BiophysicalJournal Volume 71 October 1996 1869-1876
Reversible
Adsorption
and Nonreversible Insertion of Escherichia
coli
a-Hemolysin
into
Lipid
Bilayers
Laura Bakas,* Helena Ostolaza,* Winchil L. C. Vaz,# and Felix M.
Gonfi*
*Grupo Biomembranas (UnidadAsociada alC.S.I.C.), DepartamentodeBioquimica, UniversidaddelPais Vasco,48080Bilbao,Spain, and#Unidade de Cieancias ExactaseHumanas, Universidade doAlgarve, P-8000Faro, Portugal
ABSTRACT a-Hemolysin isanextracellularproteintoxin(107 kDa) produced bysomepathogenicstrains of Escherichiacoli.
Although stable in aqueous medium, it can bind to lipid bilayers and produce membrane disruption in model and cell membranes.Previous studies had shown that toxin bindingto thebilayerdid notalwayslead to membranelysis.In thispaper,
wefind that a-hemolysin maybind the membranesinatleasttwoways,areversibleadsorptionandanirreversibleinsertion. Reversibilityisdetected by the ability of liposome-boundtoxin to inducehemolysisof added horseerythrocytes; insertionis accompaniedbyanincrease intheprotein intrinsicfluorescence. Toxin insertion does notnecessarilylead tomembranelysis. Studies ofa-hemolysin insertion into bilayers formed fromavarietyofsinglephospholipids,orbinarymixturesof phospho-lipids,orofphospholipidandcholesterol, reveal that irreversible insertionis favoredbyfluidovergelstates, bylowoverhigh cholesterol concentrations, by disordered liquid phasesover gel orordered liquid phases, and by gel over ordered liquid phases. These resultsarerelevant to the mechanism ofactionofa-hemolysin andprovidenewinsightsinto themembrane
insertion oflarge proteins.
INTRODUCTION
Escherichia coli a-hemolysin (HlyA) is a 107-kDaprotein
secretedbypathogenic strains of thisbacteriumandbelongs
to the so-called RTX protein family, characterized by a glycine-rich nonapeptide repeat region near the C-terminal
end (see Coote, 1992, for a review). In addition to this repeat domain, the protein has a hydrophobic region near the N-end (Menestrina et al., 1994, 1995), andaC-terminal
signal peptide (Zhang et al., 1995; Chervaux and Holland,
1996). The mature protein contains two fatty acyl residues linked to internal lysines (Stanley et al., 1994). HlyA dis-rupts eukaryotic cell membranes andforms cation-selective
channels in planar lipid membranes (Menestrina et al., 1995). The toxin is also capable of inducing leakage of
phospholipid large unilamellar vesicles (Ostolaza et al.,
1993).Ingeneral,there is ample
experimental
evidence thatHlyA, although existing in soluble form after its
secretion,
may become membrane-associated to produce membrane
disruption.
The mechanism ofHlyAinsertion in lipidbilayers is not known in detail. Besides the fact that some cells might contain HlyA receptors, pure lipid bilayers and vesicles constitute a good model for this study. We have recently
described methods for separately
measuring
toxin bindingtomembranes and toxin-inducedlysis, and found that
bind-ing is not
necessarily
followed by membrane damage. InReceivedfor publication 2May1996and infinalform3 July1996.
Address reprint requests to Dr. Felix M. Gonii, Departamento de Bioqufmica, Universidad del Pais Vasco, Aptdo. 644, 48080 Bilbao, Spain. Tel.: 34-4-464-7700, ext. 2407; Fax: +34-4-464-8500; E-mail: gbzoseth@lg.ehu.es.
Dr.Bak6s'spennanentaddress isCktedradeBiologia,Facultad de Cien-ciasExactas, Universidad Nacional de La Plata, La Plata, Argentina. i 1996bytheBiophysicalSociety
0006-3495/96/10/1869/08 $2.00
particular, HlyA binds lipid bilayers with about the same
affinity in the presence or absence of
Ca2+,
butonly when theprotein has beenpreincubatedwith this cation does the lytic effect follow toxinbinding (OstolazaandGofii, 1995). In this paper, we explore in more detail the influence of some lipid properties onHlyAbinding tomembranes. The interest of thisstudygoes beyondthe mechanismofaction of thetoxin, because it is one case of the moregeneral,andimportant, biologicalproblem ofprotein insertionin mem-branes (see reviewsby Jain and Zakim, 1987; Hannavy et al., 1993; Isenman et al., 1995; see also, to mention but a few examples, Dibble et al., 1993; Sankaram et al., 1994;
Pottand Dufourc, 1995; Rytomaa and Kinnunen, 1995). The presentstudy isspecificallydirected toexploring the influence oflipidphasesonthebindingof HlyA. Particular
attentionispaidtothecaseofcoexistingfluidphases, such as phospholipid-cholesterol fluid ordered and fluid disor-deredphases (Sankaram andThompson, 1991; Almeida et
al., 1992; Monette et al., 1993; Mateo et al., 1995; Mc-Mullen andMcElhaney, 1995; Pott and Dufourc, 1995), and to the case ofcoexisting gel and fluid phases (Vaz et al.,
1989; Jorgensen et al., 1993; Sankarametal., 1992, 1994;
Piknova'etal., 1996). Tomita et al. (1992) have studied the
influence of membrane fluidityonthe assembly of
Staphy-lococcus aureus a-toxin, a channel-forming protein not
belonging to the RTX family, in liposomal membranes.
Followingasimilar experimental reasoning, we have estab-lishedbilayer conditions under which HlyA may bind lipid
membranes in either a reversible or an irreversibleway.
MATERIALS AND METHODS
Materials
Egg phosphatidylcholine (PC) was grade I from Lipid Products (South Nutfield, England). Dioleoyl, dimyristoyl, dipalmitoyl, and distearoylphos-1869
Volume 71 October 1996
phatidylcholine (respectively, DOPC, DMPC, DPPC, and DSPC) were
supplied by Avanti Polar Lipids (Alabaster, AL). Cholesterol (Ch) was from Sigma. I-Amino-naphthalene-1,3,6-trisulfonate (ANTS) and p-xylenebispyridiniumbromide (DPX)wereobtained fromMolecular Probes
(Eugene, OR). Plasmid-encoded a-hemolysin (HlyA) waspurified from
the culture filtrates ofanoverproducing strain ofE. coli, accordingtothe
method of Ostolazaetal. (1991); before itsuse,the proteinwasdialyzed
against150 mM NaCl,etMurea,20 mM Tris/HCl,pH7.0 (TCU buffer), towhich1mMEGTAwasadded. Horse red blood cellsweresupplied by
Microlab(Madrid, Spain).
Largeunilamellarvesicles (LUVs) of different compositionswere
pre-pared by extrusion andsized using 0.1-gmporesize Nuclepore membranes asdescribed byMayeretal.(1986);thebufferwas20 mM Tris-HCl, 150 mMNaCl,pH7.0 (TC buffer), ± EGTA, Ca24,orZn2+ as required.
100
75
50 25
0
Measurements ofintrinsic fluorescence ofHlyA Bilayer-toxin interactionsweremonitoredthroughchanges in the intrinsic fluorescenceemissionspectraof HlyA. Small aliquots ofaconcentrated
LUVsuspension wereadded toaprotein solution (0.15-0.30 JIM)ina
cuvettewith continuous stirring. Afterequilibrating for 5min,emission
spectra were recorded with anexcitation light of 295 nm (slit 5 mm).
Fluorescenceintensity measurements were corrected for light scattering
(Surewiczand Epand, 1984).
Toxin binding to liposomes and toxin transfer
to erythrocytes
The method isessentiallytheonedescribed by Tomitaetal.(1992).Horse red blood cellswereusedasindicators inassessingthehemolytic activity
ofHlyAboundtomultilamellar vesicles(MLVs).Toxin(20-30jig)and
liposomes(multilamellar vesicles,1mM)wereincubatedinTCbufferwith
10 mM CaCl2 for 30 min, at the required temperature. HlyA-liposome complexeswererecoveredby centrifugation (16,000Xg,15min, 40C)and washed threetimes toremove anyunbound toxin.The complexes were
resuspendedin 200 ,ul of cold buffer and used forassaysofhemolytic
activity,aswellasinproteinandlipiddetermination. Thiswascalled the
"centrifugation method" formeasuringtotalproteinboundtoliposomes.
For hemolytic activityassays, analiquotof theHlyA-liposome
com-plexeswasseriallydiluted withcold buffer ina96-well microtiterplate.
One hundred microliters of the dilutesuspensionsweremixedwith100,ul
ofastandardizedsuspensionofred blood cells and lefttoincubate for 30 min at 370C. The absorbance ofsupematantswasread at412nm. One
hundredpercentlysiswasestablished afterlysingthered blood cells with Triton X-100(1%finalconcentration).
100
cf)
Cf) Cll 75 50 25 0 100 75 50 25 00. 01
0.1
1 10 100BOUND
PROTEIN
(
,ag/ml)
Time-resolved experimentsand
equilibrium conditions
Preliminarytime-resolved experiments using the varioustechniques
de-scribed above showed that5min after toxin addition, the variouscelland
model systemsused in thisstudy had reachedequilibrium. This is what
would beexpectedfromadiffusion-limitedprocess,asisprobablythecase with toxinbindingand insertion. Thus allmeasurementshavebeencarried
out5mmnafterHlyA addition,exceptin thosecasesinwhich,for the sake
ofconvenience, equilibration was allowed to take placefor 30 min,as stated in each case.
RESULTS
Thecapacityofliposome-bound HlyAtoproduceredblood celllysiswastestedafterincubatingtheproteinwithDOPC, DMPC,orDPPC multilamellarliposomesat0°Cand37°C.
The results are presented in Fig. 1. The dotted line
corre-FIGURE 1 Accessibility ofliposome-bound a-hemolysin toredblood
cells. HlyA was incubated for 30 min at 0°C (0) or 37°C (0) with
multilamellar liposomesofDOPC, DMPC, orDPPC, as indicated. The
resulting liposome-protein complexes werefurther incubated with horse
erythrocytes,and thehemolytic activitywasrecorded. See Materials and
Methods for details. Dotted line: hemolyticactivityof nativetoxin, i.e., preincubatedin the absenceofliposomesand then addedtotheerythrocyte suspension.
sponds to the dose-response curve of free hemolysin, i.e., protein that has been preincubated in the absence of
lipo-somes andthen tested againstred blood cells. When HlyA
has beenpreincubated with DMPC liposomes at0°C (i.e.,
well below
Tc,
the main gel-to-fluidtransitiontemperature of the phospholipid; open circles), incubation with horseerythrocytes leads to hemolysis, although not all of the
DOPC
v v
.70
-V
VV~~~
I
-DMPC
v>S
V/L
-DPPC
,,
I a 1870 BiophysicalJournala-Hemolysin Insertion intoLipid Bilayers proteinseemstobecapableofproducingcelllysis,because
thedose-response curveis somewhat shifted to higher
pro-tein concentrations. However, when preincubation with
DMPC (Tc = 23°C)has takenplace at37°C (filled circles),
the fraction of HlyA available for hemolysis is much
smaller, andthedose-responsecurveis shiftedbyaboutan
order of magnitude. This can be quantitatively stated by
comparing theamountofprotein requiredin all three cases
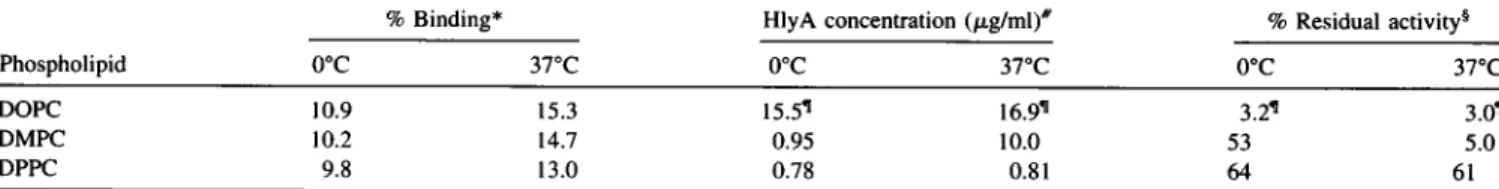
toproduce 50% hemolysis. The dataareshown in Table 1,
and the protein concentrations are, respectively, 0.5, 0.95,
and 10.0
jig/ml
for HlyA preincubated without and withliposomes at0°C andat 37°C.Intermsofpercentage, 53%
of the protein preincubated at 0°C but only 5% ofHlyA preincubated at 37°C with DMPC is "available" for
hemo-lysis. Thephenomenonappearstoberelatedtothephysical
state of the bilayer, because HlyA preincubated with
mul-tilamellar vesicles of DPPC (Tc = 41.5°C) remains
avail-able forhemolysiswhen incubatedat0°or37°C (Fig. 1 and
Table 1). The opposite situationisfound withDOPC,which
remains fluidat bothtemperatures; theproportion ofHlyA available for hemolysis is very small in both cases, and
values producing 50% hemolysis (obtainable only by
ex-trapolation) arevirtuallysimilarandcomparabletothe data forpreincubationwith DMPC in the fluidstate(37°C). Note
that protein binding to the multilamellar liposomes is
es-sentially similar in allcases (Table 1), irrespective of tem-peratureorlipid composition. Control experimentsinwhich
protein-free liposomes are incubated with red blood cells
showed that none ofthe vesicle preparations had any
he-molytic character at all.
Theseresults areinterpreted in termsof the existence of
two populations of membrane-bound HlyA molecules,
re-spectively, reversibly and irreversiblybound. Lipid bilayers
in the fluid state favor irreversible binding. Our previous
studies with LUV in the fluid state indicated that, under
conditionsleadingtomembraneleakage,the toxinremained irreversibly bound tothemembrane (Ostolazaetal., 1993).
In thecase offluidMLV (e.g., DOPC,orDMPC at37°C), virtuallyall of the toxin(>90%)remainsirreversibly bound (Table 1). For bilayers in the gel state (e.g., DPPC, or
DMPCat0WC), theapparentproportion of reversibly bound HlyA is far from 100%; this result is observed either
be-cause some of theprotein is irreversibly boundevenbelow
T,,
or because not all of the reversibly bound hemolysin binds red blood cells under conditions leading to leakage (Ostolaza and Gonii, 1995). A similar situation below Tcwasfound forS. aureus a-toxin (Tomitaet al., 1992).
Some ofthe formerexperimentswith DMPC multilamel-lar vesicles were repeated with preincubated HlyA and
liposomes in TC buffer but with 1 mM ZnCl2, or 1 mM
EGTA, instead of
Ca2+.
Theseexperimentswereperformedbecause previous studies had shown thatin the absence of divalent cations, or in the presence of
Zn2+,
HlyA wasinactive (Ostolaza et al., 1995). In agreement with the
previous observations, no hemolysis was detected under
thoseconditions,inspiteof the fact that red blood cellswere
suspendedinacalcium-containingbuffer(datanotshown). Thissuggests that, in the transfer fromMLVtoerythrocyte (whenever this occurs), the calcium-binding domain of
HlyA (Ostolazaetal., 1995) doesnotunfoldtoallowentry ofcalcium ions andsubsequent protein activation.
The hypothesis that HlyA becomes irreversibly boundto
lipid bilayers when they are in the fluid state is also
sup-portedbyanexperimentinwhichtoxinbindingisassessed
as anincreaseinthe intrinsicTrpfluorescence of theprotein
(SurewiczandEpand, 1984;Ostolaza andGoiii, 1995). Fig. 2 shows the relativechange in HlyA intrinsic fluorescence
when incubated continuously with increasingtemperatures inthepresenceofDMPC LUVs (Tc = 23°C). Fluorescence
increases steeply between 20°C and 23°C, i.e., near the
gel-fluid transition. Ifasystemequilibrated well above Tc is thengraduallycooleddown (Fig. 2, triangles), the intrinsic fluorescence doesnotvary.Our interpretation of these data
is that HlyA becomes inserted in the DMPC bilayer in a
nonreversibleway,particularly when the lipid is in the fluid
phase. The tryptophanyl residues being in a less polar
en-vironment than before, the fluorescence emission intensity increases. Once the protein is inserted above Tc, cooling
does not reverse the fluorescence effect, because binding above Tc is irreversible. The peptide becomes kinetically trapped in the gel state. Note that direct measurements of liposome-bound protein do not reveal major differences between the gel and the fluid phase (Table 1), probably
because thedirect centrifugation method gives anestimate
TABLE I Bindingofa-hemolysintophospholipid liposomes and transfer of a-hemolysin from liposome-toxin complexes to horseerythrocytes
%Binding* HlyA concentration(pLg/ml)# % Residualactivity§
Phospholipid 0°C 37°C 0°C 370C 0°C 370C
DOPC 10.9 15.3 15.5" 16.99 3.2q 3.01
DMPC 10.2 14.7 0.95 10.0 53 5.0
DPPC 9.8 13.0 0.78 0.81 64 61
*Totalprotein bound, according to thecentrifugationmethod.
#ConcentrationofHlyA, intheform of toxin-liposome complex, that produces 50% hemolysis in the standard preparation of horse red blood cells. The correspondingvalue for free HlyA is 0.5 ,tg/ml.
§Thepercentage residual hemolytic activity in the toxin-liposome complexes is calculated as (concentration of native toxin required to produce 50% hemolysis/concentration of liposome-bound toxin required to produce50%hemolysis) X 100 (Tomita et al., 1992).
sExtrapolated values.
1871 Bakais etal.
Volume 71 October 1996
15 20 25 30 35
TEMPERATURE
(C)
FIGURE 2 The influence of gel-fluid phase transition of DMPC on
HlyA bindingtoLUVs, assessedas an increase in intrinsicTrp
fluores-cenceof the protein. *,Heatingrun;V,coolingrun.
oftotal (reversibly + irreversibly) bound protein, whereas intrinsic fluorescence increases noticeably only when the
protein becomes irreversiblyembedded in alipid bilayer.
Binary phospholipid mixtures offer various possibilities of phase transitions and of coexistence of gel and fluid phases. We have studied the incorporation of HlyA into various mixtures of DMPC and DSPC at 300C. At this temperature,thebinary lipid systemis in theliquid crystal-line phase foraDSPC molar fractionx ' 0.15, and in the
gel phase forx 0.65 (Knolletal., 1981; Vazetal., 1989;
Sankarametal., 1992; Jorgensenetal., 1993; Piknovaetal., 1996); between these boundaries, the gel and liquid crys-tallinephases coexist. Total protein bound, accordingtothe centrifugation method, was 3 ± 0.1/104 (protein/lipid mol
ratio)at30°C, irrespective of thesystemcomposition. HlyA irreversiblebindingtoDMPC/DSPCLUVs is shown inFig. 3 A, as detected through changes in the intrinsic fluores-cence. Again insertion appears to be at a maximum when the system is in the fluid phase, lower when both phases coexist, and virtually zero when the lipids are in the gel phase.A smallbutreproducibleincreaseobserved between
XDSPC = 0.00andxDspc = 0.05 is attributedtoafacilitated
protein insertion when mixed-length phospholipid chains
coexist. Theproportionof insertedproteindoesnotdecrease
precisely in parallel with the theoretical fraction of fluid
phase (Fig. 3A, dotted line).Thismaybe duetofluctuations in the phase boundaries induced by the presence of the
protein. Our differential scanning calorimetry studies of
phospholipid-HlyA systems indeed show a protein-depen-dentwideningof the mainlipidgel-fluid phasetransition(P. Veigaetal., unpublished data). Insertion ofa small (25 aa)
peptideis knowntoleave thephaseboundariesunmodified
(Sankarametal., 1994),but the behavior of the muchlarger
I-C.) 0 IU o 0.0 1.0 cn CO) 0.8
CO
In 0.6 0 z In 0.4 r1 0.2 = CO cn : 0.0 0.2 0.4 0.6 0.8 1.0 XDSPCFIGURE 3 Binding of a-hemolysin to bilayers composed of binary phospholipidmixturesDMPC/DSPC. (A) Irreversible LUVbinding mea-sured as an increase in intrinsic Trp fluorescence of the protein. Data relative to fluorescence intensity of the pure protein in buffer, in the absence oflipids. (B) Accessibilityof MLV-boundHlyAtoredblood cells
(asinFig. 1). Measurementswerecarriedout at30°C. Thetwo discon-tinuousvertical lines indicate thepurelipid phaseboundaries. The dotted linecorrespondstothe calculatedmassfraction of fluidphaseas afunction
ofsystemcomposition.
and more complex HlyA may be different, particularly in
view of the small size of the fluidphasedomains in DMPC/
DSPC mixturesat30°C (Piknovaetal., 1996).Theputative
effect of the protein on the vesicle curvature and subse-quentlyonthephasebehaviorof thelipidmixture(Brumm
etal., 1996)should also be taken intoaccountinoursystem. When the reversibility ofHlyA binding to DMPC/DSPC
bilayersinthe form of MLVs is testedby incubating
liposome-bound toxin with horse
erythrocytes,
the results are ratherstraightforward (Fig.3B).Aslongasthere issomelipidin the 1.55 1.50 1.45 C o= \X 1.40 1.35 0 LL 1.30 h 1.25! 1.20 10
0
Q
X
DSPC
1872 BiophysicalJournala-Hemolysin Insertion intoLipid Bilayers
fluid phase,binding is essentially irreversible-no hemolysis is detected. Only when the mixture is in the gel phase, and particularly for
xDspc
= 1.0, does the binding become revers-ible. The pointatXDSpc
= 0.8 isinterestingbecause it shows virtually the same F/F0 asxDspc
= 1.0 (Fig. 3 A), yet the recovery ofhemolytic activity is much smaller in the former case. One plausibleexplanation is thatxDspc
= 0.8represents alimiting situation; in fact, the results in Fig. 3 may suggestthat,in thepresenceofHlyA,thelipid phaseboundariesarein fact near
xDspc
= 0.05 and 0.8, respectively. CholesteroVphosphatidylcholine mixtures are interesting because they of-fer thepossibility ofcoexisting ordered and disorderedliquid
phases (Vistand Davis, 1990; Sankaram andThompson, 1991; Almeida et al., 1992; Mateo et al., 1995; McMullen and McElhaney, 1995). ForDMPC/cholesterol, the phasediagram
publishedbyAlmeidaetal.(1992) shows,at10°C,agelphase
for 7 mol% cholesterol, an orderedliquid phase at 30mol% cholesterol, and coexisting gel and ordered liquid phases be-tween. At 37°C, the phases are disordered liquid, disordered liquid + ordered liquid, and ordered liquid, the two corre-sponding boundaries occurring at lOmol% and -30 mol% cholesterol. The total amount ofliposome-bound HlyA esti-mated by thecentrifugation method for bilayers composed of DMPC/cholesterol decreases steadily and slowly with increas-ing proportions of cholesterol (Fig. 4), apparently with little or no influence of temperature or phase transitions, as was the
casefor pure DMPCbilayers (Table 1).
When irreversible HlyA insertion into LUV bilayers is considered, assessed by the intrinsic fluorescence method (Figs. 5A and 6 A), thegeneral tendency is, as seen for total binding in Fig. 4, a decrease in irreversible binding as the cholesterol concentrationincreases. At 10°C (Fig. 5 A), the boundary between S and S +
Lo
does not markanyimpor-tant change in toxin binding, but beyond the boundary
1.50
0U-f
U.1.25
1.00
80
I-->60
U UI- 40
-J0
<20
I0
CD 2.00 -J 1.75 0 D 1.50 0 2 1.25 -@ 1.00 a 0.75 z D 0.50 0 < 0.25 I 0.00 FIGURE 4 T4 HlyAtobilayer carriedout at0
10
20
30
40
50
%
CHOLESTEROL
50
% CHOLESTEROL
FIGURE 5 Binding of a-hemolysin to bilayers composed of DMPC/
cholesterolat10°C.(A)IrreversibleLUVbindingmeasured by theintrinsic
fluorescence method. (B) Accessibility of MLV-bound toxin to erythro-cytes(asinFig. 1).Thediscontinuous verticallines correspond to thelipid
phase boundaries.
l
~~~~~~~between
S +Lo
andLo
binding
isclearly
decreased,
sug-gesting that the liquid ordered state is not a favorable solvent for
HlyA.
At37°C thebilayer ability
tobindhemo-lysin
decreasessteeply
as soon assomeLo
phase
isformed,
l l l l l
suggesting
again
the lowaffinity
ofLo
for the toxin. Note0 10 20 30 40 50 that for cholesterol concentrations ator near50%, when the
DMPC gel-fluid transition has been completely smeared
% CHOLESTEROL
out, HlyA binding at
10°C and 37°C is virtually the same.
tal binding (measured by the centrifugation method) of Tests of
accessibility
to redblood cells ofHlyA
bound to scomposedofDMPC:cholesterolmixtures. Measurements DMPC/cholesterol MLVs (Figs. 5 B and 6 B) confirm in 0°C(U)
and37°C (-). general the previous observations. At 37°C, accessibility is atBakcas et al. 1873
4
Volume 71 October 1996 A
worthy
observation is that, at the temperature of10°C,
the At
370C
liquid
orderedphase
is somehowstabilizing
themembrane-)
~\
l
l
boundform
ofthetoxin-not
only
does
the
recovery
of
he-molytic activity
decrease and remain low as soon as somecholesterol (andpresumably some
Lo
phase) is present (Fig. 5 B), but the recovery from pureLo
(50% cholesterol) is alsoL
\ |L
|L
] L lower at1O°C
than at37°C (Fig. 6 B). This is an indication that,d \ l d 0 0 for a given phospholipid/cholesterol mixture in the same phase
at two different
temperatures,
even when theproportion
ofirreversibly bound HlyA is similar (Figs. 5 A and 6 A), the fractionof
protein
transferred fromliposomes
toerythrocytes
is lower at the lower temperature, perhaps indicating a
tem-perature-dependent differential organization of the protein in the
bilayer.
Inturn,
thissuggests
that the mode ofincorporation
) I of HlyA tolipidbilayers may be more complex than the two
0 10 20 30 40 50 possibilities (reversible/irreversible) mentioned above. With
this limitation in mind, we can conclude that thelipidbilayer % CHOLESTEROL properties that facilitate the irreversible insertion of HlyA are thefluid state over the gel state (Figs. 1-3), the low over the highcholesterol concentrations (Figs. 5and6), thedisordered
B
liquid (Ld)over
theorderedliquid
(L.)
orgelstates(Figs.
5andVB
370C
_ 6), and the gel over theliquidordered state (Figs. 5 and 6).+
LoI
Lc
)0 10 20 30
%
CHOLESTEROL
FIGURE 6 Binding of a-hemolysin tobilayers comp
cholesterolat37°C. (A) Irreversible LUV bindingmeasure fluorescence method. (B) Accessibility of MLV-bound
cytes(as in Fig. 1). The discontinuous vertical linescorres phase boundaries.
a minimum when the bilayer is in the Ld s
maximumwhenthe
Lo
statepredominates (Fig.( two remarkable features are observed (Fig. 5 1veryhigh hemolytic activityforHlyAboundtof 10°C (in accordancewith the datainTable 1),
dramatic decreaseas soon as5%cholesterol isac DMPC/cholesterol mixture should be in thegel
asthepureDMPC,but thelargedecrease in hen
(withasimilarextentofbinding accordingtoth measurements; Fig. 5 A) suggests again that changingthephasebehaviorof the mixture.The
DISCUSSION
The mechanism of membrane lysis bya-hemolysin
Our previous studies on this protein have shown that 1) a
specific receptoris notrequired for membrane lysis to occur, because leakage of large molecules is induced by HlyA in vesicles of definedlipid composition, e.g., pure egg PC (Os-tolaza et al., 1993); 2) HlyA may bind lipid bilayers under conditions (e.g., absence of
Ca2+)
that do not lead to lysis(Ostolaza andGofii, 1995). The data in this paperprovide us with a closer look at the binding step. Thebinding data
ob-40 50 tained by the centrifugation method and by the intrinsic fluo-rescencemethod do not always agree (e.g., Table 1 andFig.2),
particularlybecause the fluorescenceprocedure shows differ-osed of DMPC/ encesbetween
binding
tothegel
and fluidphases,
whereas the-dbytheintrinsic
centrifugation
method does not. This had not been detected intoxin toerythro- former studies because only bilayers in the fluid phase were
spondtothelipid tested. When thebinding dataarecomplementedwith assays ofaccessibility ofliposome-bound HlyAto
erythrocytes,
two populationscanbedistinguishedin themembrane-boundtoxinfraction,onethatistransferredtothe red blood cells(reversibly tate and at a boundHlyA)andonethatisnot(irreversiblybound).Thefact 6B). At10°C, thatirreversible butnotreversible bindingischaracterized
by
3). One is the changes in intrinsic fluorescence supports the
hypothesis
thatpure
DMPCat "binding" actuallyincludestwodistinctphenomena, reversiblefollowedby a adsorption of theprotein tothebilayerand irreversible inser-lded. The 95:5 tion into thesame.Adsorptionwould bean
early
stepthat,
instate asmuch certaincases(e.g.,fluid
bilayers),
would be followedby
inser-tolytic
activity tion.Adsorption appearstobe largelyindependent
ofbilayer
efluorescence fluidity orcomposition (atleast in the absenceofnet surface the protein is charge), whereas insertion is highlydependenton thebilayer esecondnote- physicalproperties (see Results).
1.50 0
U-f
LL 1.25 1.00 80 70 60 _ 50 _Ld I
Ld
I-0
00,c
40 _ 30 - 1 20 -104 0 1874 BiophysicalJournala-HemolysinInsertion intoLipid Bilayers Bycombining data from this andpreviouspapers
(Osto-laza et al., 1995; Ostolaza and Gonii, 1995), it can be concluded thatirreversible insertion doesnotlead
automat-ically to membrane lysis (see the case of binding in the presence of EGTA, accompanied by a similar increase in fluorescence than in the presence of
Ca2+).
Afurther phe-nomenon is required that occurs only in the presence of Ca2 .In a-toxinof S. aureus(Tomitaetal., 1992), aswell as in certainpore-formingcytolysins (Bhakdi and Tranum-Jensen, 1986), insertion is followed by oligomerization.However, in the case ofHlyA, no oligomer has been iso-lated up to now, although circumstantial evidence in its favor has been produced (Ostolaza etal., 1993; Ludwig et
al., 1993). No data are currently available on the step(s) between HlyAinsertion and membrane disruption.
The insertion of
c!-hemolysin
in lipid bilayersA fraction of the membrane-associated a-hemolysin is irre-versibly bound to thebilayer, its Trpresidues appeartobe in
a less polar environment than when the protein is free in
solution,and, under theappropriateconditions (e.g.,presence of
Ca2+),
it causes bilayer leakage and/or disruption. Theseexperimental facts indicate that this particular fraction has become inserted in the bilayer. Apart from the already dis-cussed implications for the mechanism of toxin action, the
experimental data may be of interest in the framework of peptide insertion in membranes, the first stage in membrane
protein folding (Lemmon andEngelman, 1994).
Unlike the casesofproteintranslocation inprokaryotes (de Kruijff, 1994),anionicphospholipidsare notrequiredforHlyA insertion (Ostolaza et al., 1993). Jain and Zakim (1987) have
pointed out the requirement that hydrophobic regions of the
bilayerbecome transiently exposedto the aqueous phase for proteinincorporation to occur. Such transient exposure would befavored by overallchanges in thebilayer properties,suchas fluidity (Chapman, 1975), or by particular properties of a localized microenvironment, or defects (Jain and Zakim,
1987). The requirement of fluidbilayers for insertionisshown inFig. 1, and moreexplicitlyinFig. 3,where, even if gel and fluidphasesmaycoexist, theproportionofirreversiblybound
protein decreases with the fraction of fluid phase. This is in agreement with thepreferential partitioning ofgramicidinA'
influidphospholipidphases coexistingwith gelphases(Dibble et al., 1993). The uneven distribution of a transmembrane
peptide into different coexisting lipiddomains has also been described by Zhang et al. (1995). Moreover, Polozov et al. (1995) described the insertion of two amphipathic peptides in zwitterionicphospholipid bilayers in the fluid, but not in the
gelstate; thus thepreferencefor fluidbilayers by the peptides
to be inserted appears to be a rather general and predictable
phenomenon.
Defectsand intrinsic instabilitiesin thebilayermay
pro-mote protein insertion because defect sites may accommo-date a protein molecule without inducing additional
ener-lateral compression (Jain and Zakim, 1987). Local defects
may arise from lipid mixtures (McIntosh et al., 1983), or
from the presence ofimpurities (e.g., detergents) or other
proteins, etc. Our experimental results show that conditions under which local defects are likely to occur (e.g.,
disor-dered liquid state; Fig. 6) do favor irreversible protein insertion. The overall effect of cholesterol istomakeprotein insertionmoredifficult, probably because its rigidstructure does not help to accommodate the rough protein surface (Figs. 5 A and 6 A). Note also that cholesterol tends to
increase bilayer thickness (Levine and Wilkins, 1971; Nezil andBloom, 1992) andintegral proteins tendtopartition into domains ofagiven thickness (Bretscher andMunro, 1993).
The properties of cholesterol areevidentinthe
cholesterol-rich ordered liquid phases (Figs. 5 A and 6 A), which
support very little insertion. Almeida et al. (1992) have
explainedthisphenomenon intermsof cholesterol
occupy-ing free volume in the bilayer. It is interesting, in this respect,thatS.aureusa-toxin hexamerization is favoredby
these cholesterol-rich phases (Tomita et al., 1992). How-ever, the relationship between the presence of cholesterol and the reversibility of HlyA binding appears to be more
complex than the general anti-insertion effect just
dis-cussed. The effects in the DMPC/cholesterol mixture at
10°C are agoodexample, particularly the surprisingly low
recoveries ofhemolytic activity in the presence of choles-terol (Fig. 5 B) that do notcorrelate with thebinding data (Fig. 5 A). Moreover, the irreversible binding dataarevery similar for the
Lo
phaseat 10°C and 37°C (Figs. 5A and 6 A), whereas the recovery of hemolytic activity is muchhigher at the higher temperature (Figs. 5 B and 6 B). Bretscher and Munro(1993) have proposed that changes in cholesterol concentration in cell membranes may induce
segregation ofdomains withhigh and low cholesterol
con-centrations;thisidea has receivedsupportfrom the
biophys-ical studies of Virtanen etal. (1995) in model membranes. If this were the case, segregation of domains would be favoredbylowtemperatures,andHlyA would partition into
domains of a particular composition, irrespective of the
average cholesterol contents in themixture.
The fact thatprotein insertion itself helpsto promotenew structural defects is important because it would support a
cooperativeprocessofinsertion, which inturnis relatedtothe putativeoligomerization of HlyA (Ostolazaetal., 1993; Lud-wigetal., 1993).Pott and Dufourc(1995) have underlined the
creation ofdefectstructuresinthe membraneupontheaddition ofmelittin thatmightmodify the overall elastic properties of the membrane, andMonette et al. (1993) proposed thathigh cholesterol concentrations inducetight lipidpacking, which in
turn prevents penetration of melittin into the bilayer. In our
system,eventhegelstateismorepronetoHlyA insertion than
the
Lo
one,probably becauseofthestructural defects thatareknowntoexistinliposomes below
T,.
This workwassupported inpartby DGICYTgrantPB91/0441 and bya HumanCapital andMobilityNetworkfrom theEuropeanUnion.
getically unfavorable general disorder, desolvation, or
1876 Biophysical Journal Volume71 October 1996
REFERENCES
Almeida, P. F. F., W. L. C. Vaz, and T. E. Thompson. 1992. Lateral diffusion in the liquid phases of dimyristoylphosphatidylcholine/ cholesterol lipid bilayers: a free volume analysis. Biochemistry. 31: 6739-6747.
Bhakdi, S., and J. Tranum-Jensen. 1986. Membrane damage by channel-forming proteins. Staphyloccal alpha-toxin, streptolysin-O and the C5B-9 complement complex. J. Immunol. 136:2999-3005.
Bretscher, M. S., and S. Munro. 1993. Cholesterol and the Golgi apparatus. Science. 261:1280-1281.
Brumm,T., K. Jorgensen,0. G. Mouritsen, and T. M. Bayerl. 1996. The effect of increasing membrane curvature on the phase transition and mixing behaviour of a DMPC/DSPC lipid mixture as studied by Fourier transform infrared spectroscopy and differential scanning calorimetry. Biophys.J.70:1373-1379.
Chapman, D. 1975. Phase-transitions and fluidity characteristics of lipids and cell membranes. Q. Rev.Biophys.8:185-235.
Chervaux, C., and I. B. Holland. 1996. Random and directed mutagenesis to elucidate the functional importance of helix II and F-984 in the C-terminalsecretion signal of Escherichia coli hemolysin. J. Bacteriol. 178:1232-1236.
Coote, J. G. 1992. Structural and functionalrelationships among the RTX toxin determinants of Gram-negative bacteria. FEMS Microbiol. Rev.
88:137-162.
deKruijff,B. 1994. Anionicphospholipids andprotein translocation. FEBS
Lett.346:78-82.
Dibble, A. R. G., M. D. Yeager, and G. W.Feigenson. 1993. Partitioning ofgramicidinA'betweencoexisting fluid and gel phospholipid phases. Biochim. Biophys. Acta. 1153:155-162.
Hannavy, K., S. Rospert, and G. Schatz. 1993. Protein import into mitochondria: aparadigm for the translocation ofpolypeptides across membranes. Curr. Opin. Cell.Bio.5:694-700.
Isenman, L., C. Liebow, and S. Rothman. 1995. Transport of proteins
across membranes. Aparadigm in transition. Biochim. Biophys.Acta. 1241:341-370.
Jain, M. K., and D. Zakim. 1987. The spontaneous incorporation of proteins into preformed bilayers. Biochim.Biophys. Acta. 906:33-68. Jorgensen, K., M. M. Sperotto, 0.G.Mouritsen, J. H. Ipsen,and M. J.
Zuckermann. 1993. Phaseequilibria and localstructureinbinary lipid bilayers.Biochim. Biophys.Acta. 1152:135-145.
Knoll, W., K. Ibel, and E. Sackmann. 1981. Smallangleneutronscattering study oflipid phase diagrams by the contrastvariation method.
Bio-chemistry. 20:6379-6383.
Lemmon, M. A., and D. M. Engelman. 1994.Specificity and promiscuity in membranehelix interactions. FEBS Lett.346:17-20.
Levine, Y. K., andM. H. F. Wilkins. 1971. Structure of orientedlipid bilayers.NatureNewBio. 230:69-71.
Ludwig, A., R. Benz, andW.Goebel. 1993.Oligomerization of Esche-richiacolihemolysin (HlyA) is involved in pore formation. Mol. Gen. Genet. 241:89-96.
Mateo,C. R.,A. U.Acufia,and J. C. Brochon. 1995.Liquid-crystalline phases ofcholesterol/lipid bilayers asrevealedbythefluorescence of trans-parinaric acid. Biophys. J. 68:978-987.
Mayer,L.D.,M. J.Hope, and P. R. Cullis. 1986. Vesicles of variable sizes produced byarapid extrusionprocedure.Biochim.Biophys.Acta. 858: 161-168.
McIntosh, T. J., R. V.McDaniel, and J. A.Simon. 1983. Induction ofan
interdigitated gel phase infullyhydratedphosphatidylcholine bilayers. Biochim.Biophys.Acta. 731:97-108.
McMullen, T. P. N., and R. N. McElhaney. 1995. New aspects of the interaction of cholesterol withdipalmitoylphosphatidylcholine bilayers
as revealed byhigh-sensitivitydifferential scanning calorimetry. Bio-chim.Biophys.Acta. 1234:90-98.
Menestrina,G.,C.Moser, S.Pellet,andR.Welch. 1994. Poreformationby Escherichia coli hemolysin (HlyA) and other members of the RTX toxinsfamily.Toxicology. 87:249-267.
Menestrina, G., M. Ropele, M. Dalla Serra, C. Pederzolli, F. Hugo, S. Pellet, and R. A. Welch. 1995. Binding of antibodies to functional
epitopes on the poreformed by Escherichia coli hemolysin in cells and modelmembranes. Biochim. Biophys. Acta. 1238:72-80.
Monette, M., M. R.Van Calsteren, and M. Lafleur. 1993. Effect of cholesterol on the polymorphism of dipalmitoylphosphatidylcholine/melittin complexes: an NMRstudy.Biochim. Biophys. Acta. 1149:319-328. Nezil, F. A., and M. Bloom. 1992. Combined influence of cholesterol and
synthetic amphiphilic peptides upon bilayer thickness in model mem-branes. Biophys. J. 61:1176-1182.
Ostolaza, H., B. Bartolome, I. Ortiz de Zarate, F. de la Cruz, and F. M. Gonli. 1993. Release of lipid vesicle contents by the bacterial protein toxina-hemolysin. Biochim. Biophys. Acta. 1147:81-88.
Ostolaza, H., B. Bartolome, J. L. Serra, F. de la Cruz, and F. M. Gonii. 1991. a-Hemolysin from E. coli. Purification and self- aggregation properties. FEBSLett. 280:195-198.
Ostolaza, H., A. Soloaga, and F. M. Gofii. 1995. The binding of divalent cations to Escherichia coli a-hemolysin.Eur.J.Biochem. 228:39-44. Ostolaza, H., and F. M. Gofni. 1995. Interaction of the bacterial protein
toxin a-hemolysin with model membranes: protein binding does not always leadtolyticactivity. FEBS Lett. 371:303-306.
Piknova, B., D.Marsh, and T. E. Thompson. 1996. Fluorescence quench-ing study of percolation and compartmentalization in two-phase lipid bilayers. Biophys.J. Inpress.
Polozov, I. V.,A.I.Polozova, Y. G. Molotkovsky, G. M. Anantharamaiah, J.P. Segrest, and R. M. Epand.1995. Amphipathic peptide effects on the lateraldomainreorganization of lipid bilayers. Biophys. J. 68:455A. Pott, T., and E. J. Dufourc. 1995. Action of melittin on the
DPPC-cholesterolliquid-ordered phase: asolidstate2Hand3'P-NMRstudy. Biophys. J.68:965-977.
Rytomaa,M.,and P. K. J. Kinnunen.1995. Reversibility of the binding of cytochrome c to liposomes. Implications for lipid-protein interaction. J.Bio. Chem.270:3197-3202.
Sankaram, M. B., D. Marsh, L. M.Gierash,and T. E.Thompson. 1994. Reorganization oflipid domainstructurein membranesbya
transmem-branepeptide: anESRspin label studyontheeffect of the Escherichia coli outer membraneprotein A signal peptideonthefluidphasedomain connectivity inbinarymixtures ofdimyristoylphosphatidylcholineand distearoylphosphatidylcholine. Biophys. J. 66:1959-1968.
Sankaram,M.B., D.Marsh,and T.E.Thompson.1992. Determination of fluid andgeldomain sizes in two-component, two-phaselipid bilayers. Anelectronspin resonancespin label study. Biophys. J.63:340-349. Sankaram, M. B., and T. E. Thompson. 1991. Cholesterol-induced
fluid-phase immiscibility in membranes. Proc. Natl. Acad. Sci. USA. 88: 8686-8690.
Stanley, P., L. C. Packman, V. Koronakis, and C.Hughes. 1994. Fatty acylationoftwointernallysineresiduesrequiredfor the toxicactivityof Escherichia colihemolysin.Science. 266:1992-1995.
Surewicz, W. K., and R. M. Epand. 1984. Role ofpeptidestructure in lipid-peptide interactions:afluorescencestudy of thebindingof penta-gastrin-related pentapeptides to phospholipid vesicles. Biochemistry. 23:6072-6077.
Tomita, R., M.Watanabe, and T. Yasuda. 1992. Influence of membrane fluidityontheassembly ofStaphylococcusaureusa-toxin,a channel-forming protein, in liposome membrane. J. Biol. Chem. 267: 13391-13397.
Vaz, W. L.C., E. C. C.Melo, and T. E. Thompson. 1989. Translational diffusion and fluid domainconnectivityinatwo-component,twophase phospholipidbilayer. Biophys. J. 56:869-876.
Virtanen, J. A., M. Ruonala, M. Vauhkonen, andP. Somerharju. 1995. Lateral organizationofliquid-crystallinecholesterol- dimyristoylphos-phatidylcholine bilayers. Evidence for domains with hexagonal and centered rectangular cholesterol superlattices. Biochemistry. 34: 11568-11581.
Vist, M. R., and J.H.Davis. 1990.Phase-equilibriaof cholesterol dipalmi-toylphosphatidylcholine mixture. 2H-Nuclear magnetic resonance and differentialscanning calorimetry. Biochemistry. 29:451-464.
Zhang,Y.P., R. N. A. H. Lewis,R. S.Hodges, and R. N. McElhaney. 1995.Peptidemodels of helicalhydrophobictransmembrane segments of membraneproteins. 2. Differentialscanningcalorimetric andFrIR spectroscopicstudies of theinteraction ofAc-K2-(LA)12-K2-amidewith phosphatidylcholinebilayers. Biochemistry. 34:2362-2371.