63 63 63 63 63 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 92(1): 63-68, Jan./Feb. 1997
Characterization of
Endotrypanum
Parasites Using Specific
Monoclonal Antibodies
Antonia Maria Ramos Franco/
+, Gerzia MC Machado, Roberto D Naiff*, Celia
FS Moreira, Diane McMahon-Pratt**, Gabriel Grimaldi Jr
Departamento de Imunologia, Instituto Oswaldo Cruz, Av. Brasil 4365, 21045-900 Rio de Janeiro, RJ, Brasil *Instituto Nacional de Pesquisas da Amazônia, Caixa Postal 478, 69083-000 Manaus, AM, Brasil **DEPH,
Yale University School of Medicine, PO Box 3333, New Haven, CT 06510, USA
A large number of Endotrypanum stocks (representing an heterogeneous population of strains) have been screened against a panel of monoclonal antibodies (MAbs) derived for selected species of Endotrypanum or Leishmania, to see whether this approach could be used to group/differentiate further among these parasites. Using different immunological assay systems, MAbs considered specific for the genus Endotrypanum (E-24, CXXX-3G5-F12) or strain M6159 of E. schaudinni (E-2, CXIV-3C7-F5) reacted variably according to the test used but in the ELISA or immunofluorescence assay both reacted with all the strains tested. Analyses using these MAbs showed antigenic diversity occurring among the Endotrypanum strains, but no qualitative or quantitative reactivity pattern could be consistently related to parasite origin (i.e., host species involved) or geographic area of isolation. Western blot analyses of the parasites showed that these MAbs recognized multiple components. Differences existed either in the epitope density or molecular forms associated with the antigenic determinants and therefore allowed the assignment of the strains to specific antigenic groups. Using immunofluorescence or ELISA assay, clone E-24 produced reaction with L. equatorensis (which is a parasite of sloth and rodent), but not with other trypanosomatids examined. Interestingly, the latter parasite and the Endotrypanum strains cross-reacted with a number of MAbs that were produced against members of the L. major-L. tropica complex.
Key words: Endotrypanum - Kinetoplastida - Trypanosomatidae - mammalian reservoirs - molecular characterization - monoclonal antibodies
Endotrypanum parasites are unique among the Kinetoplastida in that they infect erythrocytes of their mammalian host, the two and three-toed sloths (genera Choloepus and Bradypus, respectively). Inside the erythrocyte the Endotrypanum assumes an epimastigote or trypomastigote form, while in the sandfly and during in vitro culture the parasite assumes promastigote morphology (Shaw 1992). Similarities at the morphological, molecular and biological levels exist between many trypanoso-matids isolated from sylvatic insect and/or verte-brate reservoir hosts that make the identification of the medical importance parasites demanding (Shaw 1985). Moreover, the neotropical tree sloths, which are reservoir of at least six Leishmania
spe-This work was supported in part by the Brazilian Na-tional Council of Scientific Development and Technol-ogy (CNPq) and CAPES/BR.
+CAPES fellowship and corresponding author. Fax:
+55-21-280.1589 Received 9 April 1996 Accepted 21 October 1996
cies pathogenic for human (review in Grimaldi & Tesh 1993), are hosts of Endotrypanum (reviewed in Shaw 1992) and several Trypanosoma species [T. cruzi, T. rangeli, T. leeuwenhoeki, T. preguici and T. legeri] (Montero-Gei 1956, Shaw 1969, Zeledón et al. 1975, Herrer & Christensen 1980, Miles et al. 1983).
64 64 64 64
64 Characterization of Endotrypanum Parasites AMR Franco et al.
MATERIALS AND METHODS
The parasites - The origin (i.e., host species in-volved or geographical area of isolation) of the 22 Endotrypanum stocks examined in this study, which included reference strains (Medina-Acosta et al. 1994) are given in Table I. The stocks were stored as stabilates at -190oC in liquid nitrogen refrigera-tors, and the freezing medium used was Schneider’s medium containing 10% fetal bovine serum (FBS) and 8% dimethylsulfoxide (Grimaldi et al. 1992).
Preparation of samples - Procedures for grow-ing Endotrypanum promastigotes in vitro and for preparation of extracts for serodeme analysis with monoclonal antibodies have been reported previ-ously (McMahon-Pratt et al. 1986). Briefly, promastigotes in the log phase of growth in Schneider’s medium were harvested by centrifuga-tion (1,500 x g for 10 min at 4oC) and washed twice in phosphate-buffered saline (PBS), pH 7.3. The fi-nal pellet was used for preparation of samples. An-tigens of each of the strains tested were prepared as promastigote homogenates containing protease in-hibitors (Leon et al. 1992). Prior to analysis, the
samples were briefly sonicated using a bath sonica-tor (RAI Research, Model 250 Ultrasonic cleaner) to homogenize the antigens, and then centrifuged (2,000 x g for 10 min, at 4oC). Protein concentra-tion was measured by the protein assay method of Bradford (1976), and all samples were resuspended to the same concentration before analysis.
Monoclonal antibodiesand immunological as-say systems - The MAbs used in this study were produced against membrane components of Endotrypanum (Lopes & McMahon-Pratt 1989) or Leishmania species(McMahon-Pratt et al. 1986). The following group- or species-specific clones were employed for typing parasites: E-24,
CXXX-3G5-F12, Endotrypanum sp.; E-2, CXIV-3C7-F5, E. schaudinni; T-11, LXIX 2D8-D7,L. (L.) major; T-12, IS1 2G7-F1, L. (L.) tropica; D-2, LXXVIII
2E5-A8, L. donovani complex; M-7, IXVIII 1D7-B8,
L. mexicana complex; B-20, 2 2F7-D3, Leishma-nia (V.) sp.; B-5, VII-2C5-C5, L. braziliensis com-plex; B-19, XLIV-5A2-B9, L. (V.) guyanensis; CR,
G2G4, cross-reactive to all kinetoplastids (Lopes & McMahon-Pratt 1989, WHO 1993, Grimaldi &
TABLE I
Origin and identification of Endotrypanum reference strains and 17 isolates from the Amazon Region, Brazil, which were characterized by monoclonal antibodies in this study
Stock code Designation Species Geographic origin Zymodeme Reference strains
E11 MCHO/CR/62/A-9b E. monterogeii Costa Rica EZ01
E12 MCHO/BR/88/M11602c E. schaudinni Pará EZ12
E14 MCHO/BR/80/M6159c E. schaudinni Pará EZ06
E15 MCHO/BR/79/M5725c E. schaudinni Pará EZ05
E09 MBRA/PA/00/415P01a Endotrypanum sp. Panama EZ01
Amazonian isolates
E01 MCHO/BR/89/RO9627d Endotrypanum sp. Rondônia EZ01 E02 MCHO/BR/89/RO1635d Endotrypanum sp. Rondônia EZ01 E03 MCHO/BR/89/RO1634d Endotrypanum sp. Rondônia EZ01 E04 MCHO/BR/89/RO1140d Endotrypanum sp. Rondônia EZ01 E05 MCHO/BR/89/RO1602d Endotrypanum sp. Rondônia EZ01 E06 MCHO/BR/89/RO1471d Endotrypanum sp. Rondônia EZ01 E07 MCHO/BR/89/RO1583d Endotrypanum sp. Rondônia EZ01 E16 MCHO/BR/85/IM2260c Endotrypanum sp. Pará EZ07 E17 MCHO/BR/85/IM2382d Endotrypanum sp. Rondônia EZ09 E18 MCHO/BR/85/IM2384d Endotrypanum sp. Rondônia EZ01 E19 MCHO/BR/85/IM2389d Endotrypanum sp. Rondônia EZ10 E21 MCHO/BR/89/IM3605d Endotrypanum sp. Rondônia EZ04 E22 MCHO/BR/89/IM3606d Endotrypanum sp. Rondônia EZ03 E31 MCHO/BR/85/IM2259c Endotrypanum sp. Pará EZ02 E32 MCHO/BR/85/IM2380d Endotrypanum sp. Rondônia EZ08 E33 MCHO/BR/85/IM2393d Endotrypanum sp. Rondônia EZ11 E36 MCHO/BR/89/IM3603d Endotrypanum sp. Rondônia EZ03 Designations: Host [M= Mammalia: BRA= a: Bradypus infuscatus;CHO= Choloepus sp. (b: C. hoffmanni; c: C. didactylus; d: C. juruanus)] /country of origin/year of isolation/original code. Zymodeme (= strain variant); EZ =
65 65 65 65 65 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 92(1), Jan./Feb. 1997
McMahon-Pratt 1996).
Typing of the Endotrypanum with MAbs was performed with an indirect immunofluorescence assay (IFAT), using whole fixed promastigotes as antigen (McMahon-Pratt et al. 1986) or by distinct immune binding assay systems such as indirect radioimmune assay (RIA) (Grimaldi et al. 1992), the ELISA technique (Jaffe & McMahon-Pratt 1987), or dot-blot ELISA (Hawkes et al. 1982), using whole parasite lysates as antigen (Grimaldi et al. 1992). Characterization of the molecules as-sociated with the specific antigenic determinants was performed by Western blot analysis (Jaffe & McMahon-Pratt 1983). Briefly, the soluble anti-gen extracts were resolved by sodium dodecyl sul-phate-polyacrylamide gel electrophoresis (SDS-PAGE) using 10% slab gels in non-reducing con-ditions, and electrophoretically transferred to ni-trocellulose paper (Schleicher and Schuell, Keene, New Hampshire, USA). The techniques have been described in detail previously (Leon et al. 1992).
RESULTS AND DISCUSSION
In this paper, the characterization of represen-tative strains of Endotrypanum, based on their re-activity patterns using specific MAbs to Endotrypanum (Lopes & McMahon-Pratt 1989) or Leishmania (WHO 1993, Grimaldi & McMahon-Pratt 1996) is presented. The genus Endotrypanum, analyzed in this study (Table I), may represent an heterogeneous complex of parasite species or strain variants, as classified by numerical zymotaxonomy (Franco et al. 1996).
Reactivity with monoclonal antibodies -Two MAbs (E-2, CXIV-3C7-F5 and E-24, CXXX-3G5-F12) tested in these experiments were produced against membrane-enriched preparations of Endotrypanum species. In studies using a dot-blot radioimmune assay, these MAbs appear to be spe-cific for the genus Endotrypanum, but monoclonal E-2 reacted exclusively with strain M6159 of E. schaudinni; no cross-reaction was observed when Leishmania species or other trypanosomatids were
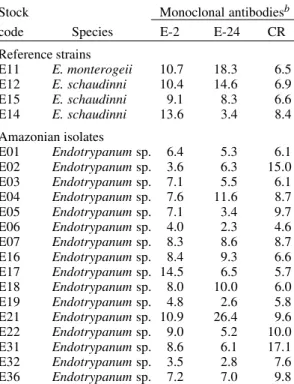
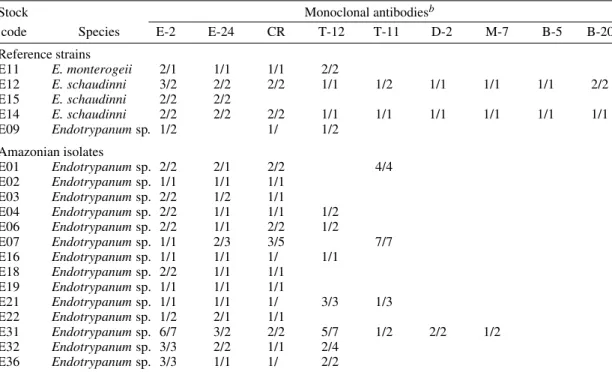
examined (Lopes & McMahon-Pratt 1989). In this study, although the two clones reacted variably according to the test used (Fig. 1; Tables II-IV), in the ELISA (Table III) as well as IFAT (Table IV) both reacted with all Endotrypanum strains tested.
Fig. 1: specificity of monoclonal antibodies, E-2 (CXIV-3C7-F5) and E-24 (CXXX-3G5-F12) for the Endotrypanum strains, as shown by dot blot enzyme binding assay. The stock codes of the Endotrypanum strains analyzed are indicated above the lanes, and their origins are shown in Table I. Stock L565 is a Leishmania guyanensis reference strain (MHOM/BR/75/M4147) and clone B-19 (XLIV-5A2-B9) is considered specific for this parasite species. Clone CR (G2G4) is cross-reactive MAbs and was used as a positive control; NS-1, hybridoma culture supernatant (negative control).
TABLE II
A comparison of the bindinga of Endotrypanum -specific monoclonal antibodies to reference strains and
Endotrypanum isolates from the Amazon Region, Brazil
Stock Monoclonal antibodiesb
code Species E-2 E-24 CR Reference strains
E11 E. monterogeii 10.7 18.3 6.5 E12 E. schaudinni 10.4 14.6 6.9 E15 E. schaudinni 9.1 8.3 6.6 E14 E. schaudinni 13.6 3.4 8.4 Amazonian isolates
E01 Endotrypanum sp. 6.4 5.3 6.1 E02 Endotrypanum sp. 3.6 6.3 15.0 E03 Endotrypanum sp. 7.1 5.5 6.1 E04 Endotrypanum sp. 7.6 11.6 8.7 E05 Endotrypanum sp. 7.1 3.4 9.7 E06 Endotrypanum sp. 4.0 2.3 4.6 E07 Endotrypanum sp. 8.3 8.6 8.7 E16 Endotrypanum sp. 8.4 9.3 6.6 E17 Endotrypanum sp. 14.5 6.5 5.7 E18 Endotrypanum sp. 8.0 10.0 6.0 E19 Endotrypanum sp. 4.8 2.6 5.8 E21 Endotrypanum sp. 10.9 26.4 9.6 E22 Endotrypanum sp. 9.0 5.2 10.0 E31 Endotrypanum sp. 8.6 6.1 17.1 E32 Endotrypanum sp. 3.5 2.8 7.6 E36 Endotrypanum sp. 7.2 7.0 9.8
a: results shown express the ratio of cpm bound mono-clonal antibodies/cpm bound control; values > 3 were considered positive.
66 66 66 66
66 Characterization of Endotrypanum Parasites AMR Franco et al.
The analysis showed antigenic variation occur-ring among the Endotrypanum strains. Significant differences between the reactive patterns with spe-cific MAbs could be observed among stocks (Tables II-III), but no qualitative or quantitative pattern could be consistently related to parasite origin (i.e., host species involved) or geographical area of isolation. These differences can be related with strain varia-tion in the level of expression of certain antigenic determinants, as recognized by these MAbs. Varia-tion in the sensitivity of the test may also occur due to the type of screening assay used.
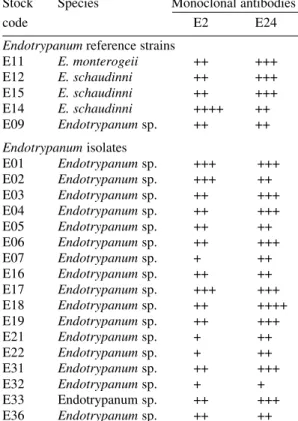
IFAT studies were carried out to confirm the specificity and binding of the MAbs to the Endotrypanum surface determinants on intact para-sites (Table IV). Each clone reacted in a continu-ous pattern on the surface of the promastigotes. Interestingly, clone E-24 produced the same im-munofluorescent pattern of reaction (data not shown) with a parasite of sloth and rodent, L. equatorensis (Grimaldi et al. 1992). As noted with L. colombiensis (Kreutzer et al. 1991), two strains of L. equatorensis cross-reacted with MAbs T1 (clone XLVI-5B8-B3), T2 (XLVI, 4H12-C2), T3
(XLVI-5A5-D4), T4 (LXVIII-1A4-G1) and T8 (LXVII-3E12-F8) (Grimaldi et al. 1992), which were originally produced against parasites in the L. major/L. tropica complex (Jaffe & McMahon-Pratt 1983). Moreover, using either IFAT (data not shown) or ELISA assay (Table III) the Endotrypanum strains reacted with MAbs specific to Leishmania, particularly with clones IS1-2C8-C7 (T-12) and LXIX-2D8-D7 (T-11) which are L. major- or L. tropica-specific respectively (WHO 1993). Our data confirm a previously reported study (Shaw 1992) showing that a number of Endotrypanum strains cross-reacted with these MAbs (Jaffe & McMahon-Pratt 1983) or mono-clonal antibody (WIC 79.3) which recognizes lipophosphoglycan components of L. major (Handman et al. 1984). Based on this results, it ap-pears that close antigenic links may exist between Endotrypanum and Leishmania. Work is now in progress to better define the phylogenetic relation-ship between these parasites. Whatever the expla-nation for the existence of these cross-reactive epitopes, these results indicate caution should be taken by all researchers working with field isolates.
TABLE III
Results of ELISA testa, employing monoclonal antibodies produced against Endotrypanum or Leishmania
species, to reference strains and selected Endotrypanum isolates from the Amazon Region, Brazil Stock Monoclonal antibodiesb
code Species E-2 E-24 CR T-12 T-11 D-2 M-7 B-5 B-20 Reference strains
E11 E. monterogeii 2/1 1/1 1/1 2/2
E12 E. schaudinni 3/2 2/2 2/2 1/1 1/2 1/1 1/1 1/1 2/2 E15 E. schaudinni 2/2 2/2
E14 E. schaudinni 2/2 2/2 2/2 1/1 1/1 1/1 1/1 1/1 1/1 E09 Endotrypanum sp. 1/2 1/ 1/2
Amazonian isolates
E01 Endotrypanum sp. 2/2 2/1 2/2 4/4 E02 Endotrypanum sp. 1/1 1/1 1/1
E03 Endotrypanum sp. 2/2 1/2 1/1
E04 Endotrypanum sp. 2/2 1/1 1/1 1/2 E06 Endotrypanum sp. 2/2 1/1 2/2 1/2
E07 Endotrypanum sp. 1/1 2/3 3/5 7/7 E16 Endotrypanum sp. 1/1 1/1 1/ 1/1
E18 Endotrypanum sp. 2/2 1/1 1/1 E19 Endotrypanum sp. 1/1 1/1 1/1
E21 Endotrypanum sp. 1/1 1/1 1/ 3/3 1/3 E22 Endotrypanum sp. 1/2 2/1 1/1
E31 Endotrypanum sp. 6/7 3/2 2/2 5/7 1/2 2/2 1/2 E32 Endotrypanum sp. 3/3 2/2 1/1 2/4
E36 Endotrypanum sp. 3/3 1/1 1/ 2/2
a: the tabulated numbers (duplicate tests) correspond to the OD ranges at absorbance 405 nm. Values ³1 were considered positive (all blanks equal negative results).
The range were: 0=0 to 0.130; 1=0.131 to 0.317; 2=0.318 to 0.504; 3=0.505 to 0.691; 4=0.692 to 0.878; 5=0.879 to 1.065; 6=1.066 to 1.252; 7=1.253 to 1.439.
67 67 67 67 67 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 92(1), Jan./Feb. 1997
Identification of Endotrypanum antigens by MAbs - Fig. 2 shows the results of the Western blot
analysis identifying the molecules associated with the antigenic determinants recognized by MAbs E-2 and E-E-24. While E-E-2 recognized two weak com-ponents with an apparent molecular mass of 24 kD and 30 kD, the latter MAb identified multiple mo-lecular components with apparent relative mobility (Mr) values ranging from 25 to more than 80-kDa. These results are consistent with previous studies using these MAbs (Lopes & McMahon-Pratt 1989). However, variation occurred between strains ana-lyzed in this study, in either the Mr, intensity or num-ber of components observed. These observations indicated that differences existed either in the epitope density or molecular forms associated with the spe-cific antigenic determinants and therefore allowed the assignment of the strains to specific antigenic groups. These strains formed a polymorphic popu-lation according to their enzyme profiles and were grouped into 12 zymodemes. However, all of the isolates from the same species of sloth (C. juruanus) and geographical origin (Rondônia, Brazil) had the same enzymic profile (Franco et al. 1996). In con-trast, this group of parasites (zymodeme EZ01) when characterized by serodeme analysis, appeared to be a heterogenous population. An explanation for these differences could be related to any of the following reasons: (1) the possibility of cross-contamination or mixed-up of samples in our laboratory, (2) a mixed infection, and (3) the fact that the two tech-niques measure different properties of parasites. Ultimately, cloning procedures, as well as genetic differences (DNA) may need to be established for these parasites.
Fig. 2: western blot analyses of promastigotes homogenates from representative strains of Endotrypanum using monoclonal antibodies E-2 (CXIV-3C7-F5) and E-24 (CXXX-3G5-F12). The stock codes of the strains analyzed are indicated above the lanes, and their origins are shown in Table I. Molecular weights are indicated in kDa beside the figure.
TABLE IV
Specificity of monoclonal antibodies, E-2 (CXIV-33C7-F5) and E-24 (CXXX-3G5-F12) for the
Endotrypanum strains by indirect immunofluorescencea
Stock Species Monoclonal antibodies
code E2 E24
Endotrypanum reference strains
E11 E. monterogeii ++ +++ E12 E. schaudinni ++ +++ E15 E. schaudinni ++ +++ E14 E. schaudinni ++++ ++ E09 Endotrypanum sp. ++ ++
Endotrypanum isolates
E01 Endotrypanum sp. +++ +++ E02 Endotrypanum sp. +++ ++ E03 Endotrypanum sp. ++ +++ E04 Endotrypanum sp. ++ +++ E05 Endotrypanum sp. ++ ++ E06 Endotrypanum sp. ++ +++ E07 Endotrypanum sp. + ++ E16 Endotrypanum sp. ++ ++ E17 Endotrypanum sp. +++ +++ E18 Endotrypanum sp. ++ ++++ E19 Endotrypanum sp. ++ +++ E21 Endotrypanum sp. + ++ E22 Endotrypanum sp. + ++ E31 Endotrypanum sp. ++ +++ E32 Endotrypanum sp. + + E33 Endotrypanum sp. ++ +++ E36 Endotrypanum sp. ++ ++
68 68 68 68
68 Characterization of Endotrypanum Parasites AMR Franco et al.
In conclusion, we have classified a total of 17 Amazon Endotrypanum isolates (from the states of Rondônia and Pará) through the application of techniques employing specific MAbs in compari-son with standard reference strains. Characteriza-tion of Endotrypanum with specific MAbs is an additional approach useful to group/differentiate further among these parasites, but problems related to the identification of stocks (the described spe-cies are indistinguishable) were encountered us-ing the MAbs employed in this study. Our results confirm previous studies (Lopes et al. 1990, Franco et al. 1996) reporting heterogeneity in this genus.
ACKNOWLEDGMENTS
To Dr JJ Shaw (Evandro Chagas Institute, PA, BR) for the donation of reference strains.
REFERENCES
Bradford MM 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
Croft SL, Chance ML, Gardener PJ 1980. Ultrasctructural and biochemical characterization of stocks of
Endotrypanum. Ann Trop Med Parasitol 74: 585-589. Fernandes O, Degrave W, Campbell DA 1993. The mini-exon gene: a molecular marker for Endotrypanum schaudinni. Parasitology 107: 219-224.
Franco AMR, Momen H, Naiff, RD, Moreira CFS, Deane MP, Grimaldi Jr G 1996. Enzyme polymor-phism in Endotrypanum and numerical analysis of isoenzyme data. Parasitology 113: 39-48.
Greig SR, Akinsehinwa FA, Ashall F, Lainson R, Shaw JJ, Miles MA, Barker DC 1989. The feasibility of discrimination between Leishmania and
Endotrypanum using total DNA probes. Trans R Soc Trop Med Hyg 83: 196-197.
Grimaldi Jr G, McMahon-Pratt D 1996. Monoclonal anti-bodies for the identification of New World Leishma-nia species. Mem Inst Oswaldo Cruz 91: 37-42. Grimaldi Jr G, Tesh RB 1993. Leishmaniases of the New
World: Current concepts and implications for future research. Clin Microbiol Rev 6: 230-250.
Grimaldi Jr G, Kreutzer RD, Hashiguchi Y, Gomez EA, Mimory T, Tesh RB 1992. Description of Leishma-nia equatoriensis sp. n. (Kinetoplastida: Trypanoso-matidae), a new parasite infecting arboreal mammals in Ecuador. Mem Inst Oswaldo Cruz 87: 221-228. Handman E, Greenblatt CL, Goding JW 1984. An
ampiphatic sulphated glycoconjugate of Leishma-nia: Characterization with monoclonal antibodies.
EMBO J 3: 2301-2306.
Hawkes R, Niday E, Gordon J 1982. A dot-immunobinding assay for monoclonal and other an-tibodies. Anal Biochem 119: 142-147.
Herrer A, Christensen HA l980. Leishmania braziliensis
in the Panamanian two-toed sloth, Choloepus hoffmanni. Am J Trop Med Hyg 29: 1196-1200. Jaffe CL, McMahon-Pratt D 1983. Monoclonal
antibod-ies specific for Leishmania tropica. I. Characteriza-tion of antigens associated with stage- and species-specific determinants. J Immunol 131: 1987-1993.
Jaffe CL, McMahon-Pratt D 1987. Serodiagnostic as-say for visceral leishmaniasis employing monoclonal antibodies. Trans R Soc Trop Med Hyg 81: 587-594. Kreutzer RD, Corredor A, Grimaldi Jr G, Grogl M, Rowton ED, Young DG, Morales A, McMahon-Pratt D, Guzman H, Tesh RB 1991. Characterization of
Leishmania colombiensis sp. n. (Kinetoplastida: Trypanosomatidae), a new parasite infecting humans, animals, and phlebotomine sand flies in Colombia and Panama. Am J Trop Med Hyg 44: 662-675. Leon LL, Machado GMC, Barral A, Carvalho-Paes LE
de, Grimaldi Jr G 1992. Antigenic differences among
Leishmania amazonensis isolates and their relation-ship with distinct clinical forms of the disease. Mem Inst Oswaldo Cruz 87: 229-234.
Lopes AHCS, McMahon-Pratt D 1989. Monoclonal antibodies specific for members of the genus
Endotrypanum. J Protozool 36: 354-361.
Lopes AHCS, Iovannisci D, Petrillo-Peixoto M, McMahon-Pratt D, Beverley SM, 1990. Evolution of nuclear DNA and the occurrence of sequences related to new small chromosomal DNAs in the trypanosomatid genus Endotrypanum. Mol Biochem Parasitol 40: 151-162.
McMahon-Pratt D, Jaffe CL, Bennett E, David JR, Grimaldi Jr G 1986. Studies employing monoclonal antibodies for the analysis of the genus Leishmania
Ross, 1903, p. 173-178. In Coll Intern CNRS/ INSERM, 1984. Leishmania. Taxonomie et Phylogenese. Applications ecoepidemiologiques. IMEEE, Montpellier, France.
Medina-Acosta E, Franco AMR, Jansen AM, Sampol M, Nevez N, Pontes de Carvalho L, Grimaldi Jr G, Nussenzweig V 1994. Trans-sialidase and sialidase activities discriminate between morphologically in-distinguishable trypanosomatids. Eur J Biochem 225: 333-339.
Miles MA, Arias JR, Valente SA, Naiff RD, de Souza AA, Povoa MM, Lima JA, Cedillos RA 1983. Ver-tebrate hosts and vectors of Trypanosoma rangeli
in the Amazon Basin of Brazil. Am J Trop Med Hyg 32: 1251-1259.
Montero-Gei F 1956. Contribucion al estudio de
Endotrypanum schaudinni (Trypanosomatidae). Rev Biol Trop 4: 41-68.
Shaw JJ 1969. The haemoflagellates of sloths. London School of Hygiene and Tropical Medicine Memoir 13, HK Lewis Co Ltd, London, 132 pp.
Shaw JJ 1985. The hemoflagellates of sloths, vermilingues (anteaters), and armadillos, p. 279-292. In GG Montegomery, The evolution and ecology of armadillos, sloths and vermilingues. Smithsonian Institution, Washington, D.C.
Shaw JJ 1992. Endotrypanum, a unique intraerythrocytic flagellate of New World tree sloths. An evolution-ary link or an evolutionevolution-ary backwater? Ciência e Cultura 44: 107-116.
WHO 1993. Report from a workshop on Use of mono-clonal antibodies in the identification of Leishma-nia of medical importance in latin America. Cali, Colombia, Dec 5-10.
Zeledón R, Ponce C, Ponce E de 1975. The isolation of
Leishmania braziliensis from sloths in Costa Rica.