Expression of Class 5 Antigens by Meningococcal Strains Obtained From Patients in Brazil and Evaluation of Two New Monoclonal Antibodies
Texto
Imagem
Documentos relacionados
Judicious application of techniques for gene-splicing, production of monoclonal antibodies, protein engineering, and so forth to solve problems in the fields of
T aenia solium (T so) antigens, and also by antibod- ies in samples of serum and cerebrospinal fluid (CSF) from patients with neurocysticercosis (NC).. Monoclonal antibodies
Distribution of human leukocyte antigen (HLA) class I antigens in the H.. Evaluation of human leukocyte antigen class I and II antigens in Helicobacter pylori -positive
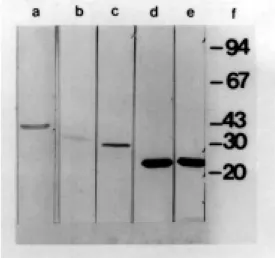
The other mAbs that failed to react in Western blot (1D12, 22C1, 4B2) reacted with antigens of the parental virus (Los Angeles) but failed to react with cells infected with
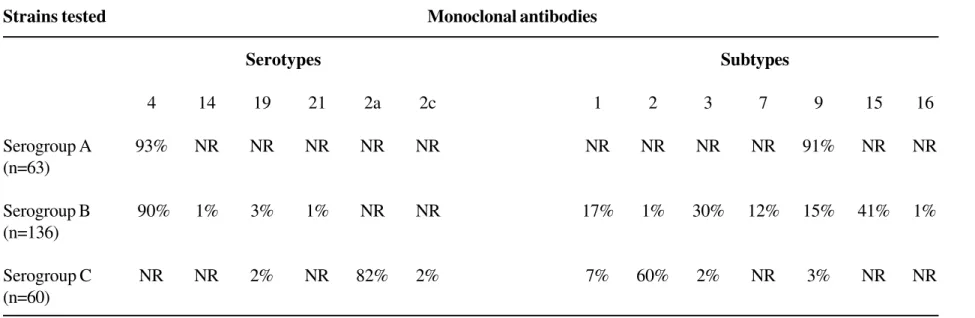
Comparison of N-terminal amino acid sequence of Neisseria meningitidis class 1 (C1) protein with deduced and translated meningococcal class 1 proteins... of the organism in
Here, we report the identification by isoenzyme electrophoresis and indirect immunofluorescence (IFA) with monoclonal antibodies of 34 Leishma- nia strains, isolated from
(e) Anti-CDV antibodies immobilized on cellulose surface and application of gold nanoparticles with antibodies solution (antigens are absent in this case) at the
FIGURE 3 – Monoclonal antibodies (including Fc-fusion proteins) currently approved by FDA and/or EMA as of October 2017, classified by (A) type of molecule, (B)