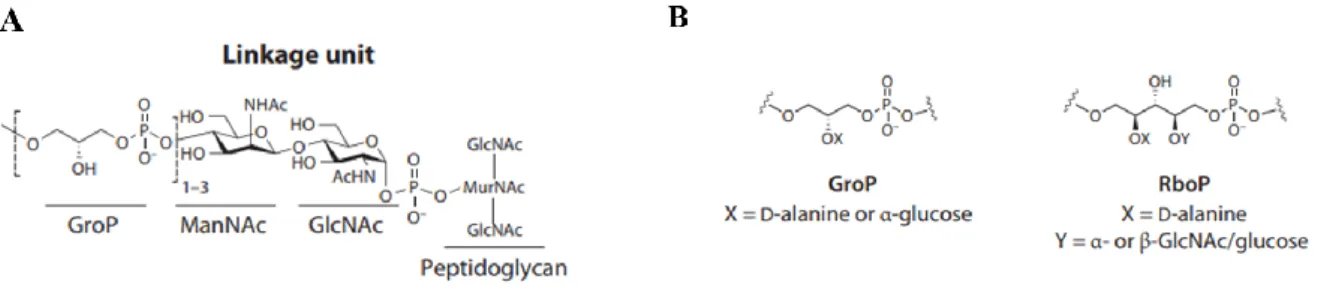
components: (1) a disaccharide linkage unit and (2) a main chain polymer composed of polyol repeat units, linked through a phosphodiester bond [109,110].The disaccharide linkage unit is conserved across bacterial species and consists in a GroP-N-acetylmannosamine (ManNAc)-β(1,4)-GlcNAc triad linked by a phosphodiester bond to the C6 hydroxyl group of MurNAc residues (Figure 6A) [105]. The biochemical and structural diversity observed between WTAs is mainly due to the type of blackbone monomers and glycosyl substituent groups. The most common structures contain a linear chain of phosphodiester-linked repeats of either glycerol 3-phosphate (GroP) or ribitol 5-phosphate (RboP) (Figure 6B) [109].
Figure 6. Structural components of WTA: (A) conserved disaccharide linkage unit composed by
GroP-ManNAc-P, which is covalently attached to PGN; (B) most common structures of WTA repeat units observed in Gram+ bacteria. GlcNAc, N-acetylglucosamine; MurNAc, acid N-acetylmuramic; ManNAc, N-acetylmannosamine; GroP, glycerol 3-phosphate; RboP, ribitol 5-phosphate. Adapted from Brown et al., 2013 [109].
The WTA biosynthesis occurs in the cytoplasmic membrane on a diphospholipid carrier (bactoprenol) that is also recruited for PGN biosynthesis [105]. The WTAs are sequentially synthetized by a number of cytosolic-exposed plasma membrane associated Tar (teichoic acid ribitol) enzymes, starting by TarO which catalysed the transfer of GlcNac-1-phosphate from cytoplasmic UDP-GlcNac to bactoprenol. Then, the enzyme TarA mediate the binding of ManNAc to GlcNAc, and TarB transfer a RboP molecule from CDP-glycerol to ManNAc,
completing the linkage unit synthesis [111]. Once the ribitol phosphate is completed, it is translocated across the plasma membrane by the TarGH complex and, in the other side of the membrane, a family of WTA ligases (LCP proteins in S. aureus) promote their attachment to the PGN and D-alanylation of WTAs. The released bactoprenol carrier is recycled (Figure 7) [111–113].
Figure 7. Schematic representation of S. aureus wall teichoic acid biosynthesis pathway [113] .
Wall teichoic acids glycosylations in L. monocytogenes and S. aureus
The repeated polymeric units of WTA possess a high diversity of non-essential sugar substituents whose absence prevents bacteriophage binding and impacts antigenicity, sensitivity to antibiotics and virulence [109]. In L. monocytogenes, WTA are mostly composed of repeated ribitol-phosphate (RboP) subunits, whose hydroxyl groups can be substituted with a diversity of monosaccharides [114]. These specific WTA substitutions are characteristic of specific L. monocytogenes serotypes: GlcNAc is common to serotype 1/2 and 3, but the serotype 1/2 also contains L-rhamnose, while the serotype 4b displays D-glucose and D-galactose (Figure 8A) [115]. In S. aureus, WTA are composed of ~40 ribitol-phosphate repeating units modified with D-alanine and/or with GclNAc via α- or β-glycosidic linkages (Figure 8B) [116–119].
WTAs play key functional roles in bacteria physiology, including cation binding, osmotic and heat tolerance, regulation of autolysin activity, phage-binding and cell-shape determination [120,121]. Furthermore, it has been shown that WTAs have an important role
for Gram+ pathogens survival, namely by coordinating mechanisms required for host infection, colonization, resistance to antimicrobial peptides (AMPs) and drug resistance [109,113,122–125].
Figure 8. Schematic representation of the wall teichoic acid structure of (A) L. monocytogenes
serotypes 1/2a and 4b and (B) S. aureus. GlcNAc, acetylglucosamine; ManNAc, N-acetylmannosamine; MurNAc, N-acetylmuramic acid.
Previous studies reported the first genome-wide transcriptome of L. monocytogenes infecting a host deep organ and revealed higher expression levels of rhamnose biosynthesis genes (rmlACBD, which encompasses genes from lmo1081 to lmo1084), during in vivo mouse infection [121,126]. It has also been demonstrated that rmlACBD locus is required for the association of L-rhamnose with L. monocytogenes WTAs, probably by providing the molecular machinery responsible for the synthesis of L-rhamnose [121].
The WTA glycosylation is catalysed by glycosyltransferases which are a class of enzymes that recognize nucleotide-sugar substrates and transfer them to a WTA subunit [127]. The decoration of L. monocytogenes WTAs with L-rhamnose requires, beside the RmlACBD biosynthetic enzymes, the expression of the rmlT gene, which has been shown to encode the rhamnosyltransferase RmlT [121]. This rhamnosyltransferase seems do not interfere with the transcription of rmlACBD locus, once the L-rhamnose is synthesized in the absence of RmlT. However, it was conveniently demonstrated that, in the absence of rmlT gene, WTAs decoration with L-rhamnose does not occur. Therefore, we know that rmlT is required for the decoration L. monocytogenes WTAs with L-rhamnose [121]. In addition, it was previously shown that WTA L-rhamnosylation is a bacterial mechanism crucial to promote resistance to AMPs by reducing the cell wall permeability to them, delaying both
their crossing by this barrier and the disruption of the plasmatic membrane, thus favouring L.
monocytogenes survival and virulence in vivo [121,128].
S. aureus can tolerate high concentrations of a number of AMPs. It is currently known
that the functional D-alanine biosynthesis pathway of WTA decoration, encoded by the
dltABCD operon, contributes for the resistance to cationic AMPs as defensins or cathelicidins,
bacteriocins and glycopeptide antibiotics such as vancomycin or teicoplanin [124,129,130]. It is also known that the proteins TarM and TarS are responsible for the glycosylation of WTA with α-O-GlcNAc and β-O-GlcNAc respectively, both exhibiting a GlcNAc-transferase activity [131,132]. Nevertheless, it was demonstrated in previous studies that the WTA glycosylations of S. aureus play a particular role in maintaining resistance to β-lactams in MRSA strains, namely by the protein TarS which is required to β-lactam resistance [132,133]. Recent studies showed that other enzyme, TarP, encodes an alternative WTA glycosyltransferase responsible by transfer GlcNAc to a different hydroxyl group of WTA RboP and has important consequences for immune recognition, namely by replacing the TarS protein function in several key interactions [134].
It is also reported that WTA are required for β-lactam resistance in many Gram+ bacteria, as S. aureus and have the ability to modulate susceptibility to cationic antibiotics and antimicrobial peptides in various organisms, such as L. monocytogenes. Thus, given its importance in pathogenicity, the WTA are promising targets for new therapeutics to overcome multi-resistant bacterial infections [109,128].
B.2 Surface proteins and anchoring mechanisms
The cell wall of Gram+ bacteria is well equipped with a diversity of surface proteins associated with their envelope or secreted to the extracellular medium, some of them shown to be involved in infection [128]. Due to their extracytoplasmic localization, these proteins are able to interact directly with host cells receptors thus establishing infection [135].
The bacterial surface proteins are synthetized in the cytoplasm for further export to the plasma membrane; once in the plasma membrane the proteins will be translocated to the other side of the lipid bilayer, where they can be associated with the cell envelope or released for the extracellular medium. The translocation is mainly carried by a specialized secretion system, the canonical Sec-dependent pathway, which recognizes and translocate protein precursors (preproteins) containing N-terminal signal peptide-containing polypeptides. Posteriorly, preproteins are cleaved by signal peptidases and, depending on the presence and the nature of surface anchoring domains, the processed protein may be membrane-associated
(such as lipoproteins, hydrophobic tail proteins or integral membrane proteins) or associated with the cell wall through covalent or non-covalent interactions (Figure 9) [128]. As shown in figure 9, the L. monocytogenes proteins are covalently linked to the cell wall peptidoglycan by their C-terminal LPXTG (Leucine-Proline-any aminoacid-Threonine-Glycine) motif, whereas other proteins are non-covalently attached by its C-terminal, including GW and hydrophobic tail proteins, anchored to the peptidoglycan and cytoplasmic membrane, respectively. In this study, we are particularly interested to study the GW proteins [128,136].
Figure 9. Major groups of L. monocytogenes surface proteins. Adapted from Cabanes et al., 2002
[49].
Non-covalently cell surface-associated proteins: GW proteins
The non-covalent interaction with the cell wall through GW modules was firstly reported for L. monocytogenes InlB protein [48]. Three GW modules (Figures 3B and 9) were found in the C-terminal region of InlB and were implicated in the interaction with LTA on the bacterial surface and also with glycosaminoglycans on mammalian cells [48,55,58]. The GW modules are essential for protein cell anchoring and for interaction with LTA, and higher number of GW modules contribute for the stronger attachment to the bacterial cell wall [137]. Other L. monocytogenes GW protein is the surface-associated protein Ami, an amidase with autolytic activity that possess eight GW modules (Figure 3A) [138]. Besides L.
monocytogenes, proteins containing GW modules have also been reported in other Gram+
pathogens such as S. aureus, as the case of the autolysin Atl [139]. Most of these proteins appear to be associated with autolytic activities, once they can degrade the PGN cell wall layer [128,140].
C. Antibiotics
Nowadays, antibiotics are the most successful drug category of antimicrobial substances used in human health care to prevent and treat bacterial infections, since the accidentally discovery of penicillin in the late 1920s by Alexander Fleming [141–143]. From that time, several classes of antibiotics have appeared with complex and different mechanism of action: (i) inhibitors of cell wall biosynthesis, (ii) inhibitors of protein synthesis, (iii) inhibitors of nucleic acids biosynthesis and (iv) antimetabolites (Figure 10) [144].
The inhibitors targeting the cell wall encompass the β-lactams and the glycopeptides families of antibiotics. Their mechanism of action is focused on target the PGN biosynthesis, in one of the three stages: (a) cytoplasmatic stage, inhibiting the production of GlcNAc and MurNAc, (b) membrane-associated stage, inhibiting the precursor of lipid intermediates responsible by the translocation of monomer units from the cytoplasm to the PGN and (c) parietal stage, inhibiting the transpeptidases (penicillin-binding proteins, PBPs) that are required for the cross-link of the PGN chains [145].
The inhibitors of protein synthesis act on the bacterial 70S ribosomes targeting the 30S subunit (aminoglycosides and tetracyclines) or the 50S subunit (macrolides, oxazolidinones and chloramphenicol) [146–148].
The class of nucleic acids biosynthesis inhibitors, such as quinolones, are able to impair the nucleic acid synthesis through the formation of intermediary compounds [148,149].
The antimetabolites such as sulfonamides and trimethoprim are mainly important to inhibit the folic acid metabolism, which is important for biosynthesis of a wide range of cellular components [148,150].
Actually, the successful use of the antibiotics is compromised not only due to the appearance of strains resistant to antimicrobials substances but also due to the overuse or inappropriate use of these antimicrobial substances [151,152]. The infections caused by antibiotic-resistance strains often occur in epidemic waves. The resistant bacterial strains develop resistance to antibiotics by four main mechanisms (Figure 10): (i) prevention of cell penetration through cell permeability alteration, (ii) inhibition of the drug-target interaction, (iii) modification of the drug-binding site in target proteins and (iv) efflux pumps, that allow the drug pumping out again before reach its target [153,154].
Methicillin-resistant S. aureus strains mainly accounts for these epidemics, being in part responsible for a large percentage of β-lactam-resistant infections [92,132]. Previous studies showed that proteins involved in cell wall biosynthesis can modulate the resistance to methicillin [155]. For L. monocytogenes, it was verified that the acquired resistance mechanisms is not related with inappropriate usage of clinically relevant antibiotics, but with
the transfer of resistance genes from other bacteria and the recent increasing minimum inhibitory concentrations (MICs) of aminopenicillins, which clearly reinforces the need for microbiological surveillance [156]. Thus, the development of new strategies and/or new therapeutics is important mainly for fight this reality.
D. Antimicrobial peptides (AMPs)
Antimicrobial peptides belong to a large family of small peptides (<10 kDa) produced by all forms of living organisms and are crucial players of the innate immune response against microbial pathogens [157]. The major advantages of these peptides are their broad-spectrum antimicrobial activity against microorganisms, ranging from Gram+ and Gram- bacteria, fungi and virus, and their low propensity for developing resistance [158,159].
Although AMPs are quite diverse in nature, they contain conserved features that are crucial for their antimicrobial activity: small size, cationic charge, hydrophobic character, amphipathicity and structural conformation [157,160]. They generally possess around 30 hydrophobic residues arranged to be amphipathic and therefore soluble in aqueous environments and lipid membrane bilayers [157,161].
AMPs cationic character is crucial for the interaction with a bacterial surface, once it an electrostatic attraction with negatively charged bacterial membrane phospholipids, as well as their hydrophobicity aids integration into the bacterial cell membrane that leads to membrane disruption and consequently bacterial death [162]. However, some AMPs can also be anionic and by this way their interaction with bacterial surfaces appears to be mediated by cationic salt bridges [163]. Previous studies demonstrated that these AMPs can cross membranes inhibiting cell wall synthesis and also targeting intracellular processes such as nucleic acid and protein biosynthesis or impair certain enzymes and chaperones functions [159,164–166].
The AMPs can adopt different structural conformations: (i) linear -helical, free of cysteine residues, (ii) packed -sheets or (iii) extended unorganized structures [167]. The first two groups are the most common ones in nature [157]. -helical AMPs maybe be unstructured in aqueous environmental, although they are able to adopt -helical structure whenever in contact with lipid membranes, such as the bacterial membranes [162,168]. An important requirement of -helical AMPs is the amidation of the AMP C-terminus that enhance the electrostatic interaction between the positively charged peptide and the negatively charged bacterial membrane. This interaction is important for the stabilization of the helical structure at the membrane interface, thus promoting a more efficient antimicrobial activity [169].
The second most abundant group of AMPs are the -sheet, which contain cysteine residues that are conserved and able to form intramolecular disulphide bonds [170].
Lastly, the unstructured extended AMPs are composed by specific amino acids such as tryptophan and arginine. The positive charges of arginine attract the peptide towards the
anionic bacterial surface, while tryptophan residues stabilize the peptide in solution via intramolecular hydrophobic interactions and promote a strong association to the interfacial region of lipid bilayers [171].
Based on several criteria, such as secondary structure, biosynthesis pathway, activity, bonding profile and cellular target, the AMPs can be classified into three major classes: bacteriocins, defensins and cathelicidins [172].
Bacteriocins are synthetize in the bacterial ribosome and secreted as cationic and hydrophobic AMPs, capable to disarm Gram+ pathogens. These heterogeneous molecules are also active against Gram- pathogens, only when the integrity of the extracellular membrane has been compromised [173,174]. Their major mechanism of action against bacteria is pore formation and cell permeabilization [175].
Defensins are found in all mammals but also in birds, reptiles, invertebrates, plants and fungi, especially in cells and tissues that are involved in host defense against microbial infections. Their major mechanism of action consist in the permeabilization of target membranes and their disulphide bonds promote the stability of its structure and avoids their proteolytic degradation [162,176,177].
Cathelicidins are one of the most diverse AMPs of vertebrates, mainly found in mammals. Beyond their antimicrobial activity, cathelicidins also playing a key role in immunomodulatory and inflammation responses [178,179]. Cationic cathelicidins can adopt the linear -helical structure or a linear unstructured conformation [180]. They are characterized by a specific cathelin-like domain and are store in the secretory granules of neutrophils and macrophages. Their secretion occurs upon cell activation, leading to the cleavage of their N-terminal domain, which includes the cathelin domain. This cleavage is responsible for peptide processing into a mature form promoting the biological peptide activation [178]. Once activated, these AMPs are able to bind and disrupt negatively charged membranes, such as the membrane phospholipids of the bacteria, leading to the cell death [181]. The human peptide LL-37 and its homologue CRAMP in mice are members of the cathelicidins family [178].
The AMPs are part of the first line of defence against a pathogenic agent since they are produced by immune cells such neutrophils and macrophages [182]. Thus, AMPs are able to modulate the immune response through the recruitment and activation of immune cells [183]. In addition, to the ability in modulate the immune response, the AMPs also can exhibit their
antimicrobial activity through direct killing of the pathogen by targeting or not the bacterial membrane [183].
Targeting the bacterial membrane permeabilization
AMPs preferentially target microbial membranes without interacting with specific receptors. They are able to interact with the components of the membrane promoting its permeabilizing [184]. The bacteria cell wall contains glycopolymers (TAs) and lipopolysaccharides (LPS) as well as membrane phospholipids that confer the negative charge to the bacterial surface, allowing their electrostatic attraction and hydrophobic interaction with cationic AMPs [162,185]. Subsequently, AMPs binds the bacterial membrane to change its permeability and thus promote bacterial death [164]. AMPs can destabilize the membrane integrity through different mechanisms, depending on their intrinsic properties and on membrane composition and architecture: (A) the toroidal-pore model, (B) the barrel-stave model and (C) the carpet model (Figure 11). The toroidal-pore model involves the attachment of AMPs and their consequent aggregation to the membrane, pushing the outer membrane leaflet inwards and thus form a positive charged curvature strain to allow peptides insertion (Figure 11A) [186]. In the barrel-stave model, the attached AMPs aggregate and penetrate into the membrane bilayer, promoting a transmembrane pore configuration, where the hydrophobic peptides with the lipid core region and the hydrophilic peptide regions are aligned in the interior region of the pore (Figure 11B) [164]. In this pore formation mechanism, the -sheet conformation is required [164]. Finally, the carpet model defends that the AMPs disrupt the membrane by orienting parallel to the surface of the lipid bilayer, covering the surface of the membrane and forming an extensive layer or carpet (Figure 11C) [187]. The cathelicidin LL-37 and CRAMP are examples of AMPs acting by this way [187].


![Figure 7. Schematic representation of S. aureus wall teichoic acid biosynthesis pathway [113]](https://thumb-eu.123doks.com/thumbv2/123dok_br/16070235.1106386/44.892.111.775.297.652/figure-schematic-representation-aureus-wall-teichoic-biosynthesis-pathway.webp)

![Figure 9. Major groups of L. monocytogenes surface proteins. Adapted from Cabanes et al., 2002 [49]](https://thumb-eu.123doks.com/thumbv2/123dok_br/16070235.1106386/47.892.239.671.365.668/figure-major-groups-monocytogenes-surface-proteins-adapted-cabanes.webp)
