Universidade de Lisboa
Faculdade de Farmácia
Antifouling strategies to prevent
catheters-associated medical infections
Sara Manuela Costa Lemos
Mestrado Integrado em Ciências Farmacêuticas
Universidade de Lisboa
Faculdade de Farmácia
Antifouling strategies to prevent
catheters-associated medical infections
Sara Manuela Costa Lemos
Monografia de Mestrado Integrado em Ciências Farmacêuticas apresentada à Universidade de Lisboa através da Faculdade de Farmácia
Orientadora: Doutora Isabel Alexandra Caldeira Ribeiro, Professora
Auxiliar
Resumo
O uso de dispositivos médicos invasivos tem vindo a aumentar com o passar dos anos, sendo que os cateteres são dos dispositivos médicos mais utilizados.
Contudo há um risco de desenvolvimento de infeções associado ao uso destes dispositivos, uma vez que os cateteres são feitos de materiais que, devido às suas superfícies hidrofóbicas, são muito propensos à adesão de microrganismos. Deste modo, a prevenção da adesão bacteriana à superfície destes materiais reveste-se de grande importância.
Este trabalho faz uma revisão de algumas estratégias anti-adesivas obtidas através da modificação das propriedades físico-químicas dos materiais. Estratégias tais como os revestimentos com polietilenoglicol, zwitteriões, polissacáridos, poliacrilamida e poliacrilatos, polímeros anfifílicos e poliuretanos modificados foram abordadas. Também a Topografia de Sharklet e as Superfícies Porosas Escorregadias Infundidas em Líquido (SLIPS) foram alvo de revisão.
Apesar de todas as estratégias abordadas terem demonstrado ser eficazes na prevenção da adesão bacteriana, o uso de zwitteriões (mais especificamente SBSi, PDA/PMEN10 e Poly-SB), escovas Amino-PPX-PAAm, metilcelulose e a associação de diferentes polissacáridos (nomeadamente a heparina com o quitosano ou a agarose) para o revestimento das superfícies dos biomateriais são alvo de destaque. Também as SLIPS e a Topografia de Sharklet demonstraram ser candidatos promissores na área das estratégias anti-adesivas.
Quanto à aplicação deste tipo de estratégias em cateteres, será necessária a realização de mais estudos in vivo e ensaios clínicos em humanos para que se possa garantir a segurança do uso destas estratégias.
Abstract
The use of invasive medical devices is becoming more common in nowadays, with catheters representing one of the most used medical devices. However, there is a risk of infection associated with the use of these devices, once they are made of materials that are prone to bacterial adhesion. Therefore, to avoid the nefarious consequences of these infections the prevention of bacterial adherence to the surface of catheters is an important aspect.
This review is focused in some strategies that are able to modify the physical or chemical properties of materials, leading to the creation of antiadhesive surfaces. Strategies such as coating the surfaces with poly(ethylene glycol), zwitterions, polysaccharides, polyacrylamide and polyacrylates, amphiphilic polymers and modified polyurethanes were reviewed. Also, two quite different approaches were included, the Sharklet topography and the Slippery Liquid-Infused Porous Surfaces.
All the reviewed strategies have proven to be effective in preventing bacterial adhesion. However, the best examples were the use of zwitterionic polymers (more specifically SBSi, PDA/PMEN10 and Poly-SB), Amino-PPX-PAAm brushes, methylcellulose grafting and associations of polysaccharides (heparin with chitosan or agarose) on biomaterials coatings. Also, SLIPS and Sharklet Topography seem to be promising candidates in the field of antifouling strategies.
Concerning the potential application of most of these strategies in catheters, more in
vitro studies and clinical trials in humans are needed, to assure the safety in possible future use.
Acknowledgements
To my parents who always believed in me, even when I doubted of myself. For all the times that they didn’t let me give up, for the strength they have always given me and for the pillar that they are in my life.
To my family that always supported me and that always showed the pride that has for me. For all the words of comfort and affection.
To the friends I did in college. For all the motivation they have always given me, for all the moments we shared and for the certainty that they will be for life.
To those long-time friends who have seen me grow as a person and have been witnessing my achievements.
To my friend João Pedro, for all the help, the advices, support and patience.
And finally, last but by no means least, to my advisor Dr. Isabel Alexandra Caldeira Ribeiro for the continuous support during the writing process of my thesis and related research, for her patience, guidance and immense knowledge.
Abbreviations and Acronyms
HAIs Healthcare-Associated Infections
ECDC European Centre for Disease Prevention and Control
EPS Extracellular Polymeric Substances
PD Peritoneal Dialysis
PVC Polyvinylchloride
PU Polyurethane
PDMS Polydimethylsiloxane
PTFE Polytetrafluoroethylene
PEG Poly(ethylene glycol)
CFUs Colony Forming Units
SEM Scanning Electron Microscopy
PPG Poly(propylene glycol)
OA Octadecylacrylate
Poly(PEGA/OA) Poly(ethylene glycol) Acrylate Copolymer
Poly(PEG-SO3A/OA) Sulfonated Poly(ethylene glycol) Acrylate Copolymer
PDA Polydopamine
PEO Poly(ethylene oxide)
CH Chitosan
CMCS Carboxymethyl Chitosan
CAUTI Catheter-Associated Urinary Tract Infection
PCU Polycarbonate-Urethane
PIME Diamino-diamide-diol
MTT 3-(4,5) dimethylthiazol-2-yl-2,5 diphenyl tetrazolium bromide
UV Ultraviolet
MSNs Mesoporous Silica Nanoparticles
AMSNs Agarose-Loaded Mesoporous Silica Nanoparticles
AHMSNs Agarose and Heparin-Loaded Mesoporous Silica Nanoparticles
PUh Hexamethylene Diisocyanate Based Polyurethanes
HDI Hexamethylene Diisocyanate
PAAm Polyacrylamide
Amino-PPX Amino-poly(o-amino-p-xylylene-co-p-xylylene)
ATRP Atom Transfer Radical Polymerization
PA13 Poly(methylmethacrylate-co-dimethylacrylamide) PA515 Poly(methoxyethylmethacrylate-co-diethylaminoethylacrylate- -co-methylmethacrylate) PA155 Poly(hydroxyethylmethacrylate-co-dimethylaminoethylme-thacrylate) SB Sulfobetaine CB Carboxybetaine PC Phosphorylcholine
Poly-SB Polymeric Sulfobetaine
SBMA Sulfobetaine Methacrylate
SBSi Sulfobetaine Silane
MPC 2-Methacryloyloxyethyl Phosphorylcholine
NPCEMA p-Nitrophenoxycarbonyloxyethyl Methacrylate
P4VP Polymer 4-vinyl Pyridine
PCB Protected Carboxybetaine
DMA Dodecyl Methacrylate
PEGMA Poly(ethylene glycol) Methacrylate
AA Acrylic Acid
PBS Phosphate Buffered Saline
O.D. Optical Density
EA Ethanolamine
S Serinol
ED Ethylene Diamine
MDI Methylene-bis-phenyl-isocyanate
DHMPA Dihydroxymethyl-propionic Acid
PLC Polycaprolactide
PLA Poly-l-lactide
PPO Polypropylenoxide
SLIPS Slippery Liquid-Infused Porous Surfaces
TLP Tethered Liquid Perfluorocarbon
Index:
1 Introduction ... 13
2 Materials and methods ... 16
3 Catheters and their materials ... 17
4 Antifouling strategies ... 19
4.1 Hydrophilic polymers ... 19
4.1.1 Poly(ethylene glycol) and derivates ... 19
4.1.2 Polysaccharides ... 23 4.1.2.1 Dermatan Sulfate ... 23 4.1.2.2 Methylcellulose ... 24 4.1.2.3 Agarose ... 25 4.1.2.4 Chitosan ... 25 4.1.2.5 Heparin ... 26
4.1.3 Polyacrylamide and polyacrylates ... 31
4.2 Zwitterionic Polymers ... 34
4.2.1 Betaines ... 34
4.3 Other antifouling coatings ... 38
4.3.1 Amphiphilic polymers ... 38
4.3.2 Modified Polyurethanes ... 39
4.3.3 Slippery Liquid-Infused Porous Surfaces ... 40
4.3.4 Sharklet topography ... 42
5 Discussion ... 46
6 Conclusion ... 48
Bibliography ... 49
Index of Figures: Figure 1 - Steps of biofilm formation ... 14
Figure 2 - Representation of an antifouling surface ... 15
Figure 3 - Representation of a SLIPS surface ………... 41
Index of Tables: Table 1 – Types of catheters, their materials and the most common microorganisms related with the development of infection ... 18
Table 2 – Use of PEG and derivates as antifouling strategies with possible application in catheters ... 22
Table 3 – Use of polysaccharides as antifouling strategies with possible application in catheters ... 30
Table 4 – Use of PAAm and polyacrylates as antifouling strategies with possible application in catheters ... 33
Table 5 – Use of zwitterions as antifouling strategies with possible application in catheters . 37 Table 6 – Use of amphiphilic polymers as antifouling strategies with possible application in catheters ... 44
Table 7 – Use of modified PUs as antifouling strategies with possible application in catheters ... 44
Table 9 – Use of Sharklet Topography as antifouling strategy with possible application in catheters ... 45
1 Introduction
Invasive medical devices are becoming more important each day on medical modern practice since they can improve therapeutic results and save many lives. However, these type of devices also represents an important risk factor for the development of healthcare-associated infections (HAIs), particularly in more susceptible patients (1,2). HAIs, also known as “nosocomial” or “hospital infections”, are infections that patients acquire while they are receiving a treatment for a previous illness in a healthcare facility. HAIs are the most common adverse events in healthcare facilities worldwide, with an important impact on morbidity, mortality and quality of life, and also representing significant financial expenses to the health systems (3). According to the 2014 annual epidemiological report of European Centre for Disease Prevention and Control (ECDC), the prevalence of patients with at least one HAI in European acute‑care hospitals in 2013 was estimated at 3.4%, with a total of 4.2 million HAIs in the entire year (4).
Currently, one of the most important devices used in medical procedures that are related to HAIs are catheters (5). In fact, in 2013, 43.3% of bloodstream infections were reported to be catheter-related and 96.7% of urinary tract infections were associated with the use of urinary catheters in UCI (4). Some of the most frequent agents of these type of infections are bacteria, but can also be parasites, fungi, viruses and prions (6).
Vascular and urinary catheters are currently the most used invasive medical devices (2,7). However, on catheters, bacteria (and sometimes other microorganisms and cells) have the ability to adhere and promote the formation of a biofilm on the surface of these devices (1,7,8).
Biofilms are communities of either homogeneous or heterogeneous populations of bacteria that can strongly attach to both biotic and abiotic surfaces, and self-produce a matrix made up of extracellular polymeric substances (EPS), that basically consists of polysaccharides, proteins, nucleic acids, lipids and other materials. The EPS acts as a scaffold that holds the biofilm together, stabilizing the biofilm network and protecting the bacteria from external threats (6,9,10). Biofilm formation includes several stages: reversible and irreversible attachment, colonization, maturation and dispersion (Figure 1) (6,11). In the first stage – the attachment – planktonic bacteria adhere to the surface mainly by weak physical forces such as electrostatic interactions or van der Waals forces, or by some bacterial appendages like flagella, pili and fimbriae, that results in a reversible attachment. However, with the time, bacterial
appendages overcome the physical repulsive forces, making bacterial cells irreversibly attached (6,9,12). In the next stage – the colonization and maturation of biofilm – bacteria already adhered to the surface start producing autoinducer signals that allow cell-to-cell communication and start building a matrix of EPS. In this stage biofilm comes multilayered, the bacterial colony grows and its density increases (9). Then, due to changes in nutrients, temperature and oxygen levels at the latter end of the biofilm cycle, cells that were part of a complex, relatively static, slow-growing community within the biofilm become differentiated and often highly motile microorganisms. Parts of biofilm detach within the host and the dispersed cells are able to cause systemic infection, particularly in immunocompromised patients, since they can attach to new surfaces and colonize new sites (6).
The presence of a matrix in biofilms is an important obstacle because it protects bacteria from the innate immune defenses of the host, difficults antimicrobials to penetrate the full depth of biofilm conferring tolerance to antibiotic treatments, and allows a better cell-to-cell communication with transference of some drug-resistance markers and virulence factors,
Attachment Colonization Maturation Dispersion
a possible increase antibiotic resistance in long-term use of biocidal coatings therefore antifouling strategies are an interesting choice in fighting biofilms. These strategies consist in physicochemical surface modifications focusing on roughness, nanostructures and hydrophobicity of the biomaterials, resulting in antiadhesive materials that prevent bacterial attachment and consequently biofilm formation on medical devices (Figure 2) (14,16).
The aim of this work is to review antifouling strategies with possible application on biomedical devices. This review will be focused on catheters in order to prevent microbial attachment and proliferation with consequent biofilm formation on the surface of these medical devices.
Bare catheter Coated catheter
2 Materials and methods
Online databases containing medical, technical and scientific contents (i.e. Web of Knowledge, PubMed, Science Direct and Springer) were used to the development of this work. For the epidemiological data, 2014 Annual epidemiological report of the European Centre for Disease Prevention and Control (ECDC) was used.
The first stage of the research was made by using some general keywords like “nosocomial infections”, “bacteria”, “catheters”, “antifouling”, “antiadhesive”, “medical devices”, “antibiofilm” and “antiadhesive polymers”. Review articles and book sections were the materials of choice to this first phase.
After that, a more specific research was made. In this stage combinations of specific keywords such as “sulfobetaine catheter”, “polysaccharide catheter” and “PEG medical devices” were used. Although there some articles from before 2009 were referenced, bibliography sources from the last 7 years were privileged. Studies conducted specifically on catheters and also on catheters-materials were considered.
3 Catheters and their materials
The ideal catheter should have some characteristics such as high tensile strength, biocompatibility, be soft and pliable and inherently chemical-resistant. It is a fact that these properties are conferred by the materials of these devices (17,18).
There are three principal types of catheters: vascular, urinary and peritoneal dialysis (PD) catheters. However, the most commonly used ones are the first two (1,2).
Urinary catheters are inserted through the urethra into the bladder, with the purpose to collect urine during surgical procedures, measure urine output or control urinary incontinence or retention (6,9). They are made of materials such as gum-elastic, polyvinylchloride (PVC), polyurethane (PU), silicone and latex. Among these silicon, more specifically polydimethylsiloxane (PDMS) (a type of silicone), is the most used material (18,19).
The most common agents that can colonize urinary catheters are, for example, some Gram-positive bacteria such as Staphylococcus epidermidis, and some Gram-negative bacteria such as Escherichia coli, Proteus mirabilis and Klebsiella pneumoniae (9,20).
Vascular catheters may be used to administer fluids, medication or nutrients or to make dialysis. The most common materials of this medical devices are PU, silicone, polyethylene and polytetrafluoroethylene (PTFE) (also known as Teflon®). However, PU is the most used one,
due to it high biocompatibility, resistance to several chemicals and compatibility with many drugs, and also because it is thromboresistant and becomes less rigid inside the body, reducing mechanical trauma and vein irritation. S. epidermidis, S. aureus, Candida albicans and
Pseudomonas aeruginosa are some of the main reported pathogens responsible for HAI’s
associated to these catheters (6,9,17,21).
PD is a therapy used in some cases of severe renal disease, that delay the degradation of the residual renal function, at the same time that improves quality of life of patients and maintains the hemodynamics (22,23). For this technique, a PD catheter (mainly constituted by silicone) is inserted into the abdominal cavity of the patient, and a dialysis solution is introduced into this cavity, and subsequently removed. The peritoneum acts like a semi-permeable membrane (1,24,25). This type of catheters is also suitable to bacterial colonization. In fact, at least 75% of peritonitis are related to colonization of Gram-positive bacteria such as S.
epidermidis and S. aureus, followed by Gram-negative bacteria colonization including P. aeruginosa in approximately 20% of the cases (25,26).
The principal types of catheters, their materials and the most common microorganisms that are related with the development of infection are summarized in Table 1.
Table 1 – Types of catheters, their materials and the most common microorganisms related with the development of infection
Common materials Most common pathogens Ref.
Urinary Catheter
Gum-elastic, PVC, PUs, silicone, PDMS and latex.
Some Gram-positive bacteria (S. epidermidis), and some Gram-negative bacteria such as E. coli, P. mirabilis and
K. pneumoniae.
(9,18–20)
Vascular Catheter
PU, silicone, polyethylene
and PTFE (Teflon®). S. epidermidis, S. aureus, C. albicans and P. aeruginosa. (6,9,17,21) PD Catheter Mainly silicone.
Mostly Gram-positive bacteria (S. epidermidis and S.
aureus) followed by Gram-negative bacteria (P. aeruginosa)
4 Antifouling strategies
As previously mentioned, the first step on catheter-associated medical infections is the attachment of bacteria to the surface of this medical devices, since both catheter and the majority of bacteria’s surfaces are hydrophobic (which increase the strength of hydrophobic interactions) (1,8,14,27,28). With the purpose of preventing that initial attachment, antifouling polymers have been developed. These polymers have the capacity to resist to bacterial attachment by one of the following mechanisms: increasing surface hydrophilicity, adding negative charges or decreasing surface free energy (27,29,30).
There are two principal types of antifouling polymers, depending on how they interact with water molecules: hydrophilic polymers and zwitterionic polymers (18,31). These polymers create a water layer on the surface of biomaterials. When bacteria or other foulants such as proteins get closer to the surface, the polymer chains are compressed and the water molecules previously bonded to polymer coatings are released (1,32,33). These two events leads to a decrease in entropy and increase in enthalpy, respectively, which results in a thermodynamically unfavorable surface that repels bacteria (34).
Thus, antifouling polymers can avoid the development of infections on medical devices such as catheters (8).
4.1 Hydrophilic polymers
Hydrophilic polymers are polymers that have chemical affinity to water molecules (35). These polymers have the ability to bond with the surrounding water by hydrogen bonds creating a hydrated layer on their surface (31,36). However, since these bonds are relatively weak, changes in surface hydration can occur with the time, which can lead to loss of antiadhesive properties of these materials (35).
4.1.1 Poly(ethylene glycol) and derivates
Poly(ethylene glycol) (PEG) is a neutral and hydrophilic polymer that has been widely studied relatively to its antiadhesive properties (1,36–39). The ether groups of this polyether are able to bond with several water molecules through weak hydrogen bonds, forming a hydrated layer on biomaterials surfaces (29,37,40).
There are many different strategies to attach PEG to the surface of biomaterials. Covalent grafting, chemical or physical adsorption, plasma deposition and graft or block copolymerization are some of the options (29,38,41).
Through the last decades many studies have been developed aiming to evaluate the efficacy of PEG coatings on the prevention of bacterial colonization on the surface of medical devices.
One of those studies was developed by Park et al. (42) who synthetized different PEG chains with the same length but different terminal groups (PEG1k-OH, PEG1k-NH2 and
PEG1k-SO3H), and a PEG chain with a higher length (PEG3.4k-OH) (Table 2). The synthetized
PEG chains were grafted on PU beads and the number of adhered E. coli (ATCC 11775) and
S. epidermidis (ATCC 12228) cells was quantified by the number of Colony Forming Units
(CFUs) per cm2 and surface observed through scanning electron microscopy (SEM).
Comparing both controls, the bare PU and the hydrophobic poly(propylene glycol) (PPG) grafted PU surfaces (PPG1k-OH), with PEG-1k coatings, the authors concluded that S.
epidermidis and E. coli adhesion in plasma reduced. Also, both PEG1k-SO3H and PEG3.4k
surfaces showed the best antifouling results for the two types of bacteria (42).
In another study, H. Lee et al. (43) developed and characterized new PEG and sulfonated PEG acrylate copolymers containing a hydrophilic portion of PEG/PEG-SO3
acrylate and a hydrophobic portion of octadecylacrylate (OA). The obtained copolymers were named as poly(PEGA/OA) and poly(PEG-SO3A/OA), respectively. The authors synthetized
different PEG/PEG-SO3 containing acrylate copolymers with three different chain lengths (1K,
2K and 4K). The obtained copolymers (Poly(PEG1KA/OA), Poly(PEG2KA/OA), Poly(PEG4KA/OA), Poly(PEG1K-SO3A/OA), Poly(PEG2K-SO3A/OA), and
Poly(PEG4K-SO3A/OA)) were grafted onto PU films. The adhesion of S. epidermidis (KCTC 1917) and E.
More recently, in 2017, C. Xing et al. (44) immobilized PEG-OH and PEG-COOH on polydopamine (PDA) pre-coated silicon wafers, obtaining the polymers PDA/PEG-OH and PDA/PEG-COOH, respectively. To test the antibiofilm activity the authors used E. coli, S.
aureus and P. aeruginosa suspensions in the bacterial adhesion tests (Table 2). After 7 days,
the obtained results from fluorescence microscopy demonstrated that both PEG coatings presented a significant reduction on bacterial attachment of the three tested strains. However PDA/PEG-OH was the coating that demonstrated the best antifouling results (44).
The antifouling properties of a derivate of PEG was also evaluated. One of those studies was performed by J. Park et al. (45) that developed different poly(ethylene oxide) (PEO)-based multiblock copolymer/segmented PU (SPU) blends (Table 2). The multiblock copolymer was composed by an hydrophilic portion of PEO and an hydrophobic portion of poly(tetramethylene oxide) (PTMO) blocks. For this study the authors added 5, 10, 20 or 30 wt% of copolymer to the SPU, and obtained the copolymers Pell-5, Pell-10, Pell-20 and Pell-30. From the bacterial tests the authors concluded that Pell-5 was sufficient to significantly decrease the number of adherent bacteria on the surfaces (83 ± 13 x 102 CFU/cm2 versus 275 ± 31 x 102 CFU/cm2 on bare SPU). They also concluded that increasing the amount of copolymer on blends, the resistance to bacterial attachment was higher. In fact, Pell-30 was the blend with less bacterial adhesion of all the four blends tested. All four copolymers demonstrated be more effective inhibiting S. epidermidis adhesion than P. mirabilis adhesion (45).
Although PEG is a very used polymer to confer antiadhesive properties to biomaterials, it is easily oxidized in the presence of O2,forming aldehydes and ethers. This reaction results
in the degradation of PEG with a consequent decrease of it antifouling ability. For this reason, PEG-coatings have a limited utility in long-term applications (35,36,38,46).
Table 2 – Use of PEG and derivates as antifouling strategies with possible application in catheters
Strategy Catheter material
Materials used on strategy
Methods used to test the strategy
Tested
microorganisms Results Ref.
T y pe o f ca thet er Urina ry PEO/PTMO multiblock copolymer/ segmented polyurethane blends
Polyurethane Pell-5; Pell-10;
Pell-20; Pell-30 Number of CFUs/cm
2 E. coli (ATCC 11775), S. epidermidis (ATCC 12228) and P. mirabilis (ATCC 25993)
Pell-30 demonstrated < 50 x 102 CFUs/cm2 of E. coli,
P. mirabilis and S. epidermidis (versus 275 x 102
CFUs/cm2, ≈ 200 x 102 CFUs/cm2 for and ≈ 12000 x
102 CFUs/cm2 on controls, respectively).
(45)
Va
scula
r
PEG grafting Polyurethane
PPG1k-OH; PEG1k-OH; PEG1k-NH2; PEG1k-SO3H; PEG3.4k-OH Number of CFUs/cm2; SEM S. epidermidis (ATCC 12228) and E. coli (ATCC 11775)
≥ 90% reduction of the number of CFUs/cm2 on
PU-PEG1K-SO3H and PU-PEG3.4K-OH in plasma. (42)
Sulfonated PEG-acrylate copolymer surfaces Polyurethane Poly(PEG1KA/OA) Poly(PEG2KA/OA) Poly(PEG4KA/OA) Poly(PEG1K-SO3A/OA) Poly(PEG2K-SO3A/OA) Poly(PEG4K-SO3A/OA) Number of CFUs/cm2; SEM S. epidermidis (KCTC 1917) and E. coli (KCTC 2441)
> 80% reduction on S. epidermidis and > 90% on E.
coli adhesion on Poly(PEGA/OA) and
Poly(PEG-SO3A/OA) coated PUs in plasma.
(43)
PDA/PEG coating Silicone PDA; PDA/PEG-OH; PDA/PEG-COOH
Fluorescent microscopy; Quantitative analysis
E. coli, S. aureus and P. aeruginosa
PDA/PEG-OH demonstrated the best antifouling
4.1.2 Polysaccharides
Due to the lack of stability of PEG coatings for long periods of time, many studies have been made with the purpose of developing alternative polymers with antifouling properties and longer stability. One of the alternatives are polysaccharides (35,36).
Polysaccharides are molecules with a high-molecular weight that are found in abundance in nature. These biopolymers are constituted by more than ten repetitive units of monosaccharides that are linked by glyosidic bonds (15,47,48). Biocompatibility, non-toxicity and biodegradability are the main advantages presented by polysaccharides (48–50). However, these properties are influenced by the nature of the monomers and the structure of these biomolecules (47).
Cellulose, dextran, dermatan, heparin, chitosan and agarose are some examples of polysaccharides (24,27,31).
4.1.2.1 Dermatan Sulfate
Dermatan sulfate (DS) is a natural glycosaminoglycan normally found in the extracellular matrix of animal tissues, such as heart, skin and blood vessels (51–53). It has a complex structure that is similar to that of chondroitin sulfate, however instead of glucuronic acid it has iduronic acid (54).
This polysaccharide is negatively charged and very hydrophilic. Also, similarly to heparin (Hep), it has anticoagulant and antithrombotic properties as well as antifouling activity (51).
With respect to the antifouling properties of DS, there are very few studies. One of those was developed by F. Xu et al. (51) in 2011. These authors incorporated concentrations of DS of 0.66%, 1.5%, 3% and 10% into the side chain of PUs obtaining the copolymers PU/DS 0.66%, PU/DS 1.5%, PU/DS 3% and PU/DS 10%, respectively. The number of adherent E. coli (BL21 strain) on PU/DS surfaces was quantified via dsDNA assay. PEG-coated PU and Agar-coated PU were used as negative and positive controls, respectively. The obtained results showed that the incorporation of DS into PU reduced the attachment of E. coli. Moreover, copolymers PU/DS 0.66% and PU/DS 10% showed a density of adhered bacteria similar to the negative control (Table 3) (51).
4.1.2.2 Methylcellulose
Methylcellulose (MeCe) is a natural methacrylated polysaccharide found in plants (55). This polysaccharide is hydrophilic, possesses hydrogen bond acceptors and is neutral. So, MeCe has good antifouling properties. (1,56). Also, it is not degradable by human enzymes (only by cellulase, an enzyme that lacks in humans). Therefore, MeCe resists to in vivo degradation which potentially allows long-term applications (55,57).
Additionally, this biopolymer has been used for diverse applications on biomedical field without any safety concerns (56).
The antiadhesive properties of this polysaccharide have been investigated in different studies. Recently, W. Mussard et al. (56) grafted a vinyl-modified MeCe on the surface of the medical grade silicone elastomer MED-4765 by one-step reaction (Table 3). Bacterial adhesion of four bacterial strains (E. coli MG1655, S. epidermidis ATCC 35984, P. aeruginosa ATCC 15442 and S. aureus ATCC 6538) was evaluated by SEM observation and fluorescence microscopy. The results allowed the authors to conclude that MeCe-coated MED-4765 was able to reduce bacterial adhesion. In fact, coated samples showed a reduction of the number of adhered E. coli, S. epidermidis, P. aeruginosa and S. aureus of approximately 99,5%, 99%, 98.9% and 99.6%, respectively. Additionally, the MeCe grafting suppressed biofilm formation under flow conditions (56).
Three years later, another group of researchers grafted MeCe to PDMS catheters of totally implantable venous access ports (TIVAP) via one-step hydrosilylation in water (58). For the in vitro and in vivo bacterial adhesion tests, the authors used the luminescent S. aureus
Xen36 and P. aeruginosa Lm1 strains and used epifluorescence microscopy and
bioluminescence activity to evaluate the adhesion of these two microorganisms (Table 3). The obtained data from the in vitro tests demonstrated that MeCe-coated catheters significantly reduced the adhesion of both bacteria after 24 h under static conditions and after 48 h under
development on the internal surface of TIVAPs was made. The results showed an excellent antibiofilm efficacy, with a 2 to 3-log reduction of bacteria colonization on modified catheters at the end of this period (58).
4.1.2.3 Agarose
Agarose (AG) is a natural, non-ionic and hydrophilic polysaccharide that is obtained from agar or agar-bearing seaweeds such as red algae Rhodophyta. It consists in alternating (1→4) linked 3,6-anhydro-α-l-galactose and (1→3) linked β-d-galactose monomers (59–62).
This polysaccharide is biocompatible, non-toxic, inert and stable. Also, it presents antifouling and non-immunogenic properties that allows its application on biomedical and tissue engineering areas (59,60,62,63).
Aiming to investigate if AG was able to resist to bacterial adhesion, a group of investigators coated silicone surfaces with agarose (AG) films and AG hydrogel (2% w/v) (64).
In vitro bacterial tests were performed with strains of P. mirabilis B2 (isolated from an
encrusted catheter), P. mirabilis HI4320 (wild-type) and P. mirabilis HI4320 ure- (a urease-negative mutant). The attached bacterial cells were observed in a parallel-plate flow-cell chamber and quantified in terms of the number of cells per high power field and per cm2 of surface (Table 3). Bacterial tests were performed with urine or phosphate buffer. The experimental results obtained from urine cultures using P. mirabilis HI4320 ure- revealed a reduction on bacterial adhesion of ≈ 94%. After 6 h, the bacterial adhesion in both media was significantly lower compared to bare silicone. Relatively to AG hydrogel coating, data from tests with buffer revealed a low adhesion of P. mirabilis B2, and in urine cultures no bacterial adhesion was observed for the first 4 h. In spite of the good results demonstrated by both AG films and hydrogels, AG hydrogel demonstrated the best antifouling properties (64).
4.1.2.4 Chitosan
Chitosan (CH) is a natural polysaccharide that is obtained by partial deacetylation of chitin (1,30,54,65).
This biopolymer is composed by units of N-acetyl-D-glucosamine and D-glucosamine linked via β-(1,4) glycosidic bonds. The amine groups in its structure confers a positive charge
to CH that interacts with negatively charged bacteria, disrupting the cell membrane, being through this mechanism that CH reveals bactericidal activity (49,54,66).
CH has many applications in the biomedical field once it is biocompatible, biodegradable, non-toxic and low-immunogenic (49,54,67).
Several studies have been performed aiming to characterize the bactericidal activity of CH through the last years. However, antiadhesive properties of this polysaccharide are poorly investigated. Nevertheless, in 2012, R. Wang et al. (68) performed a study with the purpose of evaluating the ability of CH in inhibiting E. coli and P. mirabilis adhesion and biofilm formation on medical grade silicone surfaces (Table 3). Silicone samples were coated with PDA and then grafted with carboxymethyl chitosan (CMCS) through Michael addition and Schiff-base reactions. The obtained polymer was named as PDA-CMCS. After this step, some samples suffered a crosslink of the CMCS layer, obtaining PDA-CMCS-X polymers. Bacterial tests were performed under static and flow conditions, and the used microorganisms were E. coli (ATCC DH5α) and a strain of P. mirabilis (ATCC 51286) isolated from a patient with catheter-associated urinary tract infection (CAUTI). The results obtained from SEM and fluorescence microscopy allowed to conclude that PDA-CMCS and PDA-CMCS-X coatings were able to reduce P. mirabilis adhesion in 88% and 90%, respectively, and E. coli adhesion in ≈ 90% on both surfaces. Additionally, under static conditions the authors observed biofilm disruption on both PDA-CMCS-X and PDA-CMCS surfaces. Under flow conditions, PDA-CMCS surface revealed ≈ 20% coverage by P. mirabilis and ≈ 13% by E. coli biofilms (68).
Although the statement on antifouling properties by the authors, it cannot be forgotten that CH is well known by its antimicrobial characteristics. So, part of the antiadhesive results verified by the authors may be due to the interaction of this polysaccharide with the membrane of bacteria, with consequent disruption and death, resulting in a reduction of the number of viable bacteria that are capable of adhere to the surfaces.
increased the interest by using this polysaccharide on biomedical applications, such as coating blood-contacting biomedical devices (48,71).
Different techniques to immobilize this polysaccharide to these surfaces includes covalent attachment and electrostatic self-assembly (72).
In addition to the properties already mentioned above, it was proved that Hep also presents the ability of inhibiting bacterial adhesion and biofilm formation (27,54,69,71,73). This inhibition occurs because Hep negative charge repels negative charged bacteria through repulsive forces (27,69,71). Furthermore, some Gram-positive bacteria have adhesins that are able to recognize fibronectin (the main adsorbed protein on the surface of blood-contacting devices). Thus, Hep coatings leads to a reduction on fibronectin adsorption with consequent prevention of bacterial attachment to the surfaces (73).
Focusing on the resistance to bacterial adhesion of this polysaccharide, Hep was adsorbed to the surface of Bionate®-PIME samples (74). Bionate®-PIME is a polycarbonate-urethane (PCU) with low amounts of diamino-diamide-diol (PIME) in the main backbone. PIME promotes the binding of Hep to PCU surfaces mainly via ionic bonds, which results in a major stability of Hep grafting. Strains of S. aureus (Cowan 1 and 8325-4) and S. epidermidis (RP62A and HB) were used on the in vitro bacterial adhesion assays. The results were assessed by monitorization of the absorbance at 595 nm through a 3-(4,5) dimethylthiazol-2-yl-2,5 diphenyl tetrazolium bromide (MTT) test and by SEM observation. Two medical-grade PCUs were used as controls: Bionate® and Carbothane®. The obtained data revealed that heparinized Bionate®-PIME surfaces reduced S. epidermidis and S. aureus colonization. In fact, SEM images of Hep-treated surfaces demonstrated small size and dispersed clusters of bacteria, compared to the untreated surfaces that showed a dense and uniform layer of bacteria (74).
Recently, some researchers have also developed studies that associate Hep with other polysaccharides to coat the surface of some biomaterials with the aim of evaluating the antifouling properties of these associations.
One of those studies was performed in 2014 by M. Li et al. (24) that covalently immobilized and crosslinked an acrylated AG layer on both silicone films and PD silicone catheter surfaces (Table 3). Some of the samples additionally suffered an incorporation of methacrylated Hep into the crosslinked AG. For this grafting, silicone surfaces were previously activated by O2 plasma or O3 treatment and then the acrylated AG and methacrylated Hep
surfaces were named as silicone-g-AG and silicone-g-AG-Hep. Bacterial adhesion tests were performed with S. aureus (ATCC 25923), E. coli (ATCC DH5α) and P. aeruginosa (PAO1) as model bacteria, and the results were assessed by fluorescence microscopy and SEM observation. The authors concluded that both AG and AG-Hep-modified films significantly reduced S. aureus, E. coli and P. aeruginosa adhesion by ≈ 2.4, ≈ 3.3 and > 2 folds, respectively.
E. coli and S. aureus biofilm formation was prevented in both modified surfaces. Also, with the
increase of UV-induced grafting time (120 minutes), E. coli and S. aureus adhesion reduced to ≈ 99% and ≈ 98%, respectively. Similar to modified silicone films, AG and AG-Hep modified catheters showed a reduction on E. coli adhesion of more than 3 orders of magnitude (24).
In another study, silicone films were modified with mesoporous silica nanoparticles (MSNs) loaded with AG or AG and Hep via electrostatic interaction (75). The obtained nanoparticles were named as Agarose-Loaded Mesoporous Silica Nanoparticles (AMSNs) and Agarose and Heparin-Loaded Mesoporous Silica Nanoparticles (AHMSNs). For the in vitro assays, samples of bare silicone, AMSNs-Si and AHMSNs-Si were cultured with E. coli and S.
aureus. The bacterial adhesion was evaluated by SEM observation (Table 3). It was concluded
that both AMSN and AHMSN coatings significantly reduced microbial adhesion on silicone surfaces. Also, besides the resistance to bacterial adhesion, AHMSN coating also demonstrated good hemocompatibility due to the presence of Hep (75). This last property represents an advantage relatively to the AMSNs-coatings.
Additionally, a combination of Hep with CH was also studied by F. Kara et al. (69) to evaluate the antibacterial and antiadhesive properties of this association (Table 3). For this, the authors synthetized new hexamethylene diisocyanate based PUs (PUh) by using aliphatic hexamethylene diisocyanate (HDI) and polyol. Then, PUs surfaces were plasma activated and CH and Hep were covalently immobilized onto these surfaces via a stepwise process. The investigators used concentrations of 5 mg/mL and 20 mg/mL of CH, and a concentration of 5
reduction of 97%, 90%, 98% and 99% on E. coli, P. aeruginosa, S. aureus and S. epidermidis adhesion, respectively (69).
Table 3 – Use of polysaccharides as antifouling strategies with possible application in catheters
Strategy Catheter material
Materials used on strategy
Methods used to test the
strategy Tested microorganisms Results Ref.
T y pe o f ca thet er Urina
ry MeCe grafting PDMS MED-4765-MeCe
Fluorescence microscopy; SEM
E. coli MG1655, S.
epidermidis (ATCC 35984), P. aeruginosa (ATCC 15442)
and S. aureus (ATCC 6538)
≈ 99% reduction on bacterial adhesion.
Suppression of biofilm formation. (56)
CMCS coating Silicone PDA-CMCS; PDA-CMCS-X
SEM; Fluorescence microscopy; Number of CFUs/cm2
E. coli (ATCC DH5α) and P. mirabilis (ATCC 51286)
≈ 90% reduction of E. coli and P. mirabilis
adhesion. Disruption of biofilms. (68)
Va scula r DS PU PU/DS 0.66%; PU/DS 1.5%; PU/DS 3.0%; PU/DS 10%
dsDNA assay for adherent
bacteria E. coli (BL21 strain)
< 300 ng DNA/sample with DS contents of 0.66% and 10% (versus ≈ 700 ng DNA/sample in control) (51)
MeCe grafting PDMS Si-MeCe
Epifluorescence microscopy; Bioluminescence activity; Number of CFUs/cm2 S. aureus Xen36, P. aeruginosa Lm1
2-fold reduction on bacterial adhesion. 2 to 3-log
reduction of biofilm formation after 5 days. (58)
CH and Hep immobilization PU 0.5; PU-CH-2.0; PU-CH-Hep Number of CFUs/cm2; SEM S. aureus (ATCC 25923), S. epidermidis (ATCC 12228), E. coli (ATCC 11229) and P. aeruginosa (ATCC 27853)
90% to 99% reduction of bacterial adhesion in
PUh-CH-Hep. (69)
Heparinizable
modified PCU PCU Bionate®-PIME-Hep MTT test; SEM
S. aureus Cowan 1 and 8325-4, S. epidermidis RP62A and HB
2 to 4-log reduction of S. aureus and S.
4.1.3 Polyacrylamide and polyacrylates
Polyacrylamide (PAAm) is a neutral and polar macromolecular polymer that presents good stability, biocompatibility, hydrophilicity and at the same time is neither toxic nor immunogenic (66,76–80).
Although there are few studies that used PAAm hydrogels or brushes to coat the surface of biomaterials such as silicon, it has been proved that this polymer is able to resist to bacterial and protein adhesion (35,66,76,78,81).
With the aim of evaluating the antifouling properties of this polymer, I. Fundeanu et al. (82) covalently attached amino-poly(o-amino-p-xylylene-co-p-xylylene) (amino-PPX)-PAAm brushes to silicone rubber samples by atom transfer radical polymerization (ATRP) (Table 4). For the performance of bacterial adhesion tests two bacterial strains were selected, S. aureus (ATCC 12600) and a fluorescent strain of E. coli (E. coli 3.14) and the evaluation was conducted in a parallel plate flow chamber under moderate flow conditions. Fluorescence microscopy and initial deposition rates were used to evaluate the strategy. The obtained results showed a reduction of S. aureus and E. coli adhesion of 93% and 99%, respectively, on amino-PPX-PAAm brush-coated samples when compared to bare silicone rubber. Regarding the adhesion kinetics, S. aureus attached more than 10-fold slower after the amino-PPX–PAAm brush-coating (207 ± 58 cm-2 s-1 versus the initial deposition rate of 2515 ± 812 cm-2 s-1), and
E. coli adhesion was not detectable during the 4 h of the experiment (versus an initial deposition
rate of 34 ± 332 cm-2 s-1) (82).
In another study, the resistance to bacterial adhesion of some polyacrylates/acrylamides was investigated (83). For that a polymer microarray technique was used to identify polymers with resistance to adhesion of some clinically relevant bacteria. Of a library of 381 polyacrylates/acrylamides and PUs, only two polymers were chosen:
poly(methylmethacrylate-co-dimethylacrylamide) (PA13) and
poly(methoxyethylmethacrylate-co-diethylaminoethylacrylate-co-methylmethacrylate) (PA515). Polymer
poly(hydroxyethylmethacrylate-co-dimethylaminoethylmethacrylate) (PA155) was used as positive control. The authors coated pieces of a PU and silicone vascular catheters (Cath-1 and Cath-2, respectively) with PA13, PA515 and PA155. The efficacy of the coatings, was evaluated against two mixtures of bacteria: BacMix-1 (i.e. K. pneumoniae, Staphylococcus
saprophyticus and S. aureus) and BacMix-2 (i.e. K. pneumoniae, Streptococcus mutans, S. aureus and Enterococcus faecalis). Except for S. mutans (NCTC 10923) strain, the other
bacteria were isolated from infected medical devices. The results from bacterial tests were evaluated by confocal microscopy analysis and by SEM observation after 72 h and 12 days incubation, respectively. The obtained results showed that coating Cath-1 surfaces with PA515 reduced the attachment of bacteria in about 64% for BacMix-2, and PA13 coating showed an ≥ 96% reduction of bacterial adhesion for both BacMix-1 and BacMix-2. Which concerns to Cath-2, P515 coating has displayed a 19% reduction of bacterial adhesion with BacMix-1, and at least 82% reduction of bacterial adhesion with both bacterial mixtures on PA13-coated pieces. It was also concluded that PA13 and P515 had good potential as antiadhesive coatings for medical devices. However, PA13 was the polymer that presented better antiadhesive efficacy, making of it the best choice for catheter coating (Table 4) (83).
Table 4 – Use of PAAm and polyacrylates as antifouling strategies with possible application in catheters
Strategy Catheter material
Materials used on strategy
Methods used to test the strategy
Tested
microorganisms Results Ref.
T y pe o f ca thet er Va scula r Amino-PPX-PAAm brushes Silicone rubber Amino-PPX-PAAm silicone rubber Fluorescence microscopy; Adhesion kinetics S. aureus (ATCC 12600) and E. coli 3.14
Reduction of E. coli and S. aureus adhesion in 99%
and 93%, respectively. (82)
Polyacrylates/ acrylamides coatings
Polyurethane
and Silicone PA115; PA13; PA515
Confocal microscopy analysis; SEM K. pneumoniae, S. saprophyticus, S. aureus, E. faecalis, and S. mutans (NCTC 10923)
Observed a reduction between 19% and 64% with
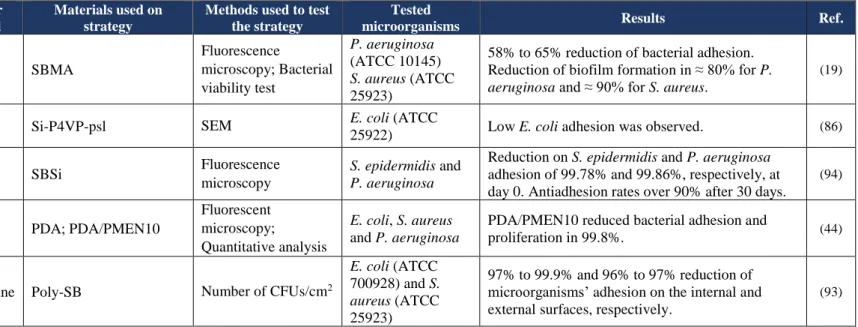
4.2 Zwitterionic Polymers
Zwitterions are neutral molecules that have both negatively and positively charged groups in the same molecular unit (18,40,46). They are very hydrophilic since they attach to water molecules through ionic bonds (1,15,31,33).
These polymers are biocompatible, stable against oxidation and, as several studies have demonstrated, they are also resistant to bacterial and nonspecific proteins adhesion. Such properties make of zwitterions good options to antifouling applications on biomedical field (1,32,46,70,84–89).
Zwitterions may be grouped into betaines and polyampholytes. However, the most commonly studied zwitterions are betaines, which are examples sulfobetaine (SB), carboxybetaine (CB) and phosphorylcholine (PC) (31,36,90–92).
4.2.1 Betaines
Of betaines, SB is the one that presents more studies with potential application on catheters or catheter-materials, followed by PC.
One of those studies was performed by R. Smith et al. (93) in 2012. This group of researchers grafted a non-leaching polymeric sulfobetaine (poly-SB) on the internal and external surfaces of a vascular catheter by redox polymerization process. To evaluate the antifouling properties of poly-SB grafting to PU catheters, the authors used suspensions of E.
coli (ATCC 700928) and S. aureus (ATCC 25923) to contaminate samples of bare and
poly-SB-coated catheters (Table 5). After a period of 24 h, the number of CFUs/cm2 on both internal and external surfaces of the samples was determined. The obtained data demonstrated that poly-SB coating reduced bacterial adhesion on 97% to 99.9% on the external surface of poly-
poly-SB-In another study, PDMS urinary catheters surfaces were modified with sulfobetaine methacrylate (SBMA) by radical polymerization (19). The antiadhesive and antibiofilm efficacy of SBMA-coating was evaluated against two bacteria models, P. aeruginosa (ATCC 10145) and S. aureus (ATCC 25923), under static and dynamic conditions. The obtained results from static condition tests were assessed by bacterial viability assessment and fluorescence microscopy. Compared to bare PDMS catheters, SBMA-coated catheters showed a S. aureus and P. aeruginosa adhesion of 42% and 35%, respectively. It was also performed a dynamic test by inserting both bare and SBMA-coated catheters into an artificial bladder model filled with artificial urine contaminated with the two types of bacteria. After 7 days, it was quantified the number of adhered bacteria on three different parts of the catheters: tip, balloon and urethra (the last one is referred to the portion below the balloon area). In case of P. aeruginosa, urethra of SBMA-coated catheters revealed a biofilm reduction of ≈ 80% compared to bare catheters. Also, it was registered a reduction of ≈ 90% in S. aureus biofilm formation in balloon portion of the SBMA-coated samples (Table 5) (19).
The efficacy of SB silane (SBSi) on resisting against bacterial adhesion was also evaluated by S. Yeh et al. (94). In that way, the researchers modified the surface of PDMS samples by covalent silanization of SBSi (Table 5). For the bacterial adhesion tests two bacterial strains were used (i.e. S. epidermidis and P. aeruginosa). The results were assessed by fluorescent microscopy. The authors concluded that the modification of PDMS surfaces with SBSi significantly reduced adhesion of both bacteria. In fact, comparing to bare PDMS, the coated samples prepared on day 0 (PDMS-SBSi-0D) presented a reduction on S. epidermidis and P. aeruginosa adhesion of 99.78% and 99.86%, respectively. In the aged samples (PDMS-SBSi-30D), P. aeruginosa adhesion remained almost the same as the fresh samples. However, the resistance to S. epidermidis adhesion registered a slight decrease. Nevertheless, the general antifouling rates of SBSi-coated samples were over 90% compared to bare PDMS (94).
More recently in 2017, C. Xing et al. (44) synthetized copolymers bearing PC and active ester groups (PMENs) by using monomers of 2-methacryloyloxyethyl phosphorylcholine (MPC) and p-nitrophenoxycarbonyloxyethyl methacrylate (NPCEMA) (Table 5) according to their reported method in 2012 (95). The synthetized polymer was named of PDA/PMEN10. Aiming to evaluate the efficacy of PDA/PMEN10 on preventing bacterial adhesion, the authors immobilized this zwitterionic polymer on the surface of PDA-precoated silicon wafers and used
E. coli, S. aureus and P. aeruginosa suspensions to perform bacterial tests. Fluorescence
reduced the adhesion of the three types of tested bacteria in 99.8%, compared with the bare silicon wafers (44).
Despite the good antifouling properties demonstrated by betaines, these compounds are prone to hydrolysis under physiological conditions, since they have ester or amide bonds in their structures (86,96).
With the purpose to find an alternative to this problem, Y. Sun et al. (86) developed a more stable and non-degradable alternative (Table 5). For this, this group of investigators first modified a silicon wafer with triethoxyvinylsilane obtaining reactive double bonds on the surface. Then, polymer 4-vinyl pyridine (P4VP) was grafted onto the silicon surface by “grafting from” technique followed by 1,3-propanesultone quaternization, obtaining the substrate Si-P4VP-psl. Aiming to evaluate the resistance to bacterial adhesion of this zwitterionic coating, the authors used an E. coli (ATCC 25922) suspension to perform bacterial tests. SEM images revealed that Si-P4VP-psl surface presented less adhered bacteria than bare silicon wafer. However, the authors pointed out that the long term effectiveness of this brushes must be explored in the future (86).
Table 5 – Use of zwitterions as antifouling strategies with possible application in catheters
Strategy Catheter material
Materials used on strategy
Methods used to test the strategy
Tested
microorganisms Results Ref.
T y pe o f ca thet er Urina ry
SBMA coating PDMS SBMA
Fluorescence microscopy; Bacterial viability test P. aeruginosa (ATCC 10145) S. aureus (ATCC 25923)
58% to 65% reduction of bacterial adhesion. Reduction of biofilm formation in ≈ 80% for P.
aeruginosa and ≈ 90% for S. aureus.
(19)
Zwitterionic
P4VP-psl brush Silicone Si-P4VP-psl SEM
E. coli (ATCC
25922) Low E. coli adhesion was observed. (86)
SBSi PDMS SBSi Fluorescence
microscopy
S. epidermidis and P. aeruginosa
Reduction on S. epidermidis and P. aeruginosa adhesion of 99.78% and 99.86%, respectively, at day 0. Antiadhesion rates over 90% after 30 days.
(94)
Va
scula
r
PDA/PMEN10
coating Silicone PDA; PDA/PMEN10
Fluorescent microscopy;
Quantitative analysis
E. coli, S. aureus
and P. aeruginosa
PDA/PMEN10 reduced bacterial adhesion and
proliferation in 99.8%. (44)
Poly-SB Polyurethane Poly-SB Number of CFUs/cm2
E. coli (ATCC
700928) and S.
aureus (ATCC
25923)
97% to 99.9% and 96% to 97% reduction of microorganisms’ adhesion on the internal and external surfaces, respectively.
4.3 Other antifouling coatings
4.3.1 Amphiphilic polymers
Amphiphilic (also known as amphipathic) polymers are molecules with high molecular weight that have a hydrophilic and a hydrophobic portion on its structure (85,97,98).
These polymers can provide a diversity of properties such as flexibility, durability, higher stability and resistance to biofouling (85,91,99,100). These characteristics increased the interest on the application of these molecules on diverse areas such as biomaterials and medical care (97).
The coating of surfaces with amphiphilic polymers may be achieved through grafting process or blending with bulk materials, for example (98,99).
Some studies have been recently developed with the purpose of investigating the antifouling properties of these type of polymers. One of those studies was performed by J. Jiang
et. al (85) that developed a novel amphiphilic PDMS-based PU networks tethered with CB
(Table 6). These networks were synthetized by a crosslink reaction of di/tri isocyanate and t-butyl protected CB (PCB) as the hard domain, and siloxane diol as the soft domain. For the synthesis of PU networks, the authors used PDMS:PCB molar ratios of (4.5:0), (3:1.5), (1.2:3.3) and (0:4.5), obtaining the networks PU-0, PU-1, PU-2 and PU-3, respectively. Finally, the samples suffered an acid hydrolysis so the t-butyl group could be removed and the zwitterionic properties of CB were acquired by the samples. For the bacterial adhesion tests, an E. coli suspension was used, and the density of bacterial cells was assessed by fluorescence microscopy images. The obtained results allowed to conclude that all the synthetized amphiphilic PU networks demonstrated excellent results on resisting to E. coli adhesion, compared to epoxy resin (control). Comparing with control ((26.6 ± 0.8) x 105 cells/cm2), PU-3 was the sample with the best antifouling properties, with an adherent bacteria density of (1.8 ± 0.4) x 105
of coated PDMS samples, two microbial strains, S. aureus (ATCC 25923) and a fluorescent mCherry-marked E. coli, were used to incubate the samples for 8 h and 24 h. WST-1 assay results demonstrated that independently of poly(DMA-mPEGMA-AA) concentration, all the tested surfaces showed a reduction on S. aureus adhesion (only 1 to 6% adhesion compared to the uncoated PDMS surfaces). Relatively to mCherry-marked E. coli, fluorescence microscopy demonstrated a reduced bacterial adhesion on all coated surfaces. The researchers also evaluated the long-term efficacy of this coating for a period of 7 days. SEM images revealed few bacterial adhesion and colonization on the surface of coated PDMS. Tests conducted on silicone urinary catheters under flow conditions revealed that poly(DMA-mPEGMA-AA) coating was able to reduce S. aureus adhesion in approximately 77% at the end of 2 days. In
vivo assays experiments were also performed. For this, the authors implanted uncoated or
polymer-coated catheters subcutaneously at the right flank of mice and 2 days later they injected phosphate buffered saline (PBS) or S. aureus suspension into the site containing the catheters. After 4 days, all the poly(DMA-mPEGMA-AA)-coated catheters previously inoculated with S.
aureus revealed low microbial colonization, with a optical density (O.D.) of 0.102 (versus 0.770
of the uncoated samples)(28).
4.3.2 Modified Polyurethanes
Segmented PUs are constituted by alternating hard and soft domains. The hard domains are often formed by diisocyanates and chain extenders, and the soft domains are formed by macrodiols (27,101–105).
These PUs have good blood-compatibility, present good flexibility and mechanical strength conferred by the soft and hard domains, respectively (27,73,103,106). Such properties have increased the use of these polymers on the manufacture of medical devices (69,73,106).
To date, very few researchers have modified these polymers to create PUs able to reduce microbial adhesion.
Still, few studies were developed and one of those was in 2012 by Francolini et al. (73) who synthetized new heparin-mimetic segmented PUs, by adding sulfate or sulfamate groups (known to be responsible for the Hep activity) to the side chain of carboxylated PUs (PEUA) (Table 7). The first step in this synthesis was the amidation of PEUA with ethanolamine (EA), serinol (S) or ethylene diamine (ED), obtaining the polymers PEUEA, PEUS and PEUED, respectively. In the second step, PEUEA and PEUS reacted with pyridine-SO3 adduct and
PEUED reacted with DMF-SO3, resulting in the sulfated polymers PEUEA–SO3H, PEUS–
SO3H and PEUED–SO3H, respectively. A suspension of S. epidermidis (ATCC 35984) was
used to perform in vitro bacterial tests. The obtained data from SEM observation demonstrated that sulfonated polymers completely inhibited bacterial colonization, and PEUS-SO3H (with a
higher composition in –SO3H groups) demonstrated excellent antifouling properties (73).
The effect of polymer hydrophilicity and the degree of hard/soft domain separation on antifouling properties of PUs was also assessed. For this, Francolini et al. (106) synthetized four new segmented PUs with the same hard domain, but a variable soft domain (Table 7). The
hard domain was constituted by methylene-bis-phenyl-isocyanate (MDI) and dihydroxymethyl-propionic acid (DHMPA). On the other hand, the soft domain was constituted by one of the following three macrodiols: polycaprolactide (PCL), poly-l-lactide (PLA) or polypropylenoxide (PPO). For the PCL and PLA-based PUs the soft domain content per repeat unit of polymer was 0.50 (expressed as molar fraction) (PCL0.50-UA and PLA0.50-UA,
respectively). With the aim of evaluating the influence of soft domain content in the polymers, PPO-based PUs were synthetized with two different contents of soft domain: 0.50 (PPO0.50
-UA) and 0.65 (PPO0.65-UA). The antifouling properties of segmented PUs was studied using a
suspension of S. epidermidis (ATCC 35984). The results obtained by SEM imaging allowed the authors to conclude that the type of soft domain had influence on bacterial adhesion, with PLA0.50-UA showing the best antifouling results (2 x 102 CFUs/cm2), followed by PCL0.50-UA
(1 x 105 CFUs/cm2) and PPO0.50-UA (1 x 107 CFUs/cm2). Relatively to the amount of soft
domain, PPO0.65-UA demonstrated only 1-log reduction on S. epidermidis adhesion comparing
to PPO0.50-UA. Additionally, after 24 h incubation, it was observed the formation of a mature
S. epidermidis biofilm on PPO0.50-UA surface, only a few single cells or small bacterial
aggregates on PCL0.50-UA and no S. epidermidis colonization on PLA0.50-UA surface, making
surface (29,92,107,108). The lubricating liquid has to be stable, non-toxic and with high affinity to the substrate (Figure 3) (29,35,100).
SLIPS surfaces started to be studied in non-medical field. More recently, the interest in the possible application of this strategy on biomedical devices increased the number of studies in this area (15,35,92,108).
One of those studies was performed by A. Epstein et al. (100) that infused a porous Teflon® membrane with Krytox® 103, a perfluorinated liquid that acts as lubricating fluid
(Table 8). P. aeruginosa PA14, S. aureus SC01 and E. coli ZK2686 strains were used to perform bacterial tests under static and flow conditions. The results were assessed by fluorescence microscopy and crystal violet absorbance. The results showed very low microbial adhesion on SLIPS surfaces. Under static conditions, SLIPS surfaces demonstrated a reduction of S. aureus and E. coli adhesion of 97.2% and 96%, respectively. After 7 days under flow conditions, SLIPS surfaces reduced P. aeruginosa biofilm formation in about 99.6%, and the fluorescence signal reduced ≈ 98% (100).
Two years later, another group of researchers covalently linked liquid perfluorocarbon (TLP) on the surface of PVC medical tubing and polyethylene terephthalate (PET) samples, and then infused it with a mobile layer of perfluorodecalin (107) (Table 8). To determine the antifouling efficacy of these SLIPS surfaces, P. aeruginosa and E. coli (ATCC 8739) were used as bacteria models on bacterial adhesion tests. The results were assessed by SEM and crystal violet staining, and it demonstrated that TLP-coated PET significantly reduced P. aeruginosa and E. coli adhesion. Also, after 6.5 weeks of incubation, TLP-coated PVC tubes showed an 8-fold reduction on P. aeruginosa biofilm development (107).
Nano/micropatterned surface Lubricating
Liquid
4.3.4 Sharklet topography
Sharklet technology is an antifouling strategy inspired on the surface topography of sharkskin (15,35,38,109,110). In fact, the skin of this aquatic animal presents a diamond-like micro-geometry that prevents the adhesion of algae and bacteria (35,90,111).
The application of this technology on biomedical surfaces has been studied through the years with the aim of reducing bacterial adhesion to these devices (15,35,110).
In one of these studies, K. Chung et al. (109) developed a Sharklet AF™ topography with protruding features on the surface of PDMS samples, by replication of silicon wafer molds. The used design presented 2 µm feature width and spacing, and 3 µm height. Aiming to evaluate the antiadhesive and antibiofilm properties of this strategy, Sharklet AF™ and bare PDMS samples were incubated in a S. aureus (ATCC 29213) suspension (Table 9). The results were assessed by SEM at the end of 0, 2, 7, 14 and 21 days. The obtained data showed a bacterial coverage on Sharklet AF™-PDMS of 0% on day 0, 7% on day 14 and 35% on day 21. Additionally, only at day 21 S. aureus biofilms started to appear in small and isolated areas of Sharklet AF™ surfaces. Until that day no evidence of early-stage biofilm formation was observed (109).
Aiming to evaluate the ability of Sharklet micropattern surfaces on prevention of E. coli (ATCC #700336) adhesion and biofilm formation on silicone urinary catheters, S. Reddy et al. (112) developed three different Sharklet topographies on the surface of silicone samples: a positive Sharklet with protruding features (2 µm width and spacing), an inverse Sharklet with recessed features (2 µm width and spacing) and a Sharklet with protruding features (10 µm width and 2 µm spacing). The bacterial adhesion and biofilm formation after 24 h, 48 h, 3 days and 7 days was assessed by SEM. The results allowed the authors to conclude that all the tested Sharklet surface modifications significantly reduced E. coli adhesion and area coverage. Also, the colonies size reduced ≈ 76% on Sharklet surfaces with 2 x 2 and 10 x 2 features and 80%
laser scanning microscopy (LSM) (Table 9). The obtained results demonstrated that, after a period of 18 h-incubation, -3SK2x2 topography reduced S. aureus adhesion in 63% to 70%, and S. epidermidis attachment in about 71%. Besides, both +3SK2×2 and -3SK2×2 PU surfaces revealed smaller bacterial colonies than control PU (111).
More recently, in 2017, A. Magyar et al. (113) and V. Arthanareeswaran et al. (114) published the results from a phase I randomized clinical trial that had the aim of investigate the efficacy of Sharklet catheters on prevention of CAUTIs. In this study, fifty eligible adult men who needed urethral catheterization during a period of 3 to 30 days were randomized into 2 groups. The first group was catheterized with unmodified silicone Foley catheters, and the second group with Sharklet micropattern catheters. After catheters removal, bacterial adhesion and biofilm formation on three different parts of the catheters (tip, middle and base) were evaluated by SEM imaging. The incidence of symptomatic urinary tract infection (UTI), pain, discomfort and the presence of bacteria in urine were also investigated. Which concerns to Sharklet micropattern catheters, SEM images revealed a significant reduction on biofilm formation. Also, the pain and discomfort in patients using the topographically modified catheters were seriously lower when compared with patients using unmodified catheters. These results have revealed promising and may lead to more studies in the area of topographical modifications of catheter surfaces in the future (113,114).