JPediatr(RioJ).2019;95(1):7---17
www.jped.com.br
REVIEW
ARTICLE
Influence
of
AIDS
antiretroviral
therapy
on
the
growth
pattern
夽
,
夽夽
Ana
Paula
Brigatto
Simões
Golucci
a,
Fernando
Augusto
Lima
Marson
a,b,
Mariana
Freitas
Fedato
Valente
c,
Maira
Migliari
Branco
c,
Camila
Carbone
Prado
c,
Roberto
José
Negrão
Nogueira
a,d,∗aUniversidadeEstadualdeCampinas(UNICAMP),FaculdadedeCiênciasMédicas,DepartamentodePediatria,Campinas,SP,Brazil bUniversidadeEstadualdeCampinas(UNICAMP),FaculdadedeCiênciasMédicas,DepartamentodeGenéticaMédica,Campinas,
SP,Brazil
cUniversidadeEstadualdeCampinas(UNICAMP),FaculdadedeCiênciasMédicas,HospitaldeClínicas,Campinas,SP,Brazil dFaculdadeSãoLeopoldoMandic,Campinas,SP,Brazil
Received18October2017;accepted7December2017 Availableonline13April2018
KEYWORDS
Growth; Children; HIV;
Antiretroviraltherapy
Abstract
Objectives: Human immunodeficiency virusinfection can resultin the earlyimpairment of anthropometricindicatorsinchildrenandadolescents.However,combinedantiretroviral ther-apyhasimproved,inadditiontotheimmuneresponseandviralinfection,theweightandheight development ininfected individuals.Therefore,theobjectivewas toevaluatetheeffectof combinedantiretroviralonthegrowthdevelopmentofhumanimmunodeficiencyvirusinfected childrenandadolescents.
Sourceofdata: Asystematicreviewwasperformed.Inthestudy,thePRISMA(Preferred Report-ing Items for Systematic Reviews and Meta-Analyses) strategy was used as the eligibility criterion.TheMEDLINE-PubMedandLILACSdatabasesweresearchedusingthesedescriptors: HIV,children,growth,antiretroviraltherapy.Theobjectivewasdefinedbythepopulation, inter-vention,comparison/control,andoutcome(PICO)technique.Inclusionandexclusioncriteria wereappliedforstudyselection.
Synthesisofdata: Ofthe549studiesindexedinMEDLINE-PubMedandLILACS,73werereadin full,and44wereincludedinthereview(33showedapositiveimpactofcombinedantiretroviral therapyonweight/heightdevelopment,tenonweightgain,andoneonheightgaininchildren andadolescentsinfectedwithhumanimmunodeficiencyvirus).However,theincreaseingrowth wasnotenoughtonormalizetheheightofinfectedchildrenwhencomparedtochildrenofthe sameageandgenderwithouthumanimmunodeficiencyvirusinfection.
夽
Pleasecitethisarticleas:GolucciAP,MarsonFA,ValenteMF,BrancoMM,PradoCC,NogueiraRJ.InfluenceofAIDSantiretroviraltherapy
onthegrowthpattern.JPediatr(RioJ).2019;95:7---17.
夽夽
StudyconductedattheFaculdadedeCiênciasMédicas,UniversidadeEstadualdeCampinas,Campinas,SP,Brazil.
∗Correspondingauthor.
E-mail:nutrigene@uol.com.br(R.J.Nogueira).
https://doi.org/10.1016/j.jped.2018.02.006
8 GolucciAPetal.
Conclusions: Combinedantiretroviraltherapy,whichisknowntoplayaroleintheimprovement ofviralandimmunologicalmarkers, mayinfluenceintheweightandheightdevelopmentin childreninfectedwithhumanimmunodeficiencyvirus.Theearliertheinfectiondiagnosisand, concomitantly,ofmalnutritionandthestartofcombinedantiretroviraltherapy,thelowerthe growthimpairmentwhencomparedtohealthychildren.
©2018PublishedbyElsevierEditoraLtda.onbehalfofSociedadeBrasileiradePediatria.Thisis anopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/ by-nc-nd/4.0/).
PALAVRAS-CHAVE
Crescimento; Crianc¸as; HIV; Terapia antirretroviral
InfluênciadaterapiaantirretroviralparaaAIDSnopadrãodecrescimento
Resumo
Objetivos: A infecc¸ão pelovírus da imunodeficiênciahumana podecomprometer, precoce-mente, os indicadores antropométricos de crianc¸as e adolescentes. No entanto, a terapia antirretroviralcombinadatemmelhorado,alémdarespostaimunológicaedainfecc¸ãoviral, oganhopôndero-estaturaldosinfectados.Dessaforma,nossoobjetivofoiavaliaroefeitoda terapiaantirretroviralcombinadanocrescimento,decrianc¸aseadolescentes,infectadaspelo vírusdaimunodeficiênciahumana.
Fontedosdados: Foirealizadaumarevisãosistemática.Noestudo,adotou-secomocritériode elegibilidadedosartigos,aestratégiaPRISMA(preferredreportingitemsforsystematicreviews andmeta-analyses). Foram consultadas asbases de dadosMEDLINE-PubMed e LILACSpelos descritores:HIV(vírusdaimunodeficiênciahumana),children,growth,antiretroviraltherapy. Oobjetivofoi definidopela estratégia PICO(population,intervention, comparison/control,
outcome).Critériosdeinclusãoeexclusãoforamaplicadosnaselec¸ãodosestudos.
Síntesedosdados: Dos549estudosindexadosnoMEDLINE-PubMedeLILACS,73foramlidosna íntegra---44incluídosnarevisão(33demonstraramimpactopositivodaterapiaantirretroviral combinadanoganhopôndero-estatural,deznoganhodepesoeumnodeestatura,emcrianc¸as eadolescentes,infectadoscomvírusdaimunodeficiênciahumana).Noentanto,oincremento nocrescimentonãofoiosuficienteparanormalizaraestaturadecrianc¸asinfectadas,quando comparadocomcrianc¸asdamesmaidadeesexo,seminfecc¸ãopelovírusdaimunodeficiência humana.
Conclusões: A terapiaantirretroviral combinadaque, conhecidamente,atua namelhorade marcadores virais eimunológicos, podeinfluenciar noganho pôndero-estatural decrianc¸as infectadas com vírus da imunodeficiência humana. Quanto mais precoce o diagnóstico da infecc¸ãoe,concomitante,desnutric¸ãoeiníciodaterapiaantirretroviralcombinada,menores serãoosprejuízosnocrescimento,quandocomparadoàscrianc¸assaudáveis.
©2018PublicadoporElsevierEditoraLtda.emnomedeSociedadeBrasileiradePediatria.Este ´
eumartigoOpenAccesssobumalicenc¸aCCBY-NC-ND(http://creativecommons.org/licenses/ by-nc-nd/4.0/).
Introduction
Overallaspects
Acquired immunodeficiency syndrome (AIDS) is an infec-tiousdiseasecausedbythehumanimmunodeficiencyvirus (HIV), which qualitatively and quantitatively affects the CD4+ T lymphocytes (CD4+ TL).1 Vertical HIV
transmis-sioninchildhoodcanoccur atthreedistinctmoments: (i) intrauterine-transplacental transmission; (ii) during labor and/ordelivery;and(iii)throughbreastfeeding.Mostcases (about 65%) occur during labor and delivery, with the remaining35%occurringthroughintrauterinetransmission, especiallyinthelastweeksofgestationandwhen breast-feeding.Breastfeeding,asatransmissionrisk,isresponsible for7%to22%ofinfectioncases.1
Global data for 2016 estimate that 36.7 (30.8---42.9) million individuals, of whom2.1 million are children, are
infected with HIV.2 In Brazil,from 1980 to 2016, 842,710
cases of HIV infection were identified, of which approxi-mately 24,900 represent children under 14 years old --- a numberthatispossiblyunderestimated.3,4
Individuals infected with HIV have a reduction in the number of CD4+ TL --- a hallmark characteristic of the
immunologicalsystem status. The mechanism that results infailuretoreconstituteCD4+TLhasnotbeenfully
eluci-dated,butitincreasestheriskofopportunisticinfections.In additiontoimmunologicalchanges,therearenutritionaland endocrine-metabolic problems in AIDS, with HIV infection beingassociatedwithgrowthimpairment.5
GrowthdeficiencyinAIDS
AIDSandgrowthpattern 9
normalrangeforage---togrowthinterruptionandwasting syndrome.6Growthretardationisadiseaseseverity
progres-sionindicatorandariskfactorfordeathinindividualswith AIDS.7Moreover,growthdeficiencyismultifactorialinAIDS,
resultingfromtheeffectofantiretroviraltherapy,aswell asotherfactorsdescribedbelow:
(i) malnutrition:mayoccurmainlyduetoinadequate nutri-tional intake secondary to anorexia, oral or upper digestivetractlesions(infectiouscause),intake reduc-tion (psychological or economic cause), or nutrient malabsorption (chronic diarrhea).8 Another important
aspect is the acceleration of protein catabolism and increased metabolic expenditure secondary to uncon-trolledviralreplicationandtheresultinginflammatory response(includingcytokinenetworkdysregulation),as wellasopportunisticinfectionsorneoplasmssecondary to immunosuppression.2 HIV-associated malnutrition
wastermedwastingsyndrome9;
(ii) gastrointestinal disorders (malabsorption): may result fromtheHIVinfection,opportunisticinfections(enteric parasites, such as Cryptosporidium, Mycobacterium avium-intracellulare,andcytomegalovirus,among oth-ers) or neoplasms. Diarrhea, abdominal pain, and dysphagia are commonly observed in HIV-infected patients;however, intestinalmalabsorptionmayoccur in children with or without diarrhea.10 Damage to
mucosal integrity and increased permeability favor intestinal dysfunction, leading to the malabsorption of fats,carbohydrates, and proteins, interfering with weightandheightdevelopment.Intestinaldysfunction mayadverselyinterferewiththeabilitytoabsorboral medications,includingzidovudine(AZT,C10H13N5O4)11;
(iii) stress: in AIDS, the expression of cytokines,including interleukins(1and6),tumornecrosisfactor-alpha,and interferonisaltered,promotingmetabolicstress,which mayleadtolossofappetite,anorexia,andcatabolism9;
(iv) chronic disease: HIV infection leads to a progressive reductionoftheimmunefunction,withareductionin thenumberofCD4+TL,concomitanttochangesinthe
function of these cells and the main immune regula-tory pathways.Therefore,the susceptibilitytorepeat infections and antibody deficiencies alter metabolism through infection and/or intake reduction and, thus, patientnutritionalmonitoringisrequired8;
(v) endocrine disorders: hormonal changes are probably caused by the viral infection itself or are secondary to endocrine glandinvolvement, due to opportunistic infections and/ormedicationsusedtotreatinfections and/ortheircomplications.12
Hence, it is important to assess factors associated with growth deficit, reinforcing the need to broaden theirunderstandinginordertominimizenutritionalstatus losses.11Adequatemonitoringbythemultidisciplinaryteam
contributes tothe early detectionof opportunistic infec-tions, metabolic and hormonal changes, decreased food intake, nutrient malabsorption, and socioeconomic prob-lems, probablyresulting in benefits to weight and height development.12
Antiretroviraltherapy---generalaspects
The1990swereamilestoneinthetreatmentofAIDS,with theuseof moreeffectiveantiretroviralregimens ---highly activeantiretroviraltherapy.Initially,antiretroviraltherapy wasbasedonAZTmonotherapy,butitshowedlowefficacy, requiringaprogressivecombinationofdrugs.Subsequently, dualtherapywasintroduced,withtheuseofnewnucleoside analogreverse-transcriptase inhibitors.Subsequently, pro-tease inhibitors and non-nucleoside reverse-transcriptase inhibitors appeared --- a three-drug regimen that was referredtoascombinedantiretroviraltherapy(cART).cART reducedHIVmortality,mainlyindevelopedcountries.6,13
Since 1996, cART has been used in childhoodto mini-mizediseaseprogression. Itisestimated thatwithout the useofantiretroviraltherapy,one-thirdofHIV-infected chil-drenwoulddiebeforeonemonth,andmorethanhalfbefore twoyearsofage.14
Antiretroviraltherapy---associationwith anthropometricandotherdata
Antiretroviral therapy in childhood and adolescence can result in metabolic disorders, mitochondrial toxicity, and adverse effects on nutritional status, especially in the firstmonthsoftreatment. Patientsmaydevelopnauseaor vomiting,lipodystrophy,andreducedbonemineralization.2
Diagnosisandtreatmentofmalnutritionisnecessary,since micronutrientdeficiency,especiallyofvitaminsA,C,E,D, andminerals (e.g.,seleniumandiron),alterstheimmune system (which is already altered in AIDS) and reduces growth.2
In adults, the duration of cART increases the risk of cardiovasculardisease(stroke,infarction,anddilated car-diomyopathy). Thus, it is suggested that children using antiretroviralsarelikelytohaveahigherriskofmorbidity andprematurecardiovascularmortality.3Moreover,immune
activation, with persistent chronic inflammation in HIV-infectedindividuals,alsoincreasestheriskofcardiovascular diseaseandotherdiseasesofinflammatoryorigin.Increased levelsofinflammatory markers (C-reactive protein, inter-leukin6, D-dimer, fibrinogen) associated with the risk of atherosclerosisandcancer,for instance, arealsofound in AIDS, and remain elevated regardless of the response to cART.15Finally,treatmentadherence,whichincludestheuse
ofcART, constitutesa challenge,mainly in childhoodand adolescence,due tothe chronic drug use, esthetic alter-ationsrelatedtolipodystrophy,andpsychosocialaspects.16
Growthinchildhoodandadolescenceis crucial;hence, inthepresence ofAIDS, thepositive andnegativeimpact of cART should be assessed. Thus, the aim of this sys-tematic review was to evaluate the association between antiretroviraltherapyand growthin HIV-infectedchildren andadolescents.
Method
10 GolucciAPetal.
Titles identified after searching the ME DLINE-PubMed database (n = 98)
Titles identified after searching the LILACS
database (n = 63)
Evaluated titles (n = 161)
Titles after removal of duplicates and read of abstracts (n = 92)
Evaluated full-text articles in details (n = 73)
Articles included in the systematic review (n = 44) Articles excluded by titles
and abstracts (n = 69)
Excluded after full-text read (n = 19)
Iden
tifi
c
a
ti
on
Scr
e
en
ing
El
ig
ib
ili
ty
Inc
lus
io
n
Evaluated full-text articles (n = 92) Articles identified in the databases (n =
549)
Excluded after full-text reading in details (n = 29)
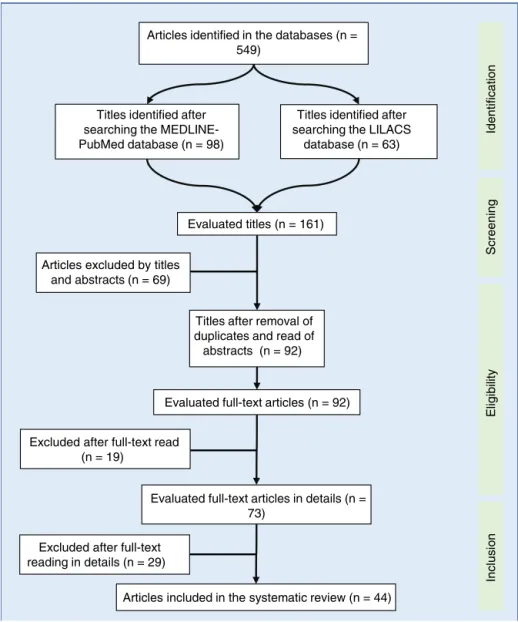
Figure1 Flowdiagramofarticleselectionusedinthesystematicreview.LILACS,LatinAmericanandCaribbeanLiteraturein
HealthSciences;MEDLINE-PubMed,MedicalLiteratureAnalysisandRetrievalSystemOnline---PublicMedline.
outcome(PICO)techniquewasusedtoestablishthestudy objective,asfollows:P,childrenandadolescentsfrom0to 19yearsofage;I,individualsusingcART;C,childgrowth; O,changeingrowthpattern.
In the PRISMA strategy, the inclusion of studies pub-lished from 1996 (year of cART start) to September 2017 wasselected.Originalarticlespublishedinjournalsindexed in the English languagewere included. It wasdecided to includealltypesofstudies,andamongtheselectedarticles, theclassificationwasmadeby degreeof recommendation andlevelofevidence.
The searches were carried out in the Medical Litera-tureAnalysisandRetrievalSystemOnline(MEDLINE-PubMed) and Latin American and Caribbean Health Sciences Liter-ature (LILACS)databases,using the following descriptors: antiretroviraltherapy,children,growth,HIV.Thefollowing filterswereused:(i)presenceofthedescriptorsinthetitle and/orabstract;(ii)publicationsinthe last21years;(iii) being a scientific article. The excluded articles were: (i) thosethathadaduplicatein anotherelectronicdatabase
ofthebibliographicsearch;(ii)thoseaboutthecostofAIDS treatment,withoutaddressinggrowth;(iii)thoseincluding childrenexposedtoHIV,butnotinfected;(iii)thoseon peri-natalgrowth;(iv)thoseaboutcARTuseinpregnantwomen; (v)thoseabouttheexclusiveuseofoneortwoantiretroviral drugs.
AIDSandgrowthpattern 11
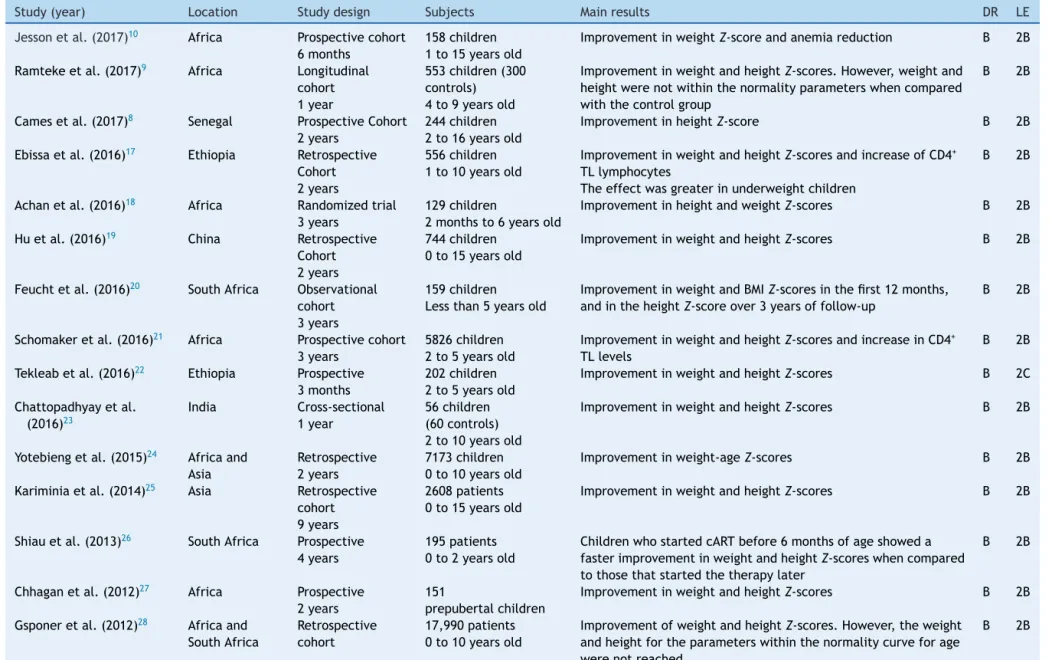
Tables 1 and 2.17---57 Therapeutic interventions performed
withatleastthreeantiretroviraldrugswereincluded, with-outconsideringthecARTtype.
Results
ThedataobtainedaresummarizedinTables1and2.After the summation of the assessed studies, a population of 51,992HIV-infectedindividualswasincluded.Inthestudies, the positive responseto cART, withgrowth improvement, wasobservedin34articles.However,concurrent improve-ment in weight and height development occurred in 33 studies. Moststudies emphasizedthe importance ofearly diagnosisofHIVinfectioninchildrenandadolescents, aim-ingtodelayAIDS progressionandstart theuseofcARTas earlyaspossible.
In theliterature, cARTisassociated withimprovement in anthropometric and survival indexes. In the review, it wasobservedthat42,203/51,992(81.17%)childrenshowed improvementinthegrowthpatternwiththeuseofcART.
Ofthearticlesincludedinthereview,42haveadegree ofrecommendationB([i]onewithlevelofevidence2A;[ii] 35withlevelofevidence2B;[iii]sixwithlevelofevidence 2C)andtwoarticleshave levelof evidence1B(degreeof recommendation A). Article classification for the level of evidencewasperformedaccordingtorecommendationsof theOxfordCentreforEvidence-basedMedicine.
Fig.2brieflyshowsthegrowth-relatedoutcomes regard-ingtheuseofcARTinHIV-infectedchildrenandadolescents.
Discussion
Changesin growtharecommonin HIV-infectedchildren.58
Growth disorders may be associated with: (i) opportunis-tic infections associated with the disease; (ii) absence of immunological and viral control at the follow-up; (iii) metabolicchangesassociatedwithHIV.50However,afterthe
startofcART,changesingrowthpatternhavebeenusedas effectivenessparametersinthetreatment ofchildrenand adolescentswithHIV.
Basedonthisreviewcarriedoutoverthelast21years, itcanbeobservedthatmostofthestudiesshoweda pos-itiveassociation inthe height-ageandweight-ageZ-score withtheuse ofcART(Table1). Inadditiontothe anthro-pometric improvements, the literature demonstrates that cARThasshownadecreaseinmorbidity,mortality,and hos-pitalizationlevelsofpatientsinfectedwithHIV.Treatment withcARTincreasesCD4+TLcounts,reducesviral
replica-tion,andpartiallyrestorestheimmunesystem.Asaresult, itreducestheincidenceofopportunisticinfections.54,58 In
2003,Benjaminetal.reportedthatchildrenusingcARThad feweralterationsintheimmunesystem,abetterresponse tothevirus,andabetterheight-ageZ-scorecurve.4Verweel
etal.,in2002,showeddecreasesof63.69%in hospitaliza-tionsaftertheintroductionofcART.54
The literaturesuggeststhatchildren whowere treated in the past with monotherapy or dual therapy showed a temporaryimprovementinthegrowthrate.However,cART results in a lasting increase in the weight and height of infectedchildren.Thisincrease,however,didnotreachthe values closeto those of the general population. In 2008,
Buonoraetal.demonstratedthat,evenwithcART,infected patients had lower growth parameters than the non-HIV infected population.41 Another study assessed that, after
theintroduction ofcART, weight-ageZ-score wascloseto thatofthepopulationwithoutHIVinfection,regardlessof thetimeoftreatmentstart.Inturn,theheight-ageZ-score remainedlow.26 Similarfindingswereobserved inEurope,
whereaftercomparinggrowthcurvesofinfectedand non-infectedchildrenfor tenyears,itwasconcludedthatHIV infectionresultsinslowergrowthratesandthatthe differ-encesincreasewithage.53
Younger children may show greater weight and height recoveryafterthestart ofcART. Nachmanetal. reported in2005thatchildrenyoungerthantwoyearsofagehada higherheightZ-scoreduringtherapy.49Weightgainwasalso
predominantlyhigherinyoungerpatients.49 In2013,Shiau
etal.evaluatedafour-yearfollow-upof195AIDSpatients andshowedthatpatientswhostartedcARTbefore6months of age showed a faster improvement in weight-age and height-ageZ-scoresthanthosewhostartedtherapylater.26
Therefore,theearlierthecARTisinitiated,thelowerthe growthdeficit.26,37
Anotherimportantfindingindicatedinthestudiesrefers tothenutritionalstatusbeforetreatment, asitcan influ-encethe anthropometricoutcomes afterthe introduction ofcART.BandyopadhyayandBhattacharyyashowedin2008 thatmalnourishedchildren haveworse resultsin the evo-lution of weight-age and height-age Z-scores compared tothose who started therapy under adequate nutritional conditions.44
Inthereview,oneofthemainlimitationswasthe inclu-sionofmanystudiesconductedinAfrica,wherenutritional deficiencymayindirectlyinfluencegrowthcontrolandmay representabiasintheuseofcART.Malnutritionandgrowth retardationoccurearlyinHIVverticaltransmission,andthe negativeimpact of chronic infection and nutritional defi-ciencyongrowthmaybeirreversible.32 However,thelack
ofgrowthpatternimprovementoccurredinstudiescarried outduringashortperiodoftime.Forinstance,inthe2011 studybyDevietal.,whoassessed102patientsonlyinthe firstyearofcARTuse.32
Furthermore,thereviewdidnotcomparetheimpactof differentcombinedantiretroviraltherapieswitheachother. Furthermore,itwasnotpossibletoanalyzegrowthbyage group. None of the studies compared groups undergoing cART,withandwithoutvirologicfailure,toassessgrowth.
Some studies have alsohighlighted the need to assess drug interactions in children and the potential adverse effectsofcARTonmetabolism,butstudiesarestillscarcein thisagegroup.Itisnoteworthythatthepossiblemetabolic effects of cART include insulin resistance, dyslipidemia, hyperlactatemia, and that such changes may lead to an increasedriskforcardiovasculardiseasesanddiabetes.The elevationoftotalcholesterolandlipodystrophyassociated withtheuseofcARTremainsachallengeinthetreatmentof thesepatients.Factorsassociatedwithchildren’sadherence tocARTshowedthatlowadherencewasstronglyassociated withthe caregivers’profile(low levelof schoolingand/or degreeofpoverty).
12
Golucci
AP
et
al.
Table1 Dataobtainedfromthestudiesaccordingtoyearofpublication,studylocation,studydesign,assessedsample,mainresultsobtained,degreeofrecommendation,
andlevelofevidence.
Study(year) Location Studydesign Subjects Mainresults DR LE
Jessonetal.(2017)10 Africa Prospectivecohort
6months
158children 1to15yearsold
ImprovementinweightZ-scoreandanemiareduction B 2B
Ramtekeetal.(2017)9 Africa Longitudinal
cohort 1year
553children(300 controls) 4to9yearsold
ImprovementinweightandheightZ-scores.However,weightand heightwerenotwithinthenormalityparameterswhencompared withthecontrolgroup
B 2B
Camesetal.(2017)8 Senegal ProspectiveCohort
2years
244children 2to16yearsold
ImprovementinheightZ-score B 2B
Ebissaetal.(2016)17 Ethiopia Retrospective
Cohort 2years
556children 1to10yearsold
ImprovementinweightandheightZ-scoresandincreaseofCD4+
TLlymphocytes
Theeffectwasgreaterinunderweightchildren
B 2B
Achanetal.(2016)18 Africa Randomizedtrial
3years
129children
2monthsto6yearsold
ImprovementinheightandweightZ-scores B 2B
Huetal.(2016)19 China Retrospective
Cohort 2years
744children 0to15yearsold
ImprovementinweightandheightZ-scores B 2B
Feuchtetal.(2016)20 SouthAfrica Observational
cohort 3years
159children Lessthan5yearsold
ImprovementinweightandBMIZ-scoresinthefirst12months, andintheheightZ-scoreover3yearsoffollow-up
B 2B
Schomakeretal.(2016)21 Africa Prospectivecohort
3years
5826children 2to5yearsold
ImprovementinweightandheightZ-scoresandincreaseinCD4+
TLlevels
B 2B
Tekleabetal.(2016)22 Ethiopia Prospective
3months
202children 2to5yearsold
ImprovementinweightandheightZ-scores B 2C
Chattopadhyayetal. (2016)23
India Cross-sectional 1year
56children (60controls) 2to10yearsold
ImprovementinweightandheightZ-scores B 2B
Yotebiengetal.(2015)24 Africaand
Asia
Retrospective 2years
7173children 0to10yearsold
Improvementinweight-ageZ-scores B 2B
Kariminiaetal.(2014)25 Asia Retrospective
cohort 9years
2608patients 0to15yearsold
ImprovementinweightandheightZ-scores B 2B
Shiauetal.(2013)26 SouthAfrica Prospective
4years
195patients 0to2yearsold
ChildrenwhostartedcARTbefore6monthsofageshoweda fasterimprovementinweightandheightZ-scoreswhencompared tothosethatstartedthetherapylater
B 2B
Chhaganetal.(2012)27 Africa Prospective
2years
151
prepubertalchildren
ImprovementinweightandheightZ-scores B 2B
Gsponeretal.(2012)28 Africaand
SouthAfrica
Retrospective cohort
17,990patients 0to10yearsold
ImprovementofweightandheightZ-scores.However,theweight andheightfortheparameterswithinthenormalitycurveforage werenotreached
AIDS
and
growth
pattern
13
Table1 (Continued)
Study(year) Location Studydesign Subjects Mainresults DR LE
Sutcliffeetal.(2011)29 Zambia Prospectivecohort
2years
193patients 0to16yearsold
ImprovementinweightandheightZ-scores.Theeffectwas higherinunderweightchildren,andthelowerheightZ-score occurredinchildrenolderthan5years.After2yearsof treatment,alargeproportionofthechildrenremainedbelow weightandheightforage
B 2B
McGrathaetal.(2011)30 Kenya Prospectivecohort
4years
173patients 0to10yearsold
ImprovementinweightforageZ-score,whichwashigherin childrenunder6years.Therewasnoimprovementinheight Z-score
B 2B
Dinizetal.(2011)31 Brazil Retrospective
3years
196patients 0to12yearsold
BetterimmuneresponseandweightandheightZ-scoreswere observedattheendofthefollow-up
B 2B
Devietal.(2011)32 India Prospective
1year
102patients
Meanage=74months
InthefirstyearofcART,therewasimprovementintheimmune responseandweightZ-score,butnotheightZ-score
B 2B
Naidooetal.(2010)33 SouthAfrica Retrospective
8months
120children ImprovementinweightandheightZ-scores.Therewasnoeffect ontheheightofchildrenwithseveremalnutrition.Therewasan increaseinCD4+TLlymphocytesandareductioninviralload
B 2C
Chantryetal.(2010)34 USA Prospective/cohort
---48weeks
97patients--- 1month to13yearsold (incomplete)
IndividualsstartingorswitchingtocARTwerecomparedtothe controlgroup(HIV-exposedbutuninfectedchildrenand
demographicandanthropometricdatafromanAmericanstudy). TherewasimprovementinweightandheightZ-scores,butno changeinBMIZ-score.However,weightandheightwerelower thanthoseofthehealthypopulation
B 2B
Yotebiengetal.(2010)35 SouthAfrica Prospectivecohort
---4years
1394patients 0to15yearsold
ImprovementinweightZ-score,butnochangeinheightZ-score. Additionally,therewasanimprovementinCD4+TLcount
A 1B
Weigeletal.(2010)36 Malawi,Africa Prospective---24
months
307patientsyounger than15yearsold
Therewasanassociationbetweenimmunologicalresponseand weightandheightZ-scoreimprovementattheendofthe follow-up
B 2C
Musokeetal.(2010)37 Uganda,Africa Prospective---48
months
130patients 6monthsto12years old
IncreasedweightandheightZ-scores,withtreatment,especially inindividualswhostartedthetherapyatayoungerage
B 2B
Aurpibuletal.(2009)38 Thailand Cohort--- 5years 225patients
0to15yearsold
ComparedwithanthropometricreferencedataforThaichildren, withnocontrolgroup.Therewasapositiveeffectonweightand heightZ-scores.Atthesametime,therewasanincreaseinCD4+
TLcount
B 2B
Daviesetal.(2009)39 SouthAfrica Prospectivecohort
--- 9years
6078patients 0to16yearsold
ImprovementinweightandheightZ-scores B 2B
Kabueetal.(2008)40 Uganda Retrospective--- 3
years
749patients--- 0to19 yearsold
Improvementinweight-ageandheight-ageZ-scores.Theviralor immunologicalresponsewasnotassessed.Moreover,thestudy doesnotspecifytheuseofHAART
B 2B
Buonoraetal.(2008)41 Brazil Retrospective--- 97
months
108patients 10to20yearsold
EvenwithHAART,height-ageandweight-ageZ-scoreswerelower thanthoseofhealthyindividuals
14
Golucci
AP
et
al.
Table1 (Continued)
Study(year) Location Studydesign Subjects Mainresults DR LE
Kekitiinwaetal.(2008)42 UnitedKingdom Prospective---12
months
1458children Meanage5and7.6 yearsold
Improvementinweight-ageandheight-ageZ-scores,especiallyin youngerchildren
B 2A
Jaspanetal.(2008)43 SouthAfrica Retrospective
cohort---4years
391patients 9to59months
ImprovementinweightZ-score,butheightwasnotevaluated. Additionally,increasedCD4+TLlevelswereobserved
B 2B
Bandyopadhyayand Bhattacharyya(2008)44
India Prospective---18 months
123
2monthsto8yearsold
MalnutritionbeforethestartofcARTmightimpairtheoutcomein nutritionalparameters;therewasanimprovementonlyinthe weightZ-score
B 2B
Zhangetal.(2007)45 China Prospective---12
months
83children---7to13 yearsold
ImprovementinweightandheightZ-scoresandthe immunologicalresponseofpatients
B 2B
Wamalwaetal.(2007)46 Kenya,Africa Prospective---9
months
67children
18monthsto12years old
Improvementinweight-ageandheight-ageZ-scores.Additionally, theclinicalresponseandviralcontrolshowedbetterresultswith theuseofHAART
B 2B
Songetal.(2007)47 Kenya,Africa Retrospective---1
year
29children---Mean age=8.5yearsold
Improvementinweight-ageZ-score B 2B
Guillenetal.(2007)48 Spain Retrospective---5
years
264patients 0to18yearsold
ImprovementinweightandheightZ-scoresandviralcontrolin thefirst5yearsofcART
B 2B
Nachmanetal.(2005)49 USA Prospective---2
years
192children 4monthsto17years old
HAARTallowedanincreaseinweight-ageZ-scoreatweek48and height-ageatweek96.Youngerchildrengainedweightinless time
B 2B
Lodhaetal.(2005)50 India Prospective---6
months
48children---mean age=5.6yearsold
Improvementinweight-ageZ-scoreduringthe6-monthperiod B 2C
Ghaffarietal.(2004)51 USA Prospective---2
years
40children 2to18yearsold
BetterimmuneresponseandhigherweightandheightZ-scoresat theendoffollow-up
B 2B
Klineetal.(2004)52 Romania Prospective---67
weeks
452children 0to18yearsold
ImprovementinweightandheightZ-scoresandimmunological response
B 2C
Newelletal.(2003)53 Europa(11
countries)
Prospective---10 years
1587patients 0to10yearsold
ImprovementinweightandheightZ-scoreswithcART,regardless ofclinicalclassification
B 2B
Verwelletal.(2002)54 Netherlands Prospectivestudy 24children---4months
to16yearsold(96 weeksoftreatment)
Nocontrolgroupwasincluded,butratherreferencecurvesfrom theNetherlands.ImprovementinweightandheightZ-scoresin patientswithreducedviralloadandBMIZ-scoreinpatientswith advancedstageofdiseaseandmalnutrition
B 2C
Nachmanetal.(2002)55 USA Prospective---120
weeks
197patients---2to17 yearsold
Itwasfoundthatthegrowthdeficitincreasedovertime,evenin theindividualswithbetterviralloadcontrol
A 1B
Milleretal.(2001)56 USA Cohort---3years 67patients---0to3.8
yearsold
Controlgroupwasnotincluded.Improvementinweightand weight/heightZ-scores,aswellasreductioninviralload,but therewasnoeffectonheight
B 2B
Buchaczetal.(2001)57 USA Prospective---3
years
906patients---3 monthsto18yearsold
ImprovementinweightandheightZ-scoreswithcARTcontaining proteaseinhibitor,regardlessofclinicalclassification
B 2B
USA,UnitedStatesofAmerica;DR,degreeofrecommendation;LE,levelofevidence;BMI,bodymassindex;CD4+TL,CD4+Tlymphocytes;HAART,highly-activeantiretroviraltherapy;
AIDSandgrowthpattern 15
Table2 Outcomerelatedtoweight/heightdevelopmentinHIV-infectedpatients(totalnumberofinfectedindividuals=51,992
childrenandadolescents),submittedtotherapywithcART.
Outcome Numberofstudies Positive---n(%)
Heightimprovement 1/44(2.27%) 244/51,992(0.47%)a
Weightimprovement 10/44(22.73%) 9789/51,992(18.83%)b
Weightandheightimprovement 33/44(75%) 41,959/51,992(80.7%)c
cART,combinedantiretroviraltherapy;HIV,humanimmunodeficiencyvirus.
a Camesetal.,2017.8
b Jessonetal.,201710;Yotebiengetal.,201524;Macgrathaetal.,201130;Devietal.,201132;Yotebiengetal.,201035;Jaspanetal., 200843;BandyopadhyayandBhattacharyya,200844;Songetal.,200747;Lodhaetal.,200550;Nachmanetal.,2002.55
c Ramtekeetal.,20179;Ebissaetal.,201617;Achanetal.,201618;Huetal.,201619;Feuchtetal.,201620;Schomakeretal.,201621; Tekleabetal.,201622;Chattopadhyayetal.,201623;Kariminiaetal.,201425;Shiauetal.,201326;Chhaganetal.,201227;Gsponer etal.,201228;Sutcliffeetal.,201629;Dinizetal.,201131;Naidooetal.,201033;Chantryetal.,201034;Weigeletal.,201036;Musoke etal.,201037;Aurpibuletal.,200938;Daviesetal.,200939;Kabueetal.,200840;Buonoraetal.,200841;Kekitiinwaetal.,200842; Zhangetal.,200745;Wamalwaetal.,200746;Guillenetal.,200748;Nachmanetal.,200549;Ghaffarietal.,200451;Klineetal.,200452; Newelletal.,200353;Verweeletal.,200354;Milleretal.,200156;Buchaczetal.,2001.57
Figure2 Outcome associatedwiththegrowthrelated totheuseofcARTinchildrenandadolescentsinfected byHIV. cART,
combinedantiretroviraltherapy;HIV,humanimmunodeficiencyvirus;CD4+TL,CD4+Tlymphocytes.
inHIV-infectedchildren.cARTisknowntoplayaroleinthe improvementofviralandimmunologicalmarkersandmay influencethegrowthofHIV-infectedchildren.However,the increaseinanthropometricmarkers(weightandheight)did not normalizethe growth when compared to the healthy population. The earlier the diagnosis of the HIV infection and,concurrently,ofmalnutritionandtheinitiationofcART, thelowerthegrowthdeficitwhencomparedtohealthy chil-dren.PatientswithAIDSandwithoutprevioustreatmentfor antiretroviraltherapyshowbetteranthropometricresponse tocARTthanpreviouslytreatedpatients.However, unfavor-able socioeconomic and intrafamilial conditions influence treatment adherence, representing a limiting factor for
moresignificantandlong-lastingresultsoftheuseofcART inHIVinfectiontreatment.Finally,mainlyincountrieswith low economic resources to quantify the viral load, the improvementofanthropometricdataduringtheuseofcART asaparameteroftherapeuticefficacyismoreevident.
Funding
16 GolucciAPetal.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.LimaVD,LourencoL,YipB,HoggRS,PhillipsP,MontanerJS. TrendsinAIDSincidenceand AIDS-relatedmortalityinBritish Columbiabetween1981and2013.LancetHIV.2015;2:e92---7. 2.JessonJ,LeroyV.Challengesofmalnutritioncareamong
HIV-infectedchildrenonantiretroviraltreatmentinAfrica.MédMal Infect.2015;45:149---56.
3.GiménezRS,MorilloVJ.SocialepidemiologyinHIV/AIDS:what elseshouldweconsidertopreventtheHIV/AIDSprogression? SocWorkPublicHealth.2017;14:1---11.
4.Boletim Epidemiológico HIV/Aids, do Departamento de
Vig-ilância, Prevenc¸ão e Controle das Infecc¸ões Sexualmente
Transmissíveis,doHIV/AidsedasHepatitesVirais(DIAHV),da
SecretariadeVigilânciaemSaúde(SVS),doMinistériodaSaúde
(MS).2016;AnoV,Número1.
5.Benjamin DK Jr, Miller WC, Benjamin DK, Ryder RW,Weber DJ,WalterE,etal.Acomparisonofheightandweight veloc-ityasapartofthecompositeendpointinpediatricHIV.AIDS. 2003;17:2331---6.
6.Leonard EG, McComsey GA. Metabolic complications of antiretroviral therapy in children. Pediatr Infect Dis J. 2003;22:77---84.
7.SilvaRA,DuarteFH,NelsonAR,HolandaJR.AIDSepidemicin Brazil:analysisofcurrentprofile.JNursUFPE.2013;7:6039---46. 8.Jesson J, Coulibaly A, Sylla M, N’Diaye C, Dicko F, Masson D, et al. Evaluation of a nutritional support intervention in malnourishedHIV-infectedchildreninBamako,Mali.JAcquir ImmuneDeficSyndr.2017;76:149---57.
9.CamesC,PascalL,DiackA,MbodjH,OuattaraB,DiagneNR, etal.RiskfactorsforgrowthretardationinHIV-infected Sene-galesechildrenonantiretroviraltreatment.PediatrInfectDis J.2017;36:87---92.
10.RamtekeSM,ShiauS,FocaM,StrehlauR,PinillosF,PatelF,etal. Patterns ofgrowth,bodycomposition, andlipidprofilesina SouthAfricancohortofhumanimmunodeficiencyvirus-infected and uninfectedchildren: a cross-sectional study. J Pediatric InfectDisSoc.2018;7:143---50.
11.PodaGG,HsuCY,ChaoJC.MalnutritionisassociatedwithHIV infectioninchildrenlessthan5yearsinBobo-DioulassoCity, BurkinaFaso.Medicine.2017;96:e7019.
12.BeraldoRA,SantosAP,GuimarãesMP,VassimonHS,PaulaFJ, MachadoDR,etal.Bodyfatredistributionandchangesinlipid andglucosemetabolisminpeoplelivingwithHIV/AIDS.RevBras Epidemiol.2017;20:526---36.
13.Leonard EG, McComsey GA. Antiretroviral therapy in HIV-infected children: the metabolic cost of improved survival. InfectDisClinNorthAm.2005;19:713---29.
14.Eisenhut M. Anupdate on HIV in children. J Paediatr Child Health.2012;23:109---14.
15.TienPC, ChoiAI,ZolopaAR, BensonC,Tracy R,Scherzer R, etal.InflammationandmortalityinHIV-infectedadults: anal-ysisoftheFRAMStudyCohort.JAcquirImmuneDeficSyndr. 2010;55:316---22.
16.Rosenvinge MM, Doerholt K. HIV in mothers and children. Medicine(Baltimore).2013;41:461---5.
17.Ebissa G, Deyessa N, Biadgilign S. Impact of highly active antiretroviraltherapyonnutritionalandimmunologicstatusin HIV-infected childreninthelow-income countryofEthiopia. Nutrition.2016;32:667---73.
18.AchanJ,KakuruA,IkileziG,MwangwaF,PlentyA,Charlebois E, et al. Growth recovery among HIV-infected children
ran-domized to lopinavir/ritonavir or NNRTI-based antiretroviral therapy.PediatrInfectDisJ.2016;35:1329---32.
19.HuR,MuW,SunX,WuH,PangL,WangL,etal.Growthof HIV-infectedchildrenintheearlystageofantiretroviraltreatment: aretrospectivecohortstudyinChina.AIDSPatientCareSTDS. 2016;30:365---70.
20.FeuchtU,BruwaeneLV,BeckerPJ,KrugerM.Growthin HIV-infectedchildrenonlong-termantirretroviraltherapy.TropMed IntHealth.2016;21:619---29.
21.Schomaker M, Davies MA, Malateste K, Renner L, Sawry S, N’Gbeche S, et al. Growth and mortality outcomes for dif-ferent antiretroviral therapy initiation criteria in children aged 1---5 years: a causal modelling analysis. Epidemiology. 2016;27:237---46.
22.TekleabAM,TadesseBT,GirefAZ,ShimelisD,GebreM. Anthro-pometricimprovementamongHIVinfectedpre-schoolchildren followinginitiationoffirstlineanti-retroviraltherapy: implica-tionsforfollowup.PLOSONE.2016;11:1---14.
23.ChattopadhyayA,BhattacharyyaS,DharS.Agrowthand nutri-tional study of HIV seropositive children from West Bengal under direct care of medical caregivers. J Clin Diagn Res. 2016;10:14---6.
24.YotebiengM,MeyersT,BehetsF,DaviesMA,KeiserO,Ngonyani KZ,etal.Age-specific andsex-specificweight gainnorms to monitorantiretroviraltherapy inchildreninlow-incomeand middle-incomecountries.AIDS.2015;29:101---9.
25.Kariminia A, Durier N, Jourdain G, Saghayam S, Do CV, NguyenLV,etal. Weightaspredictorsofclinicalprogression andtreatmentfailure:resultsfrom theTREATAsiaPediatric HIV Observational Database. J Acquir Immune Defic Syndr. 2014;67:71---6.
26.ShiauS,ArpadiS,StrehlauR,MartensL,PatelF,CoovadiaA, etal.Initiationofantiretroviraltherapybefore6monthsofage isassociatedwithfastergrowthrecoveryinSouthAfrican chil-drenperinatallyinfectedwithhumanimmunodeficiencyvirus. JPediatr.2013;162:1138---45.
27.ChhaganMK,KauchaliS,VandenBroeck J.Clinicaland con-textualdeterminantsofanthropometricfailureatbaselineand longitudinalimprovementsafterstartingantiretroviral treat-ment among South African children. Trop Med Int Health. 2012;17:1092---9.
28.GsponerT, WeigelR,DaviesMA,BoltonC,MoultrieH,VazP, etal. Variability ofgrowthin childrenstartingantiretroviral treatmentinsouthernAfrica.Pediatrics.2012;130:e966---77. 29.Sutcliffe CG, van Dijk JH, Munsanje B, Hamangaba F,
Siny-wimaanzi P, Thuma PE, et al. Weight and height Z-scores improve after initiating ART among HIV-infected children in ruralZambia:acohortstudy.BMCInfectDis.2011;11:54. 30.McGrathaCJ,ChungMH,RichardsonBA,Benki-NugentS,Warui
D,John-StewartGC. Youngerageat ART initiationis associ-ated with morerapid growth reconstitution.AIDS. 2011;25: 345---55.
31.DinizLM,MaiaMM,CamargosLS,AmaralLC,GoulartEM,Pinto JA.ImpactofARTongrowthandhospitalizationratesamong HIV-infectedchildren.JPediatr(RioJ).2011;87:131---7. 32.Devi NP, Chandrasekaran K, Bhavani PK, Thiruvalluvan C,
Swaminathan S. Persistence of stunting after highly active antiretroviraltherapyinHIV-infectedchildreninSouthIndia. IndianPediatr.2011;48:333---4.
33.NaidooR,RennertW,LungA,NaidooK,McKerrowN.The influ-enceofnutritionalstatusontheresponsetoARTinHIV-infected childreninSouthAfrica.PediatrInfectDisJ.2010;29:511---3. 34.ChantryCJ,CerviaJS,HughesMD,AlveroC,HodgeJ,BorumP,
etal.PredictorsofgrowthandbodycompositioninHIV-infected childrenbeginningorchangingantiretroviraltherapy.HIVMed. 2010;11:573---83.
AIDSandgrowthpattern 17
treatmentresponses in HIV-infected South African children. AIDS.2010;24:139---46.
36.WeigelR,PhiriS,ChiputulaF,GumuliraJ,BrinkhofM,Gsponer T,et al.Growth responsetoantiretroviraltreatmentin HIV-infectedchildren:acohortstudyfromLilongwe,Malawi.Trop MedIntHealth.2010;15:934---44.
37.MusokePM, Mudiope P, Barlow-Mosha LN, Ajuna P, Bagenda D,Mubiru MM,etal. Growth,immuneand viralresponsesin HIVinfectedAfricanchildrenreceivinghighlyactive antiretro-viraltherapy:aprospectivecohortstudy.BMCPediatr.2010; 10:56.
38.Aurpibul L, Puthanakit T, Taecharoenkul S, Sirisanthana T, SirisanthanaV.ReversalofgrowthfailureinHIV-infectedThai children treated with non-nucleoside reverse transcriptase inhibitor-basedantiretroviraltherapy.AIDSPatientCareSTDS. 2009;23:1067---71.
39.DaviesM-A,KeiserO,TechnauK,EleyB,RabieH,vanCutsem G,etal.,OutcomesoftheSouthAfricanNationalantiretroviral treatmentprogrammeforchildren:theIeDEASouthernAfrica collaboration.SAfrMedJ.2009;99:730---7.
40.KabueMM,KekitiinwaA,MagandaA,RisserJM,ChanW,Kline MW.Growth inHIV-infected childrenreceiving antiretroviral therapyatapediatricinfectiousdiseasesclinicinUganda.AIDS PatientCareSTDS.2008;22:245---51.
41.BuonoraS,Nogueira S, PoneMV, AloeM,OliveiraRH, Hofer C.GrowthparametersinHIV-vertically-infectedadolescentson antiretroviraltherapyinRiodeJaneiro,Brazil.AnnTrop Paedi-atr.2008;28:59---64.
42.KekitiinwaA,LeeKJ,WalkerAS,MagandaA,DoerholtK,Kitaka SB,etal.Differencesinfactorsassociatedwithinitialgrowth, CD4,and viralloadresponsestoARTinHIV-infectedchildren inKampala,Uganda,andtheUnitedKingdom/Ireland.JAcquir ImmuneDeficSyndr.2008;49:384---92.
43.Jaspan HB, Berrisford AE, Boulle AM. Two-year outcomes ofchildrenon non-nucleosidereversetranscriptase inhibitor and proteaseinhibitorregimens ina South Africanpediatric antiretroviralprogram.PediatrInfectDisJ.2008;27:993---8. 44.Bandyopadhyay A, Bhattacharyya S. Effect of pre-existing
malnutrition on growth parameters in HIV-infected chil-dren commencing antiretroviral therapy. Ann Trop Paediatr. 2008;28:279---85.
45.ZhangF,HabererJE,ZhaoY,DouZ,ZhaoH,HeY,etal. Chi-nesepediatrichighlyactiveantiretroviraltherapyobservational cohort:a1-yearanalysisofclinical,immunologic,andvirologic outcomes.JAcquirImmuneDeficSyndr.2007;46:594---8. 46.WamalwaDC,FarquharC,ObimboEM,SeligS,Mbori-NgachaDA,
RichardsonBA,etal.Earlyresponsetohighlyactive antiretrovi-raltherapyinHIV-1-infectedKenyanchildren.JAcquirImmune DeficSyndr.2007;45:311---7.
47.SongR,JelagatJ,DzomboD,MwalimuM,MandaliyaK,Shikely K,etal.EfficacyofhighlyactiveantiretroviraltherapyinHIV-1 infectedchildreninKenya.Pediatrics.2007;120:e856---61. 48.GuillenS,RamosJT,ResinoR,BellonJM,MunozMA.Impacton
weightandheightwiththeuseofARTinHIV-infectedchildren. PediatrInfectDisJ.2007;26:334---8.
49.Nachman SA, Lindsey JC, Moye J, Stanley KE, Johnson GM, KrogstadPA,etal.Growthofhumanimmunodeficiency virus-infectedchildrenreceivinghighlyactiveantiretroviraltherapy. PediatrInfectDisJ.2005;24:352---7.
50.LodhaR,UpadhyayA,KabraSK.AntiretroviraltherapyinHIV-1 infectedchildren.IndianPediatr.2005;42:789---96.
51.GhaffariG,PassalacquaDJ,CaicedoJL,GoodenowMM, Sleas-man JW. Two-year clinicaland immune outcomes in human immunodeficiencyvirus-infectedchildrenwhoreconstituteCD4 Tcells withoutcontrolofviralreplicationaftercombination antiretroviraltherapy.Pediatrics.2004;114:e604---11.
52.Kline MW, Matusa RF, Copaciu L, Calles NR, Kline NE, Schwarzwald HL. Comprehensivepediatric human immunod-eficiency virus care and treatment in Constanta, Romania: implementation of a program of highly active antiretrovi-ral therapy in a resource-poor setting. Pediatr Infect Dis J. 2004;23:695---700.
53.NewellML,BorjaMC,PeckhamC.Height,weight,andgrowthin childrenborntomotherswithHIV-1infectioninEurope. Pedi-atrics.2003;111:e52---60.
54.VerweelG,vanRossumAM,HartwigNG,WolfsTF,Scherpbier HJ,deGrootR.Treatmentwithhighlyactiveantiretroviral ther-apyinhumanimmunodeficiencyvirustype1-infectedchildren is associated with a sustained effecton growth.Pediatrics. 2002;109:E25.
55.NachmanSA,LindseyJC,PeltonS,MofensonL,McIntoshK, Wiz-niaA,etal.Growthinhumanimmunodeficiencyvirus-infected childrenreceiving ritonavir-containing antiretroviraltherapy. ArchPediatrAdolescMed.2002;156:497---503.
56.MillerTL,MawnBE,OravEJ,WilkD,WeinbergGA,Nicchitta J, etal. Theeffectof proteaseinhibitortherapy ongrowth and bodycompositioninhumanimmunodeficiencyvirustype 1-infectedchildren.Pediatrics.2001;107:e77.
57.BuchaczK,CerviaJS,LindseyJC,HughesMD,SeageGR3rd, Dankner WM, et al. Impact of protease inhibitor-containing combination antiretroviral therapies on height and weight growthinHIV-infectedchildren.Pediatrics.2001;108:e72. 58.van Rossum AM, Niesters HG, Geelen SP, Scherpbier HJ,