UNIVERSIDADE FEDERAL DO CEARÁ CENTRO DE CIÊNCIAS
DEPARTAMENTO DE BIOQUÍMICA E BIOLOGIA MOLECULAR PROGRAMA DE PÓS-GRADUAÇÃO EM BIOQUÍMICA
MOHIBULLAH SHAH
PROTEOME ANALYSIS OF DEVELOPING SEEDS OF Jatropha curcas L.
MOHIBULLAH SHAH
PROTEOME ANALYSIS OF DEVELOPING SEEDS OF Jatropha curcas L.
Tese apresentada ao Curso de Doutorado em Bioquímica do Departamento de Bioquímica e Biologia Molecular da Universidade Federal do Ceará, como parte dos requisitos para obtenção do título de Doutor em Bioquímica. Área de concentração: Bioquímica vegetal.
Orientador: Prof. Francisco A. P. Campos. Co-orientador: Prof. Fabio C. S. Nogueira.
Dados Internacionais de Catalogação na Publicação Universidade Federal do Ceará
Biblioteca de Ciências e Tecnologia S537p Shah, Mohibullah.
Proteome analysis of developing seeds of Jatropha curcas L. / Mohibullah Shah. – 2014. 168 f. : il., color., enc. ; 30 cm.
Tese (doutorado) – Universidade Federal do Ceará, Centro de Ciência, Departamento de Bioquímica e Biologia Molecular, Programa de Pós-Graduação em Bioquímica, Fortaleza, 2014.
Área de Concentração: Bioquímica vegetal.
Orientação: Prof. Dr. Francisco de Assis de Paiva Campos. Coorientação: Prof. Dr. Fabio César Sousa Nogueira.
ACKNOWLEDGEMENTS
First of all I would like to thank to ALLAH (SWT), for blessing me good health, courage, strength during the difficult moments and making me enable to reach here.
I would also like to thank and appreciate some people who directly or indirectly support me in successful completion of this work.
First and foremost, I express my wholehearted gratitude to my respected supervisor, Prof. Francisco A. P. Campos (Prof. Chico), for his trust, unfading interest, consistent encouragement, stimulating suggestions, expert advices, guidance and above all for his friendly relationship throughout this period. I am also thankful to his family, especially his wife Dr. Ursula Wille, for her respect and loving attitudes.
Thanks to Prof. Gilberto B. Domont (UFRJ), for keeping the doors of his lab (Proteomic Unit) open for me whenever I visited, also for his friendship, guidance, encouragement and being a source of inspiration. My gratitude to his wife Prof. Solange Guimaraes (UFRJ), for her loving and friendly attitude, and for inviting me a number of times in her home during my stay in Rio de Janeiro.
Special respect for Prof. Fabio C.S. Nogueira (UFRJ), my friend and co-supervisor, among the few competent young scientists I met so far, for his guidance and sharing of knowledge with me. In each of our discussions I always learned new things from him. I am also very thankful for his continuous guidance and help in the development of this project.
Warm thanks to Dr. Paulo C. Carvalho (FIOCRUZ-PR), a competent young scientist and a great example of simplicity, for his help and collaboration in the analysis of data. I highly acknowledge his prompt reply whenever I contacted him for assistance in data analysis during the entire period of my PhD course.
I am thankful to Prof. Arlete A. Soares (Department of Biology, UFC), for her help in histological analysis of the seeds.
I appreciate Emanoella Lima Soares (Manu) and Camila B. Pinheiro (minha amigas de mais tempo), for their great company, friendship, fruitful discussions, and continuous support since my first day in the Lab. I want to specially thank Manu, for her help in the analysis of data and preparation of this thesis.
My appreciation for Dr. Fabiano M. Texeira (meu amigo de mais tempo), and his wife Alice and mother Antonia, for the friendship, accepting me as a member of their family, inviting me in all the moments of happiness and for the help I needed during difficult moments. I am also thankful to Fabiano, for sharing his apartment with me after my arrival in Brazil, and for his great company.
Thanks to all the former and new members of the Bioplant lab, including Veronica, Magda, Carlos, Isabelle, Washington, Antonio, Tiago and Muciana, for their pleasant company in the lab.
I would like to extend my special thanks to all the members of the Proteomic unit (UFRJ), especially Gabriel and Prof. Magno, for their help in conducting mass spectrometry experiments.
I am also thankful to my friends Anwar and Rizwan (UFRJ) for giving me space in their apartment, for their moral support during my stay in Rio de Janeiro and for conducting the mass spectrometry experiments.
Special regards to my close friends, Mr. Javeed (South Korea), Dr. Umar (Unicamp), Dr. Asif (USP) and Dr. Asifullah (China) for their great moral support during completion of this work.
Very special respect to my loving parents, who have always been so close to me and I found them whenever I needed, for their unconditional love, dedication and support throughout my carrier; a continuous source of motivation to set higher goals in life.
I would like to pay my very special thanks to my life partner, for her loving company, that greatly helped me in understanding the true meaning of life and without her moral help the completion of my PhD would not have been possible. Thank you for every thing.
My Parents in law, for their affection, dedication and love. I am also thankful to my Brother in law Mr. Noman, for solving all my problems, especially bureaucratic things that I needed from Pakistan.
Special thanks to Third World Academy of Sciences (TWAS) and Brazilian National Council for Scientific and Technological Development (CNPq), for their financial support.
ABSTRACT
Physic nut (Jatropha curcas L.) is an important crop due to its ability of storing high content of oil in the seeds, which can serve as raw material for biodiesel production. Because of the presence of toxic constituents like phorbol esters (PEs) and curcins, the seed cake produced as a result of oil extraction cannot be utilize for animal feed. Development of the genotypes better suited for the industrial applications and biodiesel production as well as with lower level of toxic constituents is being hampered by a lack of understanding about the a) proteins related to the biosynthesis and degradation of fatty acids (FAs) and triacylglycerides (TAGs), b) role of proteins deposited during seed development and c) proteins related to the synthesis and storage of toxic compounds during seed development. Agreeing with this, we have performed the anatomical analysis of the developing seeds of J. curcas, followed by the proteome analysis of the endosperm isolated from the seeds of J. curcas at five different developmental stages, which resulted into the identification of the 1517, 1256, 1033, 752 and 307 proteins, from Stage 6, 7, 8, 9 and 10, respectively, summing up to a total of 1760 proteins. Proteins with similar expression pattern were grouped into five different clusters and protein quantification based on spectral counts was determined. Besides identification of the proteins involved in the biosynthesis and degradation of the FAs and TAGs, we also identified a large number of proteins involved in the metabolism of the carbohydrates, which are important for supplying energy and carbon source for the synthesis of TAGs in heterotrophic seeds. Among the members of different classes of seed storage proteins (SSPs), we have identified four SSPs named as nutrients reservoir, which in contrast to the other SSPs showed decreasing deposition pattern during seeds development and revealed to have special role during seed development. In addition, peptidases belong to different mechanistic classes were identified, which have a range of functions, highlighting the role in reserve mobilization during germination. Isoforms of curcin were also identified in this proteome analysis which were absent in our previous proteome analysis of the other tissues from these seeds, suggesting that the deposition of these toxic proteins only occur in the endosperm. Similarly, several enzymes involved in the biosynthesis of diterpenoid precursors were identified in this proteome analysis but, like in our previous proteome analysis of the other tissues from J. curcas seeds,we were unable to identify any terpene synthase/cyclase, enzymes responsible for the synthesis of PEs, which collectively suggesting that the synthesis of PEs may not occur in seeds of this plant. In conclusion, the strategy used here enabled us to provide a first in depth proteome analysis of the endosperm from J. curcas developing seeds, which along with providing information regarding important aspects of the seed development, also set the foundation of a proteomic approach to study biotechnologically important plant species.
RESUMO
Pinhão manso (Jatropha curcas L.) é uma cultura importante devido à sua habilidade em armazenar alto conteúdo de óleo nas sementes, as quais podem servir como matéria-prima para a produção de biodiesel. Devido à presença de constituintes tóxicos como ésteres de forbol e curcina, a torta da semente produzida como resultado da extração do óleo não pode ser utilizada na alimentação animal. O desenvolvimento de genótipos mais adequados a aplicações industriais e à produção de biodiesel assim como apresentando baixos níveis de constituintes tóxicos está sendo prejudicado pela falta de entendimento sobre a) proteínas relacionadas a biossíntese e degradação de ácidos graxos e triacilgliceróis, b) o papel de proteínas depositadas durante o desenvolvimento da semente e c) proteínas relacionadas à síntese e reserva de compostos tóxicos durante o desenvolvimento da semente. Diante disso, nós realizamos uma análise anatômica de sementes em desenvolvimento de J. curcas, seguido por uma análise proteômica do endosperma isolado de sementes dessa espécie em cinco diferentes estágios de desenvolvimento, o que resultou na identificação de 1517, 1256, 1033, 752 e 307 proteínas, dos estágios 6, 7, 8, 9 e 10, respectivamente, somando um total de 1760 proteínas. Proteínas com padrão de expressão similar foram agrupadas em cinco grupos diferentes e a quantificação das proteínas baseada na contagem dos espectros foi determinada. Além da identificação das proteínas envolvidas na biossíntese e degradação de FAs e TAGs, nós identificamos um grande número de proteínas envolvidas no metabolismo de carboidratos, as quais são importantes para o fornecimento de energia e fontes de carbono para a síntese de TAGs em sementes heterotróficas. Entre os membros de diferentes classes de proteínas de reservas de sementes (SSPs), nós identificamos quatro SSPs denominadas reservatórios de sementes, que em contraste as outras SSPs mostraram decréscimo no padrão de deposição e revelaram ter um papel especial durante o desenvolvimento da semente. Em adição, peptidases pertencentes a diferentes classes mecanísticas foram identificadas destacando o papel da mobilização de reservas durante a germinação. Isoformas da curcina ausentes em nossas análises proteômicas prévias de outros tecidos da semente foram identificadas sugerindo que a deposição dessas proteínas tóxicas só ocorre no endosperma. Similarmente, várias enzimas envolvidas na biosíntese de precursores de diterpenóides foram identificadas nessa análise proteômica, mas como em nossas prévias análises proteômicas de outros tecidos de sementes de J. curcas, nós não fomos capazes de identificar sintases/ciclases de terpenos, enzimas responsáveis pela síntese de PEs, o que coletivamente sugere que a síntese desses compostos pode não ocorrer nas sementes dessa planta. Em conclusão, a estratégia utilizada nos fornece a primeira análise proteômica profunda do endosperma de sementes em desenvolvimento de J. curcas, o que além de fornecer informações sobre aspectos importantes do desenvolvimento da semente, também estabelece a base para uma pesquisa proteômica com o objetivo de estudar espécies vegetais importantes biotecnologicamente.
LIST OF FIGURES
Figure 1 Components of Jatropha curcas fruit that could be utilized for the production of different kind of bio-fuels. Figure adapted from Singh et al., (2008).
25
Figure 2 Structure of PEs backbone (Tigliane and Phorbol) and the various types of PEs identified from Jatropha curcas (C1 to C6) (Evans 1986 apud Goel et al., 2007; Haas et al., 2002).
26
Figure 3 Jatropha curcas ovule and developing seed structures (Singh,
1970). 29
Figure 4 Nuclear endosperm development (Olsen, 2001). 31
Figure 5 Morphology of Jatropha curcas seed collected at 40 days after
pollination. 35
Figure 6 Fatty acid (FA) and triacylglycerides (TAG) biosynthesis in oilseeds (Weselake et al., 2009).
38
Figure 7 General workflow summarizing the process from seeds
collection to proteomics analysis of the extracted proteins. 52
Figure 8 Workflow showing steps used for data analysis. 58
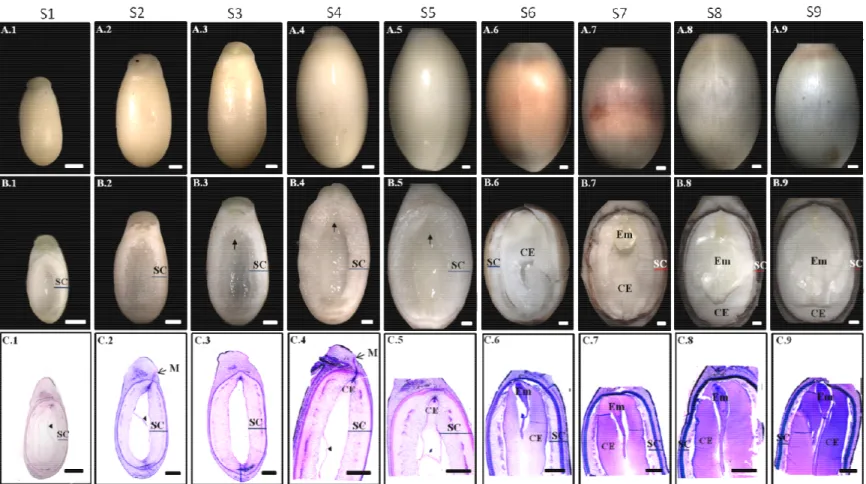
Figure 9 Jatropha curcas seeds development. Seed external morphology (A.1-A.9), internal part of the seeds (B.1-B.9) and histological analysis of the seeds (C.1-C9) at different developmental stages S-I to S-IX.
63
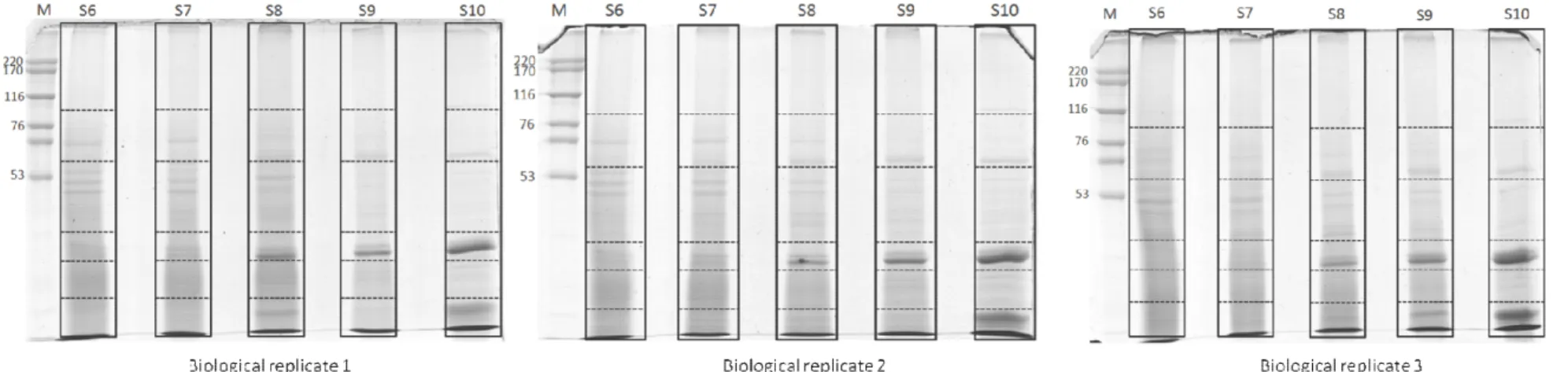
Figure 10 SDS-PAGE of proteins extracted from the endosperm of
Jatropha curcas seeds at five different developmental stages. 64 Figure 11 Distribution of the 1760 proteins appeared in at least two
biological replicates among the five developmental stages used for proteomic analysis.
67
Figure 12 Gene Ontology annotation of the proteins identified from Jatropha curcas endosperm at five developmental stages.
Figure 13 Gene ontology annotation of the unique proteins, present in each developmental stage of Jatropha curcas seed.
69
Figure 14 Functional classification of the identified proteins according to KEGG functional classification.
71
Figure 15 Functional classification of the identified proteins from the Jatropha curcas endosperm based on MapMan classification.
72
Figure 16 Spectral count based expression comparison of the proteins identified from Stage 7, 8, 9 and 10 to those identified in Stage 6, using T-test.
74
Figure 17 Cluster analysis of the proteins identified in Stage 6, 7, 8 and 9. Proteins with similar expression profile were grouped into five different clusters.
75
Figure 18 Distribution of the identified peptidases and peptidase inhibitors into different mechanistic classes.
105
Figure 19 Venn diagram showing proteins common to endosperm proteome and three other proteomes analyzed in our lab (A). Venn diagram showing comparison of the endosperm proteins with proteins identified in other proteomic analysis of the Jatropha curcas (B). Venn diagram showing comparison of the four proteome results obtained in our lab with proteins identified in other proteomic studies of Jatropha curcas (C).
LIST OF TABLES
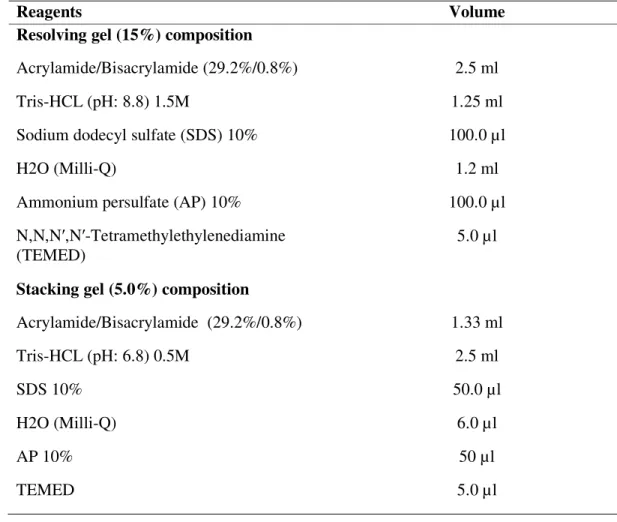
Table 1 Composition of polyacrylamide mini gels. 54
Table 2 Proteins identified from the endosperm of Jatropha curcas seeds at five different developmental stages.
65
Table 3 Proteins related to the metabolism of carbohydrates, identified from the endosperm of developing Jatropha curcas seeds.
77
Table 4 Proteins related to the lipids metabolism, identified from the endosperm of developing Jatropha curcas seeds.
87
Table 5 Seed Storage Proteins (SSPs) and proteins related to seed maturation, identified from the endosperm of developing Jatropha curcas seeds.
94
Table 6 Peptidases and peptidase inhibitors, identified from the endosperm of developing Jatropha curcas seeds.
100
Table 7 Proteins related to the terpenoids biosynthesis identified from the endosperm of developing Jatropha curcas seeds.
LIST OF SUPPLEMENTARY TABLES
Supplementary Table I Table represents total number of proteins including common contaminants and decoys, identified from the endosperm of Jatropha curcas developing seeds at five different developmental stages.
Supplementary Table II This table shows total number of proteins and their peptides, identified from the endosperm of Jatropha curcas developing seeds at five different developmental stages.
Supplementary Table III This table contains proteins appeared in at least two biological replicates, identified from the endosperm of Jatropha curcas developing seeds at five different developmental stages. These proteins were considered as true representatives of each developmental stage used here. Supplementary Table IV This Supplementary Table summarizes grouping of the
maximum parsimony groups to different clusters and spectral count based pairwise comparison of the proteins identified from Stage 8, 9 and 10, to Stage 6.
LIST OF ABBREVIATIONS
2-DE Two-dimensional electrophoresis
AACT Acetoacetyl-CoA thiolase
AAPVD Approximately Area Proportional Venn Diagram
ACBP Acyl-CoA binding protein
ACN Acetonitrile
ACP Acyl carrier protein
ACS Acetyl synthetase
ADP-glucose Adenosine diphosphate glucose
AP Ammonium persulfate
ATP Adenosine triphosphate
BH Benjamini-Hochberg
BSA Bovine serum albumin
cDNA Complementary DNA
CID Collision-induced dissociation
CoA Coenzyme-A
CS Casbene synthase
DAG Diacyl glycerol
DDA Data-dependent analysis
DGAT Diacylglycerol acyltransferase
DNA Deoxyribonucleic acid
DOXP 1-deoxy-D-xylulose-5-phosphate
ER Endoplasmic reticulum
ESI Electrospray ionization
FAs Fatty acids
FAD2 Oleate desaturase
FAD3 Linoleate desaturase
FDR False discovery rate
FPP Farnesyl diphosphate
FT-ICR Fourier transform ion cyclotron resonance
GGPP Geranylgeranyl diphosphate
GOEx Gene ontology explorer
GPAT Glycerol-3-phosphate acyltransferase
GPP Geranyl diphosphate
GUI Graphical user interface
HCL Hydro chloridric acid
HIV Anti-human immunodeficiency virus
HMGCS Hydroxymethylglutaryl-CoA synthase
IAA Iodoacetamide
IPP Isopentenyl diphosphate
KAAS KEGG automatic annotation server
KAS Ketoacyl ACP-synthase
LC Liquid chromatography
LCACS Long chain acyl-CoA synthetase
LEA Late embryogenesis abundant
LPAAT Lysophosphatidic acid acyl transferase
LTQ Linear trap quadropole
M Molar
MALDI Matrix assisted laser desorption ionization
MDD Mevalonate diphosphate decarboxylase
mM Millimolar
mRNA Messenger ribonucleic acid
MS Mass spectrometry
MVA Mevalonate
NADH Nicotinamide adenine dinucleotide
NADPH Nicotinamide adenine dinucleotide phosphate
NAHCO3 Sodium bicarbonate
NCBI National Center for Biotechnology Information
NH4HCO3 Ammonium bicarbonate
OB Oil body
OPP Oxidative pentose phosphate
PAGE Polyacrylamide gel electrophoresis
PCD Programmed cell death
PDHC Pyruvate dehydrogenase complex
PKC Protein kinase C
PL Phospholipids
PTMs Post-translational modifications
PVPP Polyvinyl-polypyrrolidone
RIP Ribosome-inactivating proteins
RNA Ribonucleic acid
ROS Reactive oxygen species
rRNA Ribosomal ribonucleic acid
SAD Stearoyl-ACP desaturase
SDS Sodium dodecyl sulfate
SEPro Search engine processor
SSPs Seed storage proteins
TAG Triacylglycerides
TCA Tricarboxylic acid pathway
TEMED N,N,N′,N′-tetramethylethylenediamine
TOF Time of flight
tRNA Transfer ribonucleic acid
VPE Vacuolar processing enzyme
TABLE OF CONTENTS
1 INTRODUCTION ... 19
1.1 Biotechnological importance of Jatropha curcas ... 20
1.2 Toxicity of Jatropha curcas seeds ... 24
1.3 Ovule structure and endosperm development ... 28
1.4 Role of endosperm in reserves deposition ... 32
1.5 Endosperm role in seed development ... 34
1.6 Triacylglycerides metabolism and accumulation in seeds ... 36
1.7 Plants proteomics ... 40
1.8 Mass spectrometry ... 42
1.8.1 ESI LTQ-Orbitrap ... 43
1.9 Post-genomic era and Jatropha curcas ... 45
2 OBJECTIVES ... 50
2.1 General objective ... 50
2.2 Specific objectives ... 50
3 MATERIALS AND METHODS ... 51
3.1 Plant material ... 51
3.2 Histological analysis and selection of developmental stages for the endosperm isolation... 51
3.3 Endosperm isolation and protein extraction ... 53
3.4 1D-SDS-PAGE and samples preparation for LC-MS/MS ... 53
3.6 NanoLC-MS/MS analysis ... 57
3.7 DATA analysis ... 57
3.7.1 Proteins idenitifcation ... 57
3.7.2 Functional classification ... 59
3.7.3 Cluster analysis and quantification ... 60
3.8 Data reposition ... 61
4 RESULTS AND DISCUSSIONS ... 62
4.1 Anatomical analysis of developing seeds of Jatropha curcas ... 62
4.2 Proteins identification and data analysis ... 62
4.3 Functional classification of the identified proteins... 66
4.4 Proteins quantification and cluster analysis ... 73
4.5 Major functional classes ... 76
4.5.1 Proteins related to the metabolism of carbohydrates ... 76
4.5.2 Proteins related to lipids metabolism ... 86
4.5.3 Seed Storage and Desiccation related proteins ... 93
4.5.4 Peptidases and peptidase inhibitors ... 99
4.5.4.1 Serine peptidases ... 106
4.5.4.2 Metallo peptidases ... 107
4.5.4.3 Aspartic peptidases ... 108
4.5.4.4 Cysteine peptidases ... 109
4.5.4.5 Threonine peptidases ... 110
4.6 Contribution of this study to the establishment of the deep proteome of
developing Jatropha curcas seeds ... 114
5 GENERAL CONCLUSIONS ... 118
6 FUTURE PERSPECTIVES... 120
7 REFERENCES... 122
8 PUBLICATIONS, WORKSHOPS AND CONFERENCES………....142
1 INTRODUCTION
Jatropha curcas L. belongs to family Euphorbiaceae, and is a multipurpose small tree with substantial economical and pharmacological potential. It is commonly known as Physic nut, Purging nut, Black vomit nut, Barbados nut or big purge nut (Makkar et al., 1998a). This plant is native to tropical regions of America but later was introduced to various tropical and sub-tropical parts of the world (Openshaw, 2000; Pramanik, 2003). J. curcas has few pests and diseases, and is adapted to grow under a wide range of climatic conditions including dearth of rains where it sheds leaves as a response to drought. This plant can be used tfor the control of soil erosion, land reclamation and as a barrier around other plants for protecting them from animals. This plant is also planted as a commercial crop but the most important aspect of the plant is the accumulation of the viscous oil in its seeds. This oil, besides other industrial uses like soap making and cosmetics industry, has a potential to be used as a raw material for the production of biodiesel (Openshaw, 2000).
The genus Jatropha is derived from Greek words iatrós which stands for doctor and trophé for food, shows that this plant was initially famous for its medicinal uses. Almost all parts of the plant were found to have medicinal importance and their use against various health problems have been extensively reviewed in literature (Villegas et al., 1997; Debnath and Bisen, 2008).
Different parts of J. curcas have also been evaluated for their chemical constituents and different biological activities, showing its pharmacological importance. For example, skin irritant compounds were isolated and partially characterized from seed oil of J. curcas (Adolf et al., 1984) which were further proved to have tumor promoting activity on mouse skin (Hirota et al., 1988). Anti-human immunodeficiency virus (HIV) activity were checked for the extracts of branches and leaves of J. curcas (Matsuse et al., 1999) and it was found that water extract of the branches and methanolic extracts of the leaves have potent inhibitory activity against HIV induced cytophatic effect in cultured cells.
alkaloids and flavonoids (Igbinosa, 2009; Nayak and Patel, 2010). Presence of these active compounds was found to be related to the antibacterial, antifungal and other bioactivities of this plant (Igbinosa, 2009).
In summary, an extensive literature is available highlighting the pharmacological importance of this plant but currently this plant is getting famous for its biotechnological aspects that is related to the use of its oil for biodiesel production.
1.1 Biotechnological importance of Jatropha curcas
The economic development and increased industrialization of the world resulted in a high demand for energy, which is mainly derived from the fossil fuel reserves like petroleum, coal and natural gas. Limited reserves of these different fossil fuels drawn attention of the scientific community to search for alternative fuels that could be obtained from renewable feedstocks (Openshaw, 2000; Pramanik, 2003; Demirbas, 2005). Biodiesel is one such option that could be used as an alternative to diesel fuels (Pinto et al., 2005; Gui et al., 2008). On chemical basis biodiesel is the alkyl esters of fatty acids (FA) produced through the transesterification of oils or fats from animals or plants, with short chain alcohols like methanol and ethanol (Pinto et al., 2005).
primarily because its drought resistant and can be grown in abandoned land under diverse environmental conditions (Sudhakar Johnson, et al., 2011), but obviously for high quantity of oil production it needs soil with suitable nitrate, phosphate and potassium (Carels, 2009).
J. curas seed kernel contains about 60% of oil that can replace the fossil diesel in the form of biodiesel (Makkar et al., 1997; Pramanik, 2003). Its oil can be combusted without making any refinement and has been tested successfully as a fuel for the diesel engine (Jain and Sharma, 2010).
Oil of this plant has been used during the Second World War in Madagascar, Cape Verde and Benin as a petroleum diesel substitute (Foidl et al., 1996; Gubitz et al., 1999). In Thailand the engine tests with J. curcas oil were done, showed satisfactory engine performance. The feasibility of the production of FA ethyl esters from J. curcas oil was studied for African countries, and the economic evaluation has shown, that biodiesel production from this plant is profitable, provided its by product could be sold as valuable material (reviewed in Kumar and Sharma, 2008). A preliminary investigation on the suitability of various non-edible oilseeds to be used as a source of biodiesel in Cuba revealed, that based on oil yield and FA composition, J. curcas was found to be the most promising candidate for biodiesel production (Martin et al., 2010). In countries like India J. curcas is becoming the future source of biodiesel and government of India already initiated programs for growing J. curcas on waste land for biodiesel production (Jain and Sharma, 2010). In different states of India 1.72 million hectares has been allocated for J. curcas cultivation and small quantities of J. curcas biodiesel have already been sold to local oil companies (Gui et al., 2008).
may turn yellow upon standing. The biodiesel produced from J. curcas oil release lesser amount of carbon dioxide than the petroleum based diesel which makes J. curcas biodiesel superior over the ordinary diesel (Abdulla et al., 2011).
Fuel characteristics of J. curcas are very similar to that of the diesel and cope with the American (ASTMD6751) and European (EN14214) standards (Kumar Tiwari et al., 2007). The ability of biodiesel to meet the American and European standard criteria is mainly determined by the FA composition. Properties like, cetane number (CN), cold flow, cloud point, viscosity and stability are influenced by the FA composition. Petroleum diesel consisted of hydrocarbons with carbon chains of 8-10 carbon atoms while J. curcas oil mainly contain FA with carbon chains of 16-18, which inferred high CN to J. curcas biodiesel (King et al., 2009). A factor that makes the biodiesel production less efficient is the presence of high level of free FA (14%) in crude J. curcas oil. However, pretreatment methods to overcome this problem are well developed (Koh and Ghazi, 2011). Another problem related to the oil of J. curcas is its high viscosity compared to petroleum diesel and hence its direct use in the engine needs further treatments. This issue has been addressed in many ways, like preheating the oils, blending or dilution with other fuels, transesterification and thermal cracking/pyrolysis. These further treatments make this oil expensive in comparison to the diesel oil. To reduce the cost of the process proper use of the byproducts from the plant is of great importance (Pramanik, 2003: Gui et al., 2008).
al. (2009). Among the other genes, JcFAD2-1 was found to be the most important, as it mediates the conversion of oleic acid to linoleic acid, making it a good candidate for genetic manipulation. Silencing of this gene was performed through RNA interference that causes a dramatic increase in oleic acid from 34% to more than 78%, and a corresponding reduction in polyunsaturated FA from 41% to less than 3% (Qu et al., 2012).
J. curcas oil is a renewable source with energy potential close to petroleum diesel, but, like other vegetable oils, main problem associated with it is the cost of production which makes it economically less feasible compared to diesel fuel. To add to the economic viability of the overall production process of the J. curcas biodiesel, development of the methods for the proper use of its byproducts such as seed cake, glycerin and fruit husks, are of supreme importance (Achten et al., 2007). Seed cake obtained after the extraction of oil from seeds is an important byproduct that could be utilized in multiple ways to make the biodiesel production process cost effective (King et al., 2009). J. curcas produce 1ton of seed cake per hectare and for instance, in India it is expected to grow this plant on 20 million hectares in coming years that can consequently produce 20 million tons of seed cake each year (Carels, 2009).
One of the possible uses of the seed cake is organic fertilizer (Openshaw 2000; King et al., 2009; Sharma et al., 2009), where it will not only increase the agricultural productivity but will also save the foreign exchange by replacing mineral fertilizers. However, investigation about the impact of the toxic constituents in this seed meal on the soil ecology will be important before its consideration as organic fertilizer (King et al., 2009). After detoxification seed meal could be an efficient animal feed due to its rich protein contents which is another alternative to add value to the biodiesel production from the oil of this plant (Achten et al., 2007; Debnath and Bisen 2008; King et al., 2009). A study on the utilization of various parts of J. curcas fruits and seeds showed, that seed cake is rich in organic matter and has excellent potential to be used for biogas production (Sing et al., 2008). This study revealed that biogas generation ability of the J. curcas seed cake is higher than that of the cattle dung and this biogas has more calorific value than cattle dung.
obtained from the fermentation of sugar sources like molasses, cereals and fruits (Mishra et al., 2011). J. curcas seed cake is a carbohydrates rich source that could serve as raw material for the bio-ethanol production (Mishra et al., 2011; Wever et al., 2012). Use of J. curcas seed cake for bio-ethanol production will not only solve the problem of safe disposition of this byproduct but will also provide opportunity of obtaining two important renewable fuels at the price of one starting material (Mishra et al., 2011). Along with the possibilities of the generation of energy from the seed cake it was also found to be an efficient source for the production of industrial enzymes like proteases and lipases, due to its suitable composition for the microbial growth (Mahanta et al., 2008).
According to Sing et al., (2008) J. curcas fruit consists of about 37.5% shell and 62.5% seed, while seed further contains 42% hull/husk and 58% kernel (Figure 1). On whole fruit weight basis it gave 17-18% oil while rest of the material produced as a byproduct that constitute about 53.7% of the whole fruit. These various parts of the fruit are rich sources of lignocelluloses and hence can be used as a raw material for the bio-ethanol production (Visser et al., 2011; Singh et al., 2008). The authors further suggested a holistic approach for utilizing all parts of J. curcas fruit in the energy production in order to achieve maximum benefit out of it.
1.2 Toxicity of Jatropha curcas seeds
Although, the protein rich seed meal obtained after oil extraction could be utilized for the animal feed but this use is currently hampered due to the presence of toxic and anti-nutritional constituents in this meal. J. curcas seeds were found to be toxic to mice, rats, calves, sheep, goats, chickens and human (Aderibigbe et al., 1997).
PE (Goel et al., 2007). So far six PE have been isolated and characterized from J. curcas oil (Figure 2) (Haas et al., 2002). PE are distributed in different parts of the plant, however, they are found in high quantity in the seed kernel, of which, 70% is in the oil and rest in the deoiled seed cake (Makkar and Becker, 2009).
PE are known for their tumor promoting activity and mimic the diacyl glycerol (DAG) mechanism of action which is an activator of the protein kinase C (PKC), and regulate different cellular metabolic pathways including signal transduction pathways. These ester molecules act as the analogs for the DAG and strongly activate PKC which in turn triggers cell proliferation and amplify the efficacy of carcinogens (Goel et al., 2007).
Curcin is another toxin commonly found in the seeds of J. curcas and represent another barrier to use seed cake as animal feed (King et al., 2009). It belongs to the family of ribosome-inactivating proteins (RIPs) which are classified as type I RIP when consisted exclusively of single RNA N-glycosidase (A chain) or type II RIP when additionally possess a domain with carbohydrate-binding activity (B chain) (Peumans et al., 2001). Curcin is a type-I RIP that halts the protein synthesis by damaging the ribosome through rRNA N-glycosidase activity and cleaves the specific glycosidic bond of adenine residue (Juan et al., 2003; Lin et al., 2003; Luo et al., 2006). Ricin is a type II RIP found in castor oil (R. communis) seeds that possess extreme toxicity due to the binding of the B chain to a sugar-containing receptor on the cell surface (Peumans et al., 2001). Due to the absence of B chain, curcin does not present the ability of binding to sugar-containing receptor on the cell surface and hence its toxicity is not as high as ricin. It has been reported that both recombinant and the crude curcin isolated from the J. curcas seeds have anti-tumor activity and further research is needed for its application as an anti-tumor medicine (Lin et al., 2003; Luo et al., 2006).
Numerous attempts have been made to detoxify the seed meal in order to make it suitable for animal consumption. Trypsin inhibitors are heat labile, and it was found that trypsin inhibition activity can be deactivated through heat (Makkar et al., 1998b), however, heating did not affect other anti-nutritional components (Aderibigbe et al., 1997). Double solvent extraction using hexane and ethanol combined with moist heat treatment enabled the complete inactivation of both trypsin inhibition and lectin activity (Chivandi et al., 2004). Martínez-Herrera et al. (2006) tested the effects of various treatments on the toxic factors in the seed cake of J. curcas and found that, trypsin inhibitors could be easily deactivated with moist heating at 121 oC for at least 25 minutes. They also reported that ethanol extraction coupled with 0.07% sodium bicarbonate (NAHCO3)notonly decreased the lectin activity but also decreased the PE content to 97.9%. They further showed that along with other treatments, irradiations could also reduce the phytate and saponin contents. In another study seed meal was subjected to alkali and heat treatment and was found that PE concentration was reduced up to 89%. They further checked the toxicity of the treated and untreated seed meal on rats and concluded that the mortality rate of the rats fed with treated meal was decreased compared to the ones fed with untreated meals (Rakshit et al., 2008).
Much efforts have been made (Oskoueian et al., 2011; Joshi et al., 2011; Kumar et al., 2011; Xiao et al., 2011) for the detoxification of the seed cake to make it suitable for animal consumption but commercial exploitation of these procedures have not been materialized yet.
1.3 Ovule structure and endosperm development
Ovule is the precursor of seeds and play a central role in the sexual reproduction of both angiosperms and gymnosperms. In angiosperms, ovule harbor female gametophyte, embryo sac, which is surrounded by nucellus and one or two integuments (Figure 3) (Gasser et al., 1998). After fertilization, the female gametophyte will form embryo and triploid endosperm, while integuments will differentiate to maternally derived seed coat (Ingram, 2010).
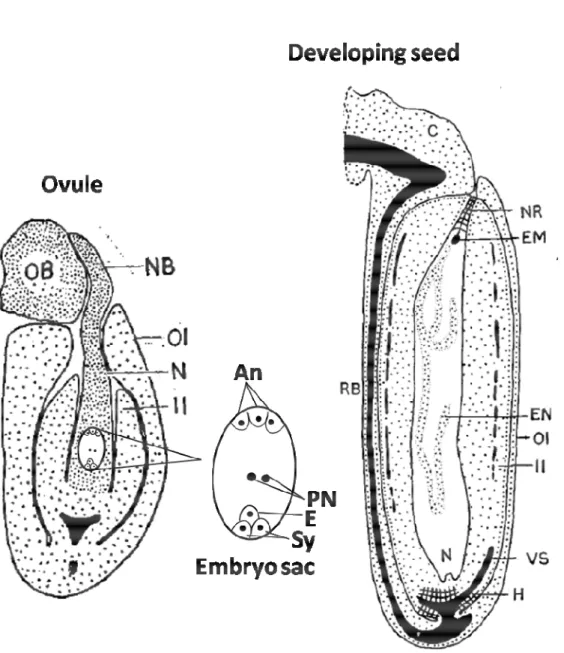
Figure 3: Jatropha curcas ovule and developing seed structure (Singh, 1970). An, antipodal cells; C, caruncle; E, egg cell; EM, embryo; EN, endosperm; H, hypostase; II, inner integument; N, nucellus; NB, nucellar beak; NR, nucellar remains; OB, obturator; OI, outer integument; PN, polar nuclei; RB, raphe bundle; Sy, synergids; VS, vascular supply.
embryo sac where one male gamete fertilizes the female gamete forming embryo of the daughter plant while other male gamete fuses with the diploid central cell. The triploid product of the second fertilization eventually develops to endosperm (Lopes and Larkins, 1993).
Level of persistency of the endosperm during development depends on the pattern of seed formation and the fate of endosperm in that particular species. For example, in cereal species, endosperm store reserves and remains as storage tissue in the mature seeds while in many dicotyledoneous species, endosperm is formed but partly degraded with the maturation of embryo. In peas, endosperm is absorbed at the free nuclear state as it has a non persistent endosperm (Lopes and Larkins, 1993; Olsen, 2001).
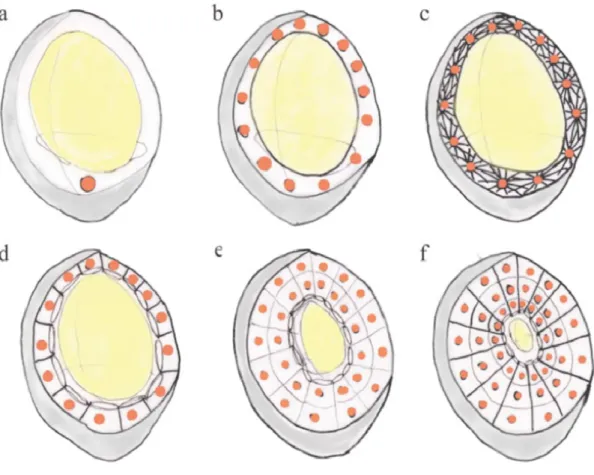
Endosperm development can be differentiated into three different types i.e. nuclear, cellular and helobial. Nuclear type is the most common one, where primary nucleus undergoes several divisions without cellularization, forming a large number of free nuclei organized at the sides of the central cavity. Cytokinesis starts from peripheral movement towards the center until whole endosperm is cellularized. In cellular type of endosperm development starting from the first nuclear division both the nuclear division and cytokinesis takes place together. In the less common, helobial type of endosperm development, the first primary endosperm cell will generate two different sized cells. The larger cell will follow the nuclear type development while the smaller one will remain undivided (Lopes and Larkins, 1993).
the central vacuole. Continued growth of the alveoli towards the central vacuole and their periclinal division results in the formation of a peripheral layer of the cells and a new layer of the alveoli towards the central vacuole. This process is repeated until the whole central vacuole get closed and replaced by new cells (Olsen, 2001).
1.4 Role of endosperm in reserves deposition
Seeds of the angiosperms constitute three basic parts: embryo proper, a tissue with stored reserves for use by the embryo till the stage of autotrophy and a seed coat that act as a protecting covering. Depending on the plant species the nutrients storage tissues may be cotyledons, endosperm or megagametophyte in the gymnosperms (Miernyk and Hajduch, 2011).
germination for the newly developing plantlet (Ekman et al., 2008). Oil palm is a monocot that stores reserves in the form of oil but the storage tissue is endosperm rather than cotyledons (Alang et al., 1988). Besides the monocots there are dicot species such as castor oil (R. communis) which retain endosperm as the main storage tissue and accumulates reserves mainly in the form of oil in this important tissue (Marriott and Northcote, 1975). Mature J. curcas seeds also accumulate oil as the main storage constituents in the endosperm. Cytohistological analysis showed that all portions of the mature seeds except seed coat, contributed to the accumulation of reserves but the main site of reserves deposition is the endosperm (Reale et al., 2012).
With the accumulation of the other products, endosperm also accumulate a variety of different proteins, most abundant of which called as storage or reserve proteins. The main purpose of these different kinds of storage proteins in the seeds is the storing of nitrogen and sulfur for the seedlings during germination, as these proteins have high amide and sulfur containing amino acids (Lopes and Larkins, 1993). Based on their extraction and solubility reserve proteins are traditionally classified into different classes like, albumins (water soluble), globulins (dilute saline solution soluble), prolamins (alcoholic solution soluble) and glutelins (dilute acid or base soluble) (Shewry et al., 1995). These proteins have some common characteristics, for example they are synthesized in higher quantity in some special tissue at the specific stage of development. Secondly they show presence of polymorphism, which may be due to the members of the multigenic families or proteolytic processing or glycosylation (Shewry et al., 1995). Thirdly these proteins are stored in special structures call protein bodies. Presence of reserve proteins in particular cellular bodies could be an adaptation to prevent these proteins from the action of enzymes responsible for the turnover of the metabolic proteins. Another advantage of the packing of these proteins in a particular membrane organelles is that they are deposited in the comparatively non aqueous conditions, which facilitates the seed desiccation (Lopes and Larkins 1993).
monocotyledon seeds and globulins are predominant in the dicotyledon seeds (Xu and Messing, 2009; Weber et al., 2005).
Storage proteins has been named in different ways, for example globulin storage proteins have further been grouped based on their sedimentation rate such as 7S and 11S (Shewry et al., 1995). Taking this as an easy and straightforward system of nomenclature it is common to find references for 3S or 12S globulins (Miernyk and Hajduch, 2011) or at the extreme for the 2.2S and 11.3S globulins (Templeman et al., 1987). In addition to this nomenclature, there are various proteins that were assigned trivial or informal names. For example cereal prolamins were named based on their Latin names i.e. zeins from maize, hordeins from barley etc. Similarly wheat prolamins were named as gliadins. 7S globulins are also called as vicilins and 11S globulins as legumins while 2S albumins were named as cactin (Reviewed in Miernyk and Hajduch, 2011).
Besides the storage proteins there are many other proteins which accumulate in the endosperm in high quantity, such as protease inhibitors, a-amylase inhibitors, lectins, thionins, RIPs, and certain enzymes, e.g., sucrose synthase, urease, etc. These proteins may be the secondary source of nitrogen and sulfur but their main role is the protection of seed from the attack of pathogens and predators (Lopes and Larkins, 1993).
1.5 Endosperm role in seed development
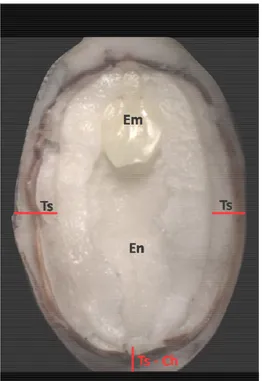
Figure 5: Morphology of Jatropha curcas seed, collected at 40 days after pollination. Em, embryo; En, endosperm; Ts, testa; Ts - Ch, testa on the chalazal region.
Embryo development depends on the proper development of the endosperm. If the endosperm fails to develop it will ultimately cause the embryo to seize its development as well (Lafon-Placette and Köhler, 2014). Hirner et al. (1998) showed that the amino acids supply to the embryo is totally dependent on the endosperm. They suggested that the amino acids transport first occurs to the endosperm which acts as a transient storage tissue and at the later stages of development these amino acids are transferred to the embryo via maternal tissue. Identification of the early expression of a specific gene around the area of embryo suggested the existence of interactions between the embryo and endosperm (Opsahl-Ferstad et al., 1997). This novel endosperm specific gene has been detected to express in a specific endosperm region around the embryo and was suggested to have role in the interaction between the two tissues. It is thus assumed that the endosperm has a critical role in the development of its neighboring embryo, though the endosperm development itself may be independent of the interaction with the embryo. This hypothesis was supported by the in vitro fertilization of the central cell (Kranz et al., 1998). The in vitro fertilized endosperm was found to follow the same developmental pattern as that of the in vivo. Although the used medium contained various nutrients and hormones but the role played by embryo cannot be excluded.
In cereals the endosperm may affects the development of embryo in a way other than nourishment. It was found that different mutations related to the endosperm development can restrict the development and size of the embryo and can exert both positive and negative effects on embryo. Some mutants were found to have a larger embryo due to reduced size endosperm while in others the larger endosperm occupies the main portion left by embryo (Hong et al., 1996).
1.6 Triacylglycerides metabolism and accumulation in seeds
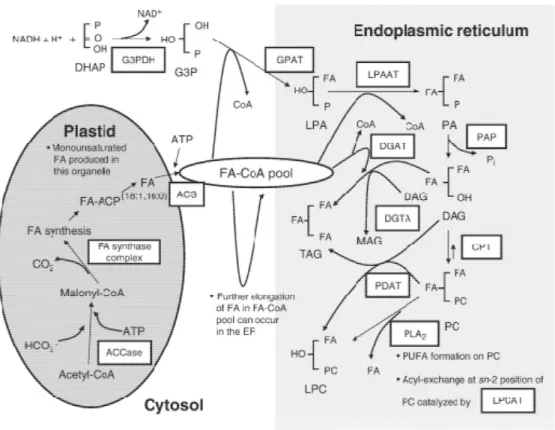
In seeds, oil is accumulated in small subcellular spherical structures called oil bodies (OBs). OBs consists of a TAG matrix surrounded by a layer of phospholipids (PLs) and special structural proteins, most abundant of which are oleosins. Small size of the OBs by providing large surface area per unit of TAG, facilitate the binding of lipases and lipolysis during seed germination. They are quite stable entities inside the cell and do not segregate (Hsieh and Huang, 2004).
Depending on the species composition of FA in vegetable oil is different. For example, R. communis oil contains 90% of ricinoleate (12- hydroxy-oleate) (Chen et al., 2007). In J. curcas, where the seeds oil content reaches to 60%, oleic acid (18:1) and linoleic (18:2) present in highest level followed by palmitic acid (16:0) and stearic acid (18:0) (Yang et al, 2009). Among the 200 different types, the most abundant FA present in the main commercial oilseeds (e.g. soybean, palm, canola and sunflower) are linoleic acid, palmitic, lauric acid and oleic acid (Thalen and Ohlrogge, 2002).
1.7 Plants proteomics
The term proteome was introduced to describe all the proteins encoded by a genome (Wilkins et al., 1996a). However, this definition does not explain that unlike genome, proteome is not a static entity and can change not only with the intracellular conditions but also with the conditions of the environment. Therefore proteome means the proteins content of a particular sample under specific time and conditions and include all the proteins isoforms and modifications (Wilkins et al., 1996b; De Hoog and Mann, 2004). Proteomics emerged as a result of the advances made by the genomics, transcriptomics and computational biology (Graves and Haystead, 2002; Zhu et al., 2003; De Hoog and Mann, 2004). One of the aim of proteomics is to identify the total expressed proteins to help in genome annotation as it is difficult to predict all the genes from genomic data. Expression studies through mRNA using various techniques is increasingly popular but the problem with these studies is that the mRNA analysis do not reflect the exact concentration of a protein in a particular sample. Other contributions of the proteomics could be: assigning proteins functions, different modifications occurring in a protein, proteins localization and compartmentalization and protein-protein interactions (See Graves and Haystead 2002, for a review).
In the field of botany, large scale proteomic studies have been possible only for some model species whose genome had been sequenced. On the other hand, proteomic analysis is still quite challenging for many economically important species with genome still not sequenced as mass spectrometry (MS) based proteomic analysis is dependent on the presence of sequence databases. With rapid advancements in the molecular biology techniques, that made possible the production of large scale genomic and transcriptomic data, plant proteomic studies acquired momentum in the last few years (Champagne and Boutry, 2013).
Recent improvements in the extraction, separation, quantification and identification of the plant proteins made it feasible to perform high throughput proteomic analysis for the plants to unravel the molecular mechanisms underlying plant growth, development and interaction with the environment (Chen and Harmon, 2006).
development, growth, productivity and can result in huge economic loss. In this regard proteomics and especially quantitative proteomics is appearing as a powerful approach, that allowing the rapid identification and quantification of the stress and tolerance related proteins (Agrawal et al., 2012; Salekdeh and Komatsu, 2007). Direct insight in to the function and understanding of the expression pattern and post-translational modifications (PTMs) of these stress related proteins can provide information which could be utilize to engineer stress tolerant plants using molecular biology strategies. Hashiguchi et al. (2010) presented a comprehensive overview of the application of proteomics to study different kind of stress related studies.
In different foods, proteins are important from both nutritional and technological point of view, and closely related to food quality, safety and nutrition (Agrawal et al., 2012). Proteomics could be used as a powerful tool in food industry for the quality, safety and nutritional assessment (Pedreschi et al., 2010). Food composition and quality could be analyzed through proteomics. Knowledge of the proteins composition of the various crops, obtained through the use of proteomics, has been successfully used for the industrial improvements (Agrawal et al., 2012). A proteomic study has been conducted to evaluate the effect of heat treatment on the proteins content of the peach fruit. This study showed, that most of the differentially expressed proteins were related to the development and repining, suggesting that this information could be used to improve the fruit quality of the peach (Zhang et al., 2011).
Food allergy is another field where proteomics has been applied at different levels. Combination of the 2-DE with immunoblotting using allergic patients sera has been the common approach used to characterize the allergenicity of certain food proteins (Akagawa et al., 2007; Salekdeh and Komatsu, 2007). Besides 2-DE based approaches, shotgun proteomics approaches have also been applied for the detection and characterization of the several food allergens (Chassaigne et al., 2007; Heick et al., 2011).
Moreover, there are many other applications of the proteomics for crops that have been extensively reported in the literature, like food authenticity through specific protein markers, assessment of the nutritional value, subcellular proteomics and many others (Salekdeh and Komatsu, 2007; Jorrin-Novo et al., 2009; Pedreschi et al., 2010; Agrawal et al., 2012).
In many plants, seeds are the important source of food, feed and biotechnologically important products, due to which one of the important goal of the agricultural research is to improve seed quality and traits in order to fulfill our needs (Moïse et al., 2005). Seed proteomics usually referred to the proteomic analysis of the storage tissues which may either be endosperm or cotyledons, as they comprise main part of the mature seeds (Miernyk and Hajduch, 2011).
Proteomics has been used for understanding the expression pattern and regulatory mechanism of the enzymes that are related to the reserves deposition in the seeds of different plants. Considerable progress has been made for the characterization of the proteomes of developing and mature seeds of the oilseed plants, which mainly accumulate oil and proteins in their seeds (Hajduch et al., 2011).
1.8 Mass spectrometry
chemistry. Mass spectrometer is very sensitive technique that can deal with the proteins mixture and offer much higher throughput (Pandey and Mann, 2000). MS consists of an ion source, mass analyzer and ion detector. Molecules are ionized in the ion source followed by separation according to their mass to charge ratios in the mass analyzer and the separated ions are detected by the detector (Nyman, 2001; Aebersold and Mann, 2003). Use of the MS has greatly increased in the proteins analysis after the invention of the two ionization techniques: MALDI (Matrix assisted laser desorption ionization) (Karas and Hillenkamp, 1988) and ESI (Electrospray ionization) (Fenn et al., 1989), also called soft ionization techniques. In MALDI-MS, matrix is a key component, which is an organic compound containing an aromatic conjugate ring and has the ability to absorb the ultraviolet light of particular wavelengths (Muddiman et al., 1997). In this technique, sample is cocrystallized with a proper matrix on a sample plate and laser beam of appropriate wavelength is fired on this mixture, which results desorption of the sample and matrix mixture. The matrix absorbs energy upon irradiation, and transfers it to the sample and eventually ionizes the sample (Cotte-Rodriguez et al., 2011). In ESI, sample dissolved in a solvent that pumped through a needle with high voltage and results into charged droplets. These droplets rapidly evaporate and impart their charge on the analyte (Mann et al., 2001; Cotte-Rodriguez et al., 2011).
Mass analyzer is an essential part of each MS because it can separate ions based their mass to charge (m/z) ratios. The important parameters for a mass analyzer are sensitivity, resolution and mass accuracy (Aebersold and Mann, 2003; Yates et al., 2009). Mass analyzers can be broadly categorized into two basic types: scanning and ion beam mass analyzers, such as time-of-flight (TOF) and quadrupoles (Q); and the trapping mass analyzers such as ion trap (IT), Orbitrap and Fourier transform ion cyclotrons resonance (FT-ICR) (Yates et al., 2009). In MS, mass analyzers are either used stand alone or in combination with other analyzer in order to increase the versatility and take advantage from the strength of each one (Aebersold and Mann, 2003; Yates et al., 2009).
ESI LTQ-Orbitrap is a hybrid MS instrument that contains ESI as the ion source and two different ion trap analyzers, the linear trap quadrupole (LTQ) and orbitrap. LTQ also called two-dimensional (2D) ion trap consists of hyperbolic quadrupole rods, each of which is divided into three successive axial sections. Three kinds of voltages are necessary for LTQ to operate as MS: three discrete DC (direct current) voltages applied to the three axial sections to produce axial trapping field, radio frequency (RF) voltage applied to the two pairs of rods to produce radial trapping field and alternating current (AC) voltage applied to the X rods for isolation, activation and ejection of the ions (Schwartz et al., 2002). In comparison to the previous three-dimensional (3D) quadrupole ion traps, LTQ has an increased sensitivity, resolution and mass accuracy (Schwartz et al., 2002; Aebersold and Mann, 2003; Yates et al., 2009).
accuracy of the Orbitrap couple with high speed and sensitivity of the LTQ (Yates et al., 2009).
1.9 Post-genomic era and Jatropha curcas
After availability of the techniques for producing large amount of genomics data in comparatively short period of time, now the focus is on the use of this data for conducting the transcriptomic and proteomic studies. J. curcas has also been the subject of different studies aiming in the understanding of the pattern of TAG and toxic components accumulation during plant development. Here we describe the progress made with the transcriptomic and proteomic studies of J. curcas, before and after its genome sequence.
J. curcas genome was sequenced in 2010 (Sato et al., 2010) using conventional Sanger method and new generation multiplex sequencing method, covering ~95% genes coding regions with 40929 presumptive proteins encoding genes, including 9870 complete genes. They reported 73 genes involved in the metabolism of TAGs and 3 genes encoding the curcins. It is also reported different genes related to the PE biosynthesis, the main toxic constituent of this plant. Later the genome was added (Hirakawa et al., 2012) by deoxyribonucleic acid (DNA) sequences generated through Illumina sequencing platform and the transcriptome data available in the public databases at that time, which considerably increased the number of genes with complete structure.
C-acyltransferase, related to the oil and carbohydrate breakdown. Besides, they also reported different proteins related to the terpenoids biosynthesis but were unable to detect any casbene synthase enzyme, the main enzyme of the PEs biosynthesis. Within two months of the appearance of this transcriptomic study, two more reports of the cDNA libraries for the developing J. curcas seeds were also published. A first study was published by Natarajan et al. (2010), where they prepared a cDNA library from developing seeds at 8 developmental stages and showed that the library had abundant genes related to stress response, disease resistance and plant development. They also identified an array of ESTs corresponding to the genes related to the FA metabolism, PL biosynthesis, carbohydrates metabolism and many other important genes involved in diverse metabolic activities. Similarly, Gomes et al. (2010) also constructed a cDNA from developing J .curcas seeds at three developmental stages and identified express sequence tags (ESTs) for the genes such as FA, terpene, quinine and hormone biosynthetic pathways. They also studied the expression profiles of the four genes i.e. palmitoyl-ACP thioesterase, 3-ketoacyl-CoA thiolase B, lysophosphatidic acid acyltransferase (LPAAT) and geranyl diphosphate (GPP) synthase, between leaves and seeds using real-time PCR (Polymerase chain reaction), and found that the expression of these genes were higher in the seeds than the leaves.
developmental stages and constructed three cDNA libraries, from which they identified 2295, 1646 and 1512 unigenes, respectively. They concluded that the proteins of lipids metabolism are not only involved in the oil accumulation but they are also important for embryogenesis, morphogenesis, defense responses and adaptive mechanisms in plants.
Eswaran et al. (2012) constructed a cDNA library from the salt stressed roots of J. curcas and compared with that of the untreated plants, for identification of the proteins involved in abiotic stress responses. They reported 1240 ESTs generated from salt stressed root cDNA library of J .curcas, which represent a diverse repository for stress related genes for this plant. This stress responsive transcriptome would help in understanding of the adaptability of J. curcas to harsh enviroments.
In addition to the construction of these cDNA libraries, various transcriptomic studies were also made for analysis of the expression profile of either selected genes involved in the lipids metabolism (Gu et al., 2011; Xu et al., 2011; Gu et al., 2012) or a global investigation of the genes during seed development (Jiang et al., 2012), which provided important information regarding the lipids metabolism during J. curcas seed development.
glyoxylate cycle, glycolysis, citric acid cycle, gluconeogenesis and pentose phosphate pathway were involved in the oil mobilization during seed germination. A comparative proteome analysis of the embryo and endosperm from the seeds of J. curcas was performed by Liu et al. (2009), using 2DE approach. 380 and 533 major protein spots were observed for the embryo and endosperm, respectively. 14 differentially expressed protein spots between the two tissues were identified through LC-MS/MS analysis and concluded that the proteins in the endosperm were catabolism related while those in the embryo were anabolism related. Oilbody proteome analysis from J. curcas identified 10 different proteins with three oleosins as the major constituents (Popluechai et al., 2011). These oleosins were characterized at the gene, transcript and protein level. They discovered two alleles for one of the oleosin, one with and other without introns. Single nucleotide polymorphisms (SNPs) were identified in the introns among different Jatropha accessions, which were suggested to serve as the markers during the phylogenetic and breeding studies. In another study the differential proteome of the endosperm and embryo from mature J. curcas seeds was analyzed using 2DE approach (Liu et al., 2011). 66 proteins spots were analyzed using LC-MS/MS that resulted in the identification of the 28 proteins, classified into 9 different functions. Authors showed that the proteins required for the germination of the seeds like proteins related to oil mobilization, signal transduction, transcription, proteins synthesis and cell cycle, were already present in the endosperm and embryo of the dry mature seeds. Recently proteome of the whole seed at six different developmental stages were analyzed using 2DE (Liu et al., 2013). Analysis of the differentially expressed proteins through MALDI-TOF/TOF resulted in the identification of the 104 proteins, classified into 10 different functional categories. They demonstrated that the proteins related to the energy and metabolism were involved in the carbon flux to the lipids accumulation in the seeds. Booranasrisak et al. (2013) investigated the seed kernel proteome at eight developmental stages using shotgun proteomic approach which resulted in the identification of the 22 proteins related to the FA metabolism.
2 OBJECTIVES
2.1 General objective
To analyze the proteome of the endosperm from developing J. curcas L. seeds through shotgun proteomics approach.
2.2 Specific objectives
• Classification and histological analysis of J. curcas seeds at different developmental stages.
• Identificationof the proteins extracted from the developing J. curcas seeds using
LC-MS/MS technique.
• Functional classification of the identified proteins.
• Classification of the proteins involved in the synthesis and degradation of the
FA.
• Classification of the proteins involved in carbohydrates metabolism and their
role in the lipids deposition.
• Classification and pattern of deposition of the J. curcas SSPs.
• Classification of the different types of peptidases involved in the reserve
mobilization and PCD of the developing endosperm.
• Classification of the proteins involved in the synthesis of toxic constituents. • Interpretation of the results and electing the possible target for the
3 MATERIALS AND METHODS
3.1 Plant material
The strategy of the seeds collection for the proteomics analysis used in this thesis is summarized in the form of a workflow (Figure 7). Seeds of J.curcas were collected at the experimental farm of the Federal University of Ceará (UFC), located at the Pentecoste, a municipality of the state of Ceará, Brazil. Based on their morphological characteristics, seeds were divided into nine different developmental stages and histological analysis was performed in order to characterize seeds in each stage. The mature Stage was called Stage 10. At Stage 1 and 2, nuclear endosperm, nucellus, and integuments were present in the seeds. At Stage 3 and 4, cellular endosperm was initiating at the micropyle side. Seeds at Stage 5 contained integuments and a thin layer of cellular endosperm around the central cavity. From Stage 6 to Stage 9, seeds contained integuments and a thicker endosperm but had different morphological characteristics. Seeds from Stage 1 to 5 had a light yellow color integuments. Seeds at Stage 6 had an orange color inner integument while the outer integument was still light yellow. Seeds at Stage 7 had an inner integument with 70 to 90% area as burgundy color while the outer integument was still light yellow. Seeds at Stage 8 had black color inner integument while light yellow outer integument. Seeds at Stage 9 had a black inner integument and an external integument with brownish ends. Morphological characteristics along with histological results are presented in results and discussion.
3.2 Histological analysis and selection of developmental stages for the endosperm isolation