Substrate effect on bacterial communities from constructed
wetlands planted with Typha latifolia treating
industrial wastewater
Cristina S.C. Calheiros
a, Anouk F. Duque
a, Alexandra Moura
b, Isabel S. Henriques
b,
António Correia
b, António O.S.S. Rangel
a, Paula M.L. Castro
a,∗aUniversidade Católica Portuguesa, Escola Superior de Biotecnologia, Rua Dr. António Bernardino de Almeida, 4200-072 Porto, Portugal bCESAM & Department of Biology, University of Aveiro, 3810-193 Aveiro, Portugal
Keywords: Bacterial communities Constructed wetland DGGE Typha latifolia Industrial wastewater
a b s t r a c t
Constructed wetlands (CWs) have been recognized as being able to effectively treat wastewater from municipal and industrial sources. This study focused on the effect of different substrates and long-term operation of horizontal subsurface flow CWs treating tan-nery wastewater on the bacterial communities. The CWs were planted with Typha latifolia in three types of substrate: two units with different types of expanded clay aggregates and one unit with fine gravel. Another unit with expanded clay was left unvegetated. Changes in the bacterial community related to the type of substrate, different hydraulic loading rates and along CW operation were examined using denaturating gradient gel electrophoresis (DGGE). Bacterial enumeration was also performed and several bacterial isolates were retrieved from the CWs. Phylogenetic affiliations of those isolates were obtained on the basis of 16S rRNA gene sequences and revealed that they were closely related to the genera Bacillus (TM1S1, TM1R3, TNR1 and TAR1), Paracoccus (TM1R2), Pseudomonas (TM1R1) and Halomonas (TM1S2). The type of substrate and the presence of T. latifolia had a major effect on the species richness and the structure of bacterial communities as inferred by numerical analysis of DGGE profiles.
Introduction
The tannery industry converts rawhide or skin, a putrescible material, into leather, a stable material, so that it can be used in the manufacture of a wide range of consumer products. Most of the steps of the tannery operations are carried out in water. Consequently, the wastewater treatment is of major concern. Tannery wastewater composition varies considerably with the production process that is engaged by the specifi-cation of the final product. Problems related to high organic content, and the presence of sulphides and chromium are
∗Corresponding author. Tel.: +351 22 5580059; fax: +351 22 5090351. E-mail address:plcastro@esb.ucp.pt(P.M.L. Castro).
often encountered imposing treatment needs in order to avoid negative impacts on the environment (COTANCE, 2002).
Constructed wetlands (CWs) are an effective method for wastewater treatment (Vymazal, 2005), inclusively for several types of industrial wastewater (Kadlec et al., 2000). In the case of the tannery industry these systems have already shown favorable performance (Calheiros et al., 2007, 2008a). The treat-ment mechanisms in CWs encompass a mix of physical, chemical, and biological processes. The vegetation is essential in wetland treatment systems (Kadlec et al., 2000); however, the main role in the transformation and mineralization of
nutrients and organic pollutants is played by microorganisms (Baptista, 2003; Stottmeister et al., 2003). The functional com-position of the different bacterial groups in subsurface flow CWs has not been fully explored (Ibekwe et al., 2003). Accord-ing toBaptista (2003)these functional bacterial groups may be largely determined by the environmental conditions and the nature of the system feed.
Culture-dependent methods are known to be insufficient for studying natural microbial community composition since numerous microorganisms are uncultured under laboratory conditions (Ward et al., 1990). The denaturating gradient gel electrophoresis (DGGE) analysis of 16S rRNA genes amplified by polymerase chain reaction (PCR) is useful for profiling com-plex microbial populations (Muyzer et al., 1993). Examples of studies of microbial communities in CWs using DGGE are those ofIbekwe et al. (2003), who characterized the micro-bial communities composition in CWs for dairy wastewater treatment, ofTruu et al. (2005), who investigated the varia-tion of microbiological parameters within a CW for domestic wastewater treatment, ofBaptista et al. (2003), who analyzed the microbial mechanisms of carbon removal in subsurface flow wetlands, and ofNicomrat et al. (2006), who assessed the microbial community in a CW receiving acid coal mine drainage.
The aim of this study was to analyze the effect of different substrates and long-term operation of horizon-tal subsurface flow CWs treating tannery wastewater on the dynamics of the bacterial communities. The bacte-rial abundance was evaluated by plate counting and the dynamics of the bacterial communities was evaluated by DGGE electrophoresis of 16S rRNA gene amplification prod-ucts. Numerical analysis of DGGE profiles allowed the estimation of species richness along the operation of each CW.
Methods
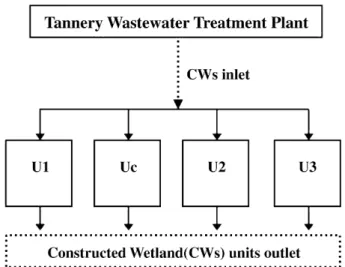
The setup conditions of the CWs, located at a tannery wastew-ater treatment plant in Portugal, are described inCalheiros et al. (2008a). Briefly, the CWs consisted of three units planted with Typha latifolia in different substrates: unit one (U1) and unit three (U3) had expanded clay aggregates being respec-tively Filtralite®MR 3–8 (FMR) and Filtralite®NR 3–8 (FNR) (from maxit, Argilas Expandidas, SA, Portugal), and unit two (U2) had fine gravel: AGH 4–8 (FG) (from Areipor-Areias Portuguesas, Lda, Portugal). An unvegetated unit (Uc), with the expanded clay aggregate FMR as substrate, was also included (Fig. 1). The CWs operated with horizontal subsurface flow and had a sur-face area of 1.2 m2. They received tannery wastewater after primary treatment under different hydraulic loading rates. The two units with FMR, U1 and Uc, had been in operation for 17 months during which two hydraulic regimes, 3 and 6 cm d−1, were tested (Calheiros et al., 2007). The units U2 and U3 had an acclimation period of 3 weeks before the tan-nery wastewater was applied. After that, all the systems were operated for 31 months under different hydraulic loadings and interruptions in feed (18, 6 and 8 cm d−1). Maintenance
Fig. 1 – Schematic representation of the constructed wetlands (CWs).
of the systems was carried out according toCalheiros et al. (2008a).
Wastewater samples were collected periodically from the inlet and outlet of the CW units and physico-chemical param-eters were determined based on Standard Methods (APHA, 1998): chemical oxygen demand (COD; Closed Reflux, Titri-metric Method), biochemical oxygen demand (BOD5; 5-day BOD Test), total suspended solids (total suspended solids, TSS; dried at 103–105◦C method), Kjeldahl nitrogen (TKN; Kjeldahl method), nitrate nitrogen (NO3−–N; nitrate electrode method), ammonia nitrogen (NH3–N; phenate method), total phospho-rus (total P; manual digestion and flow injection analysis for total phosphorus) and pH. The sulphate determination (SO42−; turbidimetric method) was done based on the method of the Association of Official Analytical Chemists (AOAC, 1995). The analyses were done immediately after sample col-lection otherwise samples were properly stored. Dissolved oxygen (DO) and conductivity were registered with a WTW handheld multi-parameter instrument 340i at the inlet and outlet of the units. The substrates used in the units were analyzed for organic matter content, based onHouba et al. (1995).
Microbiological analyses were performed simultaneously with physico-chemical analysis. Colony forming units (CFUs) were determined based on the surface-plate counting procedure. Briefly, three subsamples were pooled to form two compos-ite samples (10 g) of plant roots and substrate (from a depth between 10 and 15 cm) of each CW, being placed separately in sterile tubes with 10 mL of saline solution (0.15 mol L−1 NaCl) and shaken on a vortex mixer for 1 min at room tem-perature. Serial dilutions were made in duplicate and 0.1 mL of each dilution was spread onto nutrient agar (LABM, UK). Plates were incubated at 25◦C for 4 days after which CFU were counted. The same procedure was used for bacterial enumeration of the wastewater at the inlet and outlet of the CWs.
CWs set-up and physico-chemical analysis
Different bacterial colonies were isolated based on size, mor-phology and pigmentation, from nutrient agar plates using a streak-plate procedure. DNA of each isolate was obtained by picking a colony with a sterile toothpick, suspending the cells in 20L sterile water and incubating for 10 min at 100◦C (Henriques et al., 2006b). Molecular typing of bacterial isolates was performed by random amplified polymorphic DNA (RAPD) analysis. Amplification was performed in 25L reaction mix-tures containing: 0.75 U Taq polymerase, 1.5 mM MgCl2, 0.2 mM of each dNTP, 1.0M primer M13 (MWG-Biotech AG) and 0.5L of crude cell lysates. The thermal cycling profile was as follows: initial denaturation (94◦C for 5 min); 45 cycles of denaturation (94◦C for 1 min), annealing (34◦C for 2 min), and extension (72◦C for 2 min); and a final extension (72◦C for 10 min) (Silva et al., 2006). The reactions were carried out in a Bio-Rad iCycler Thermal Cycler (Bio-Rad Laboratories, Richmond, CA, USA) using Taq polymerase and nucleotides purchased from MBI Fermentas (Vilnius, Lithuania). Polymor-phic DNA fragments were analyzed by electrophoresis in a 1% agarose gel in Tris–acetate–EDTA (TAE) buffer, after stain-ing with ethidium bromide. Gel image was acquired usstain-ing a Molecular Image FX apparatus (Bio-Rad Laboratories, Her-cules, CA, USA).
Isolates displaying unique RAPD profiles were subsequently identified by 16S rRNA gene sequencing analysis. Amplifica-tion was performed with universal bacterial primers 27F and 1492R, as described byLane (1991). PCR products were puri-fied with Jetquick PCR Product Purification Spin Kit (Genomed, Löhne, Germany). DNA sequencing was conducted under BigDyeTM terminator cycling conditions, and analyzed using an automatic sequencer 3730xl (Macrogen Inc., Seoul, Korea). To determine the phylogenetic affiliation, similarity searches were performed using the BLAST program (Altschul et al., 1997).
Genomic DNA from substrate and root samples (six subsam-ples were pooled to form one composite sample of plant roots and substrate for each CW) was extracted using the Ultra CleanTM Soil DNA Isolation Kit (MO BIO Laboratories, Inc., USA), according to the manufacturer’s protocol. PCR amplifi-cation of bacterial 16S rRNA gene fragments was performed using primers 338F GC and 518R, as described previously (Henriques et al., 2006a). Nested PCR amplifications were per-formed using as template 1L of the DNA amplicon obtained after the first amplification round and using the same primers and conditions applied in the first PCR amplification.
DGGE analysis was performed on a DCodeTM Universal Mutation Detection System (Bio-Rad Laboratories, Hercules, CA, USA). Samples containing approximately equal amounts of nested-PCR amplicons were loaded onto 8% (w/v) poly-acrylamide gels (37.5:1, poly-acrylamide/bis-poly-acrylamide) in 1× TAE
buffer using a denaturing gradient ranging from 35% to 60% (100% denaturant solution is defined as 7 M urea and 40% (v/v) formamide (Muyzer et al., 1993)). A standard marker was also included in all gels, to serve as an indicator of the analysis quality. The standard marker was constructed using bacte-rial isolates (obtained as described above) selected to cover an adequate range of bands. Electrophoresis conditions and image acquisition were as described previously (Henriques et al., 2006b).
DGGE profiles, concerning the presence and intensity of the bands, were analyzed using GelCompar®II software (Version 4.6; Applied Maths, Sint-Martens-Latem, Belgium). Detected band patterns were transferred to an absence/presence matrix. The binary matrix was transformed into a similar-ity matrix using the Bray-Curtis measure. Dendrograms were generated by unweight pair group mean average (UPGMA) cluster analysis. Cluster analysis and multidimensional scal-ing diagram (MDS) were performed usscal-ing PRIMER 5 for Windows (Version 5.2, 2001, PRIMER-E Ltd.) (Clarke and Gorley, 2001).
DGGE banding data were used to estimate diversity, H (Shannon and Weaver, 1963) and equitability, E (Pielou, 1975) indexes to describe possible changes in the dominance among phylotypes (Fromin et al., 2002).
The 16S rRNA gene sequences of bacterial isolates obtained in this study were deposited in GenBank under the accession numbers TM1R1: EU430693, TM1R2: EU430694, TM1R3: EU430695, TM1S1: EU430696, TM1S2: EU430697, TNR1: EU430700, TAR1: EU430701.
Statistical analysis was performed using the software SPSS (SPSS Inc., Chicago, IL, USA; Version 12.0). When applicable, the data were analyzed through one-way analysis of variance (ANOVA) and Student’s t-test. To detect the statistical signifi-cance of differences (p < 0.05) between means of observation, the Duncan test was performed. When applicable, values were presented as the mean± standard error.
Results
InFig. 2the removal of several wastewater components by the CWs is illustrated. COD, BOD5and TSS inlet concentration varied between 835–2261, 430–850 and 43–110 mg L−1, respec-tively. In general, the FMR unit U1 presented higher removal levels of organic matter, followed by the FNR unit U3, the fine gravel unit U2 and the FMR unvegetated unit Uc.
The inlet concentrations of TKN, NH3and SO42− ranged between 102–160, 60–98 and 78–1070 mg L−1, respectively. The outlet concentrations of TKN, NH3 and SO42− ranged between 57–115, 35–65 and 32–1005 mg L−1, respectively. For NO3− the inlet varied between 17–59 mg L−1 and the out-let between 9–47 mg L−1. Total phosphorus ranged between Bacteria isolation, DNA extraction and RAPD typing
DNA sequence and phylogenetic analysis
Nucleotide sequence accession numbers
Data analysis
Analysis of bacterial communities of substrate and roots from CWs
Fig. 2 – Removal efficiency of (A) COD, BOD5and TSS and (B) TKN, NH3and SO42, by the constructed wetlands (CWs). Dispersion bars represent standard error of the mean. U1: CW with Typha latifolia planted in Filtralite®MR 3–8; U2: CW with T. latifolia planted in fine gravel, AGH 4–8; U3: CW with
T. latifolia planted in Filtralite®NR 3–8; Uc: unvegetated unit with Filtralite®MR 3–8. One-way ANOVA was performed in order to compare the performance in terms of removal efficiency between units for each parameter. Columns marked with different letters differed significantly according to Duncan’s multiple range tests at of p < 0.05.
0.13–0.83 mg L−1at the inlet and between 0.06–0.77 mg L−1at the outlet.
DO at the inlet and outlet was low, varying between 0.17–0.99 mg L−1 and 0.09–0.88 mg L−1, respectively. The pH values were quite stable at the outlet (7.23–8.68) although the inlet values varied between 4.76 and 8.26. Conductivity varied between 4.74–9.64 mS cm−1at the inlet and 5.00–9.61 mS cm−1 at the outlet.
The organic matter content was determined for the three substrates as being 0.30% for FMR, 0.30% for FNR and 0.04% for FG. At the inlet and outlet zone of each unit the organic matter content was also determined as being, respectively: 0.86 and 0.63% for U1, 0.37 and 0.17% for U2, 1.00 and 0.71% for U3 and 0.89 and 0.71% for Uc.
The enumeration of bacteria in the units, including roots and substrate, is shown in Fig. 3. One-way ANOVA was applied to compare the CFUs between the planted units and no sig-nificant differences were found. When Student’s t-test was applied to compare bacterial counts from roots and substrate of each unit significant differences were seen for units U2 and U3, although for U1 no significant differences were found.
The number of aerobic bacteria present in the wastewa-ter at the inlet and outlet of the CWs was also dewastewa-termined. Samples collected periodically (February, July, September, December 2005 and February, June, October 2006) showed a variation between 3.9× 101and 4.2× 105for the inlet, between 3.2× 103and 2.2× 106for the outlet of the vegetated units (U1, U2 and U3) and between 2.6× 104and 1.2× 106for the outlet of the unvegetated unit (Uc).
Dominant bacterial isolates obtained from the plates were further molecular typed by RAPD-PCR. Seven different RAPD types were then characterized through sequencing of the 16S rRNA encoding gene. According to BLAST results, 2 strains were affiliated with␥-Proteobacteria, 4 with Firmicutes and 1 with␣-Proteobacteria. These strains were isolated from plant roots (TM1R1, TM1R2, and TM1R3) and substrate (TM1S1 and TM1S2) of unit U1 and from plant roots of unit U2 (TAR1) and unit U3 (TNR1) (Table 1).
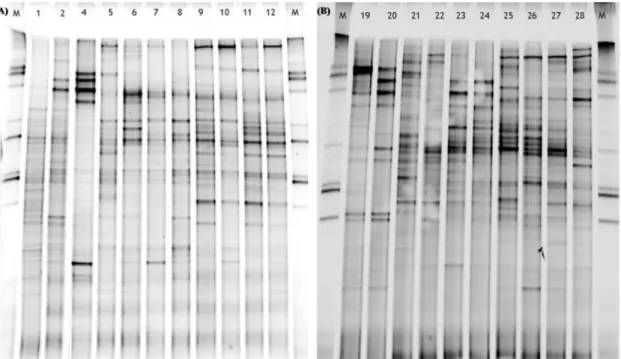
Analysis of bacterial communities from substrate and root samples, collected along the CWs operation, was performed by PCR–DGGE. Two examples of banding patterns for the 16S rRNA gene PCR–DGGE amplicons are presented inFig. 4, corre-sponding to units U1 and U2. Each gel presents the variability within each CW at different sampling periods correspond-ing to different hydraulic conditions. Reproducibility of PCR amplification and DGGE was confirmed in replicates.
Table 2shows, for each CW, the number of bands in each lane and the Shannon and equitability indexes (calculated by using the DGGE profiles) correspondent to the different sam-pling times within each gel. The total number of different band positions in the four gels was 68 (58 in gel U1, 50 in gel U2, 51 in gel U3 and 43 in gel Uc). The Shannon’s diversity index (H) was in average of 1.10± 0.07 for U1, 1.08 ± 0.03 for U2, 1.14 ± 0.05 for U3 and 1.01± 0.07 for Uc. The equitability index (E) was in average of 0.77± 0.05 for U1, 0.82 ± 0.02 for U2, 0.80 ± 0.02 for U3 and 0.73± 0.05 for Uc.
Fig. 5shows clustering analysis of DGGE banding patterns. In general, samples clustered mostly according to the place of collection (substrate or root) and the type of substrate, being the temporal factor less relevant in bacterial assemblage com-position.
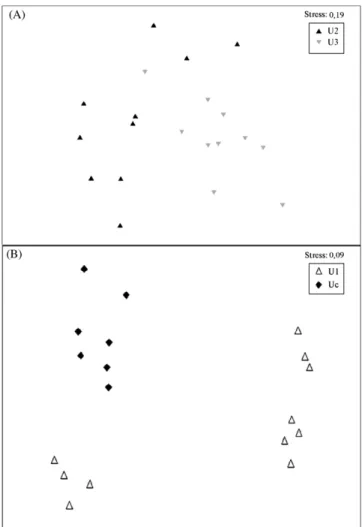
Multidimensional scaling diagrams of similarity matrices (Fig. 6), calculated from the DGGE patterns of samples, showed that the bacterial community changed according to the differ-ent substrates. Concerning the analysis between the FMR units U1 and the unvegetated control (Uc), differences between community profiles within each unit are clear, with a higher dispersion for the vegetated unit U1.
Discussion
This study intended to investigate the bacterial communi-ties in CWs established with different substrates, namely expanded clay aggregates and fine gravel. Close attention was given to bacteria, although it is known that fungi may also play an important role in the function of wetlands (Baptista, 2003). DGGE analysis of bacterial community
Fig. 3 – Bacterial enumeration analyzed by plate counts and expressed in CFUs g−1for substrate and plant roots of constructed wetlands (CWs). Dispersion bars represent standard error of the mean. U1: CW with T. latifolia planted in Filtralite®MR 3–8; U2: CW with T. latifolia planted in fine gravel, AGH 4–8; U3: CW with T. latifolia planted in Filtralite®NR 3–8; Uc: unvegetated unit with Filtralite®MR 3–8.
The complex physico-chemical composition of the tannery wastewater passing through the wetland is of major impor-tance since it can have a relevant effect on the vegetation (Calheiros et al., 2007, 2008a,b) and in the bacterial commu-nities that inhabit these ecosystems. The wastewater content varies in terms of nutrients and toxic substances and can limit or promote bacterial activity and growth. The main role concerning the direct degradation of organic chemicals in wastewater treatment is played by microorganisms despite the capacity of plants to detoxify xenobiotics (Stottmeister et al., 2003). The organic compounds degradation in the hori-zontal subsurface flow wetlands is carried out aerobically and anaerobically at different extents by bacteria attached to plant roots and substrate surfaces (Kadlec et al., 2000).
The three tested substrates have proven to be adequate for T. latifolia development although there was higher plant
propagation for the expanded clay aggregates (Calheiros et al., 2008a). The macrophytes are important in the wetlands since they provide structure and a source of reduced carbon for the microbes that mediate most of the pollutant transformations occurring in the wetlands (Kadlec et al., 2000).Collins et al. (2004)concluded that plants do have an effect on water qual-ity, in part because they affect bacterial assemblages. Higher pollutant removals, in terms of COD and BOD5, were achieved in expanded clay planted units after long-term operation. The similar behavior of the expanded clay systems (U1 and U3) concerning the pollutant removal may be attributed to the fact that they may have similar functional group of microorgan-isms.
The substrate is an important wetland component since it supports plant growth, establishment of microbial biofilms and influences the hydraulic processes (Stottmeister et al.,
Table 1 – Phylogenetic affiliation of bacterial strains isolated from the constructed wetlands.
Isolate NCBI
accession no.
Phylogenetic affiliation
Closest relative (accession no.) Similarity (%)
Origin
TM1R1 EU430693 ␥-Proteobacteria Pseudomonas stutzeri ST27MN3 (U26419)Pseudomonas stutzeri 19smn4 (U22426) 9999 SeaSea
TM1R2 EU430694 ␣-Proteobacteria Paracoccus sp. G30 (DQ667122)Paracoccus sp. G29 (DQ667121) 9898 Antarctic seawaterAntarctic seawater TM1R3 EU430695 Firmicutes Bacillus pumilus MLA14 (EF462914) 99 n.d.
Bacillus sp. NS88 (EF633174) 99 Phyllostachys edulis
TM1S1 EU430696 Firmicutes Bacillus sp. BCw063 (DQ492812) 99 Arctic sea water
Bacillus sp. GB02-29 (DQ079002) 99 Hydrothermal
sediments and plumes TM1S2 EU430697 ␥-Proteobacteria Halomonas sp. 3014 (AM110971)Halomonas sp. A3-3 (AB219125) 9999 Deep sea sedimentFermented foods TNR1 EU430700 Firmicutes Bacillus sp. BCw063 (DQ492812)Bacillus sp. GB02-29 (DQ079002) 9999 Arctic sea waterHydrothermal
sediments and plumes TAR1 EU430701 Firmicutes Bacterium 8-gw3-5 (DQ990048) 99 River waters andshallow groundwater
Fig. 4 – DGGE analysis of 16 rRNA gene fragments of total bacterial population from samples of the root and substrate of constructed wetlands planted with T. latifolia in different matrixes. (A) Gel image of root and substrate samples collected in U1 in September 2004 (lanes 1 and 2), February 2005 (substrate, lane 4), June 2005 (lanes 5 and 6), July 2005 (lanes 7 and 8), July 2006 (lanes 9 and 10), October 2006 (lanes 11 and 12) and (B) gel image of root and substrate samples collected in U2 in February 2005 (lanes 19 and 20), June 2005 (lanes 21 and 22), July 2005 (lanes 23 and 24), July 2006 (lanes 25 and 26), October 2006 (lanes 27 and 28). A DNA marker (M) was included in all the gels to serve as control.
2003). A porous matrix, such as expanded clay, provides a greater surface area for treatment contact and biofilm devel-opment. Each substrate used here has different characteristics in terms of pH, electrical conductivity, porosity, and organic matter content. The higher organic matter content verified at the inlet substrate of all units was attributed to the fact that
at this point the wastewater organic loading was higher than at the outlet.
The number of CFU found in the vegetated units is within the range of what has been published byTruu et al. (2005)
for aerobic heterotrophic bacteria in horizontal subsurface flow CW for domestic wastewater treatment, filled with coarse
Table 2 – Number of bands and Shannon diversity (H) and equitability (E) indexes, calculated for the constructed wetlands (CWs). U1: CW with Typha latifolia planted in Filtralite®MR 3–8; U2: CW with T. latifolia planted in fine gravel,
AGH 4–8; U3: CW with T. latifolia planted in Filtralite®NR 3–8; Uc: unvegetated unit with Filtralite®MR 3–8.
Samplesa U1 U2 U3 Uc
Nr bands H E Nr bands H E Nr bands H E Nr bands H E
Set R 04 35 1.40 0.91 n.d. n.d. n.d. n.d. n.d. n.d. n.a. n.a. n.a.
Set S 04 36 1.18 0.76 n.d. n.d. n.d. n.d. n.d. n.d. 17 0.82 0.66
Feb R 05 n.d. n.d. n.d. 21 0.94 0.71 17 0.81 0.66 n.a. n.a. n.a.
Feb S 05 26 0.88 0.62 14 0.92 0.80 21 1.05 0.79 25 1.06 0.76
Jun R 05 29 1.02 0.70 22 1.18 0.88 24 1.09 0.79 n.a. n.a. n.a.
Jun S 05 30 1.01 0.68 27 1.20 0.84 22 1.05 0.78 23 1.00 0.74
Jul R 05 26 1.64 1.16 18 1.05 0.84 29 1.18 0.81 n.a. n.a. n.a.
Jul S 05 24 0.76 0.55 19 1.12 0.87 25 1.21 0.87 23 1.29 0.94
Sep S 05 n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. 21 0.64 0.49
Jun S 06 n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. n.d. 30 1.04 0.71
Jul R 06 22 1.13 0.84 22 1.16 0.86 35 1.30 0.84 n.a. n.a. n.a.
Jul S 06 21 0.94 0.71 25 1.13 0.80 35 1.23 0.80 30 1.02 0.69
Oct R 06 22 1.09 0.81 25 1.10 0.79 33 1.26 0.83 n.a. n.a. n.a.
Oct S 06 22 1.02 0.76 22 1.03 0.77 26 1.19 0.84 28 1.18 0.82
n.a., not applicable; n.d., not determined.
Fig. 5 – Cluster analysis of microbial communities generated by the analysis of DGGE 16S rRNA gene patterns representing the genetic similarity of the microbial profiles acquired. Similarities were calculated using the Bray-Curtis measure. (A) Cluster analysis of bacterial communities from U1 (Filtralite®MR 3–8 substrate), U2 (fine gravel: AGH 4–8 substrate) and U3 (Filtralite®NR 3–8 substrate). (B) Cluster analysis of bacterial communities from U1 and Uc (unvegetated control with Filtralite®MR 3–8 substrate).
sand and dominated by Scirpus sylvaticus, Urtica dioica and Epilobium hirsutum. The fact that there were no significant dif-ferences in bacterial numbers between the substrate and the roots in unit U1 was attributed to the diffuse propagation of T. latifolia, since this unit was established for units longer than U2 and U3. Significantly higher numbers of CFUs were fre-quently found in the wastewater samples collected from the CWs outlet when compared to the inlet, which can be due to the fact that the water passing through the wetland washes out bacteria from the substrate. Although in this study an aer-obic plate count method was followed it would be interesting,
in the future, to undertake anaerobic enumeration consid-ering the fact that the organic degradation can occur both aerobically and anaerobically in the CW systems. Attempts to evaluate the relative importance of different microbial reactions on organic matter removal (in terms of COD), in hor-izontal subsurface flow CWs treating urban wastewater, have been carried out using a 2D simulation model (Ojeda et al., 2008). They indicated that the microbial anaerobic reactions involved in organic matter removal (methanogenesis and sul-phate reduction) occurred over larger areas of the wetlands than anoxic (denitrification) and aerobic reactions.
Fig. 6 – Multidimensional scaling diagram of DGGE patterns of samples collected in the constructed wetlands (CWs) of (A) U2 and U3 and (B) U1 and Uc. U1: CW with T. latifolia planted in Filtralite®MR 3–8; U2: CW with T. latifolia planted in fine gravel, AGH 4–8; U3: CW with T. latifolia planted in Filtralite®NR 3–8; Uc: unvegetated unit with Filtralite®MR 3–8.
The microbial diversity in the substrate and rhizosphere is of great importance since it may be influenced by the type and amount of plants. Most of the bacterial isolates retrieved from the roots and substrate of the CWs presented here were similar to environmental isolates reported from sources such as river and sea waters, sediments and soil. Two isolates affil-iated with␥-Proteobacteria were recovered from substrate and roots from the CWs.Franco et al. (2005), based on enrichment cultures with soil collected nearby the same CWs, have iso-lated bacteria able to degrade polyphenols used in the tannery process being all of them members of␥-Proteobacteria.
The dynamics of the bacterial community in the CWs over 3 years of operation were analyzed by comparison of 16S rDNA PCR–DGGE profiles. Data from this study showed that there was a diverse community of bacteria in the CWs, and that might influence to different extents the final effluent quality. Although the FMR units U1 and Uc had been in operation for a bit longer than U3 and U2, which could indicate that these sys-tems would be more stable and resistant to the stress caused
by the hydraulic alterations and wastewater contaminations, no direct relationship could be found.
In this study the community composition was not deep-ened in relation to the identification of bacterial groups. Despite that, species richness and the structure of bacterial communities have been inferred by numerical analysis of DGGE profiles (Moura et al., 2007). DGGE profiles showed a high variability among bacterial assemblages. The presence of vegetation seemed to have a major effect on the commu-nity diversity, as lower bacterial diversity (lower H values) was observed in the unvegetated unit (Uc). Similarly,Baptista et al. (2003)reported that microbial communities from inlet and outlet of horizontal subsurface flow CW with plants are dif-ferent when compared to an unplanted wetland, treating a solution of filtered beer. In this study, species richness was higher in the unit with the expanded clay FNR (U3), followed by FMR (U1) and FG (U2). The differences observed might be explained in part by the substrates that differ in characteris-tics such as pH, conductivity and porosity (Calheiros et al., 2008a,b). In general, all units showed higher diversity near root comparing to substrate, which can be related to rhi-zosphere effects on bacterial communities. Previous studies have reported the importance of plant metabolites excreted to the rhizosphere, such as vitamins, that may stimulate micro-bial growth (Stottmeister et al., 2003). Additionally,Marschner et al. (2002)have studied the microbial community structure in the rhizosphere of cluster roots of Lupinus albus and reported that changes in the community structure may be related with organic acid plant exudates.
Differences in microbial community structure were detected by using the equitability index (Pielou, 1975). The equitability was higher in the unit with the sand FG (U2) fol-lowed by the units with expanded clay FNR (U3) and then FMR (U1), and finally the unvegetated unit (Uc).Vacca et al. (2005)
have reported that when using CWs for domestic wastewater treatment, the rhizosphere of P. australis is colonized by dis-tinct communities depending on the filter material (sand and expanded clay) and have concluded that the technology, filter material and plant have a selective influence on the microbial community within the CW. The functional diversity may be linked to certain extents to the ecophysiological roles played by functional groups although the present study did not allow such conclusions. In general, equitability was higher in root samples comparing to substrate samples. Again, this may be related to the rhizosphere effect on microbial communities that may promote the growth of certain bacterial groups.
The assessment of microbial communities in CWs has been addressed by several authors (Baptista et al., 2003; Ibekwe et al., 2003; Truu et al., 2005; Nicomrat et al., 2006). Ibekwe et al. (2003)have characterized the microbial communities and composition in CW for dairy wastewater and reported that these systems are dependent on microbial communi-ties for optimal wastewater treatment. Since little information is available regarding the bacterial communities that inhabit these ecosystems, the PCR–DGGE technique employed here was of great importance in obtaining new data concerning the microbial structure and dynamics of CWs for tannery wastewater treatment. The study of dynamics of microbial communities from CWs is thus essential in understanding these treatment systems, being a valuable tool for
improv-ing its design and operation. A more extensive investigation may also be undertaken at other levels, mainly those related to the detection of microbial genes encoding enzymes linked to degradation pathways, and analysis of their quantitative expression within each CW.
Conclusions
(1) The type of substrate and the presence of T. latifolia seemed to have a major effect on the dynamics and diversity of the bacterial community.
(2) The variations introduced in the systems in terms of hydraulic regimes and wastewater contamination did not result in substantial changes in the diversity of the micro-bial communities along the systems operation.
(3) A high diversity of bacterial populations was found in the CW units (according to DGGE profiles) and that could con-tribute to the resilience and resistance of the CWs to stress created by the wastewater loadings applied.
(4) The type of substrate was more relevant in determining the bacterial composition than the temporal variability, with the planted CWs units presenting a high similarity within the same year for root and substrate.
Acknowledgements
The authors thank Dias Ruivo, Curtumes e Produtos Indus-triais, Lda for making possible the establishment of this project and to maxit, Argilas Expandidas, SA that kindly offered Filtralite®. Cristina S.C. Calheiros, Isabel Hen-riques and Alexandra Moura wish to thank the research grants from Fundac¸ão para a Ciência e Tecnologia (FCT), Portugal (SFRH/BDE/15507/2004, SFRH/BPD/21384/2005 and SFRH/BD/19433/2004). The work was supported by the project POCI/AMB/60126/2004.
r e f e r e n c e s
Altschul, S.F., Madden, T.L., Schäffer, A.A., Zhang, J., Zhang, Z., Miller, W., Lipman, D.J., 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402.
AOAC, 1995. Official Methods of Analysis, 16th ed. Association of Official Analytical Chemists Inc., Arlington, VA, USA. APHA, 1998. Standard Methods for the Examination of Water and
Wastewater, 20th ed. American Public Health
Association/American Water Works Association/Water Environment Federation, Washington, DC, USA.
Baptista, J.C., 2003. Microbial communities in subsurface flow wetlands. In: Proceedings of the 1st international seminar on the use of aquatic macrophytes for wastewater treatment in constructed wetlands, Lisbon, Portugal, pp. 265–276. Baptista, J.D.C., Donnelly, T., Rayne, D., Davenport, R.J., 2003.
Microbial mechanisms of carbon removal in subsurface flow wetlands. Water Sci. Technol. 48 (5), 127–134.
Calheiros, C.S.C., Rangel, A.O.S.S., Castro, P.M.L., 2007.
Constructed wetland systems vegetated with different plants applied to the treatment of tannery wastewater. Water Res. 41, 1790–1798.
Calheiros, C.S.C., Rangel, A.O.S.S., Castro, P.M.L., 2008a. Evaluation of different substrates to support the growth of Typha latifolia in constructed wetlands treating tannery wastewater over long-term operation. Bioresour. Tecnhol. 99, 6866–6877. Calheiros, C.S.C., Rangel, A.O.S.S., Castro, P.M.L., 2008b. The
effects of tannery wastewater on the development of different plant species and chromium accumulation in Phragmites australis. Arch. Environ. Con. Tox. 55 (3), 404–414.
Clarke, K.R., Gorley, R.N., 2001. PRIMER v5: User Manual/Tutorial. PRIMER-E, Plymouth, UK.
Collins, B., McArthur, J.V., Sharitz, R.R., 2004. Plant effects on microbial assemblages and remediation of acidic coal pile runoff in mesocosm treatment wetlands. Ecol. Eng. 23, 107–115.
COTANCE, 2002. The European Tanning Industry Sustainability Review. Confederation of Tanning Industries of the European Union Brussels, Belgium.
Franco, A.R., Calheiros, C.S.C., Pacheco, C.C., De Marco, P., Manaia, C.M., Castro, P.M.L., 2005. Isolation and characterization of polymeric galloyl-ester-degrading bacteria from a tannery discharge place. Microbial Ecol. 50, 550–556.
Fromin, N., Hamelin, J., Tarnawski, S., Roesti, D.,
Jourdain-Miserez, K., Forestier, N., Teyssier-Cuvelle, S., Gillet, F., Aragno, M., Rossi, P., 2002. Statistical analysis of denaturing gel electrophoresis (DGE) fingerprinting patterns. Environ. Microbiol. 4 (11), 634–643.
Henriques, I.S., Alves, A., Tacão, M., Almeida, A., Cunha, A., Correia, A., 2006a. Seasonal and spatial variability of free-living bacterial community composition along an estuarine gradient (Ria de Aveiro, Portugal). Estuar. Coast Shelf S 68, 139–148.
Henriques, I.S., Moura, A., Alves, A., Saavedra, M.J., Correia, A., 2006b. Analysing diversity among-lactamase encoding genes in aquatic environments. FEMS Microbiol. Ecol. 56, 418–429.
Houba, V.J.G., Van Der Lee, J.J., Novozamsky, I., 1995. Soil Analysis Procedures—Other Procedures (Soil and Plant Analysis. Part 5B). Department of Soil Science and Plant Nutrition. Sixth edition. Wageningen Agricultural University. Syllabus. Wageningen, The Netherlands.
Ibekwe, A.M., Grieve, C.M., Lyon, S.R., 2003. Characterization of microbial communities and composition in constructed dairy wetland wastewater effluent. Appl. Environ. Microbiol. 69 (9), 5060–5069.
Kadlec, R.H., Knight, R.L., Vymazal, J., Brix, H., Cooper, P., Haberl, R., 2000. Constructed wetlands for pollution
control—processes, performance, design and operation. IWA Scientific and Technical Report No. 8. IWA Publishing. London, UK.
Lane, D.J., 1991. 16S/23S rRNA sequencing. In: Stackebrandt, E., Goodfellow, M. (Eds.), Nucleic Acid Techniques in Bacterial Systematics. John Wiley and Sons Ltd., West Sussex, England. Marschner, P., Neumann, G., Kania, A., Weiskopf, L., Lieberei, R.,
2002. Spatial and temporal dynamics of the microbial community structure in the rhizosphere of cluster roots of white lupin (Lupinus albus L.). Plant Soil 246, 167–174.
Moura, A., Tacão, M., Henriques, I., Dias, J., Ferreira, P., Correia, A., 2007. Characterization of bacterial diversity in two aerated lagoons of a wastewater treatment plant using PCR–DGGE analysis. Microbiol. Res., doi:10.1016/j.micres.2007.06.005. Muyzer, G., De Waal, E.C., Uitterlinden, A.G., 1993. Profiling of
complex microbial populations by denaturating gradient gel electrophoresis analysis of polymerase chain
reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microb. 59 (3), 695–700.
Nicomrat, D., Dick, W., Tuovinen, 2006. Assessment of the microbial community in a constructed wetland that receives acid coal mine drainage. Microbial Ecol. 51, 83–89.
Ojeda, E., Caldentey, J., Saaltink, M.W., García, J., 2008. Evaluation of relative importance of different microbial reactions on organic matter removal in horizontal subsurface-flow constructed wetlands using a 2D simulation model. Ecol. Eng. 34, 65–75.
Pielou, E.C., 1975. Ecological Diversity. Wiley, New York. Shannon, C.E., Weaver, W., 1963. The Mathematical Theory of
Communication. University of Illinois Press, Urbana, IL. Silva, M.F., Tiago, I., Veríssimo, A., Boaventura, A.R., Nunes, O.C.,
Manaia, C.M., 2006. Antibiotic resistance of enterococci and related bacteria in an urban wastewater treatment plant. FEMS Microbiol. Ecol. 55 (2), 322–329.
Stottmeister, U., Wießner, A., Kuschk, P., Kappelmeyer, U., Kästner, M., Bederski, O., Müller, R.A., Moormann, H., 2003. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 22, 93–117.
Truu, J., Nurk, K., Juhanson, J., Mander, Ü., 2005. Variation of microbiological parameters within planted soil filter for domestic wastewater treatment. Environ. Sci. Health 40, 1191–1200.
Vacca, G., Wand, H., Nikolausz, M., Kuschk, P., Kästner, M., 2005. Effect of plants and filter materials on bacteria removal in pilot-scale constructed wetlands. Water Res. 39, 1361– 1373.
Vymazal, J., 2005. Horizontal sub-surface flow and hybrid constructed wetlands systems for wastewater treatment. Ecol. Eng. 25, 478–490.
Ward, D.M., Weller, R., Bateson, M.M., 1990. 16S rRNA sequences reveal numerous uncultured microorganisms in a natural community. Nature 345, 63–65.