Adaptation of the tetrazolium test method for estimating
the viability of sorghum seeds
1Tereza Cristina de Carvalho
2*, Camila Ribeiro de Souza Grzybowski
2,
Osvaldo de Castro Ohlson
3, Maristela Panobianco
2ABSTRACT – The tetrazolium test is part of internal programs of seed quality control because it allows a quick, reliable and accurate assessment of seed viability. The purpose of this study was to determine, among the various methodologies suggested in the literature, a practical and efficient procedure for carrying out the tetrazolium test in sorghum seeds, with a view toward a rapid estimate of their viability. Four seed lots of the simple hybrid Buster were used, testing two forms of seed pre-conditioning (direct immersion in water and between moistened paper towels); two types of preparation (longitudinal cut of the seed through the embryo with immersion of one of the halves in the tetrazolium solution or placement of the two halves on filter paper moistened with tetrazolium solution); two forms of staining (on paper and with direct immersion in the solution) and three concentrations of tetrazolium solution (0.1%, 0.5% and 1.0%). The tetrazolium test may be efficiently conducted for sorghum seeds through pre-conditioning between paper towels for 18 hours at 20 °C, with immersion staining of one half of the seed in tetrazolium solution at 0.1% for three hours at 40 °C.
Index terms: Sorghum bicolor (L.) Moench, quality control, physiological potential.
Adequação da metodologia do teste de tetrazólio para estimar
a viabilidade de sementes de sorgo
RESUMO – O teste de tetrazólio está inserido nos programas de controle interno de qualidade das sementes, pois permite obter a viabilidade das sementes com rapidez, confiabilidade e precisão. O trabalho objetivou determinar, dentre as várias metodologias indicadas na literatura, um procedimento prático e eficiente para a condução do teste de tetrazólio em sementes de sorgo, visando estimar rapidamente a sua viabilidade. Foram utilizados quatro lotes de sementes do híbrido simples Buster, testando-se duas formas de pré-condicionamento da semente (imersão direta em água e entre folha de papel toalha umedecida); dois tipos de preparo (corte longitudinal da semente através do embrião com imersão de uma das metades na solução de tetrazólio ou com colocação das duas metades sobre papel filtro umedecido com solução de tetrazólio); duas formas de coloração (sobre papel e com imersão direta na solução) e três concentrações de solução de tetrazólio (0,1%, 0,5% e 1,0%). O teste de tetrazólio para sementes de sorgo pode ser conduzido eficientemente mediante o pré-condicionamento entre papel por 18 horas a 20 °C, com coloração por imersão de uma metade da semente em solução de tetrazólio a 0,1%, por três horas, a 40 °C.
Termos para indexação: Sorghum bicolor (L.) Moench, controle de qualidade, potencial fisiológico.
1Submitted on 09/14/2012. Accepted for publication on 12/06/2012. 2Departamento de Fitotecnia e Fitossanitarismo,Universidade Federal do
Paraná (UFPR), 80035-050 - Curitiba, PR,Brasil.
3Laboratório Oficial de Análise de Sementes, Empresa Paranaense de
Classificação de Produtos (CLASPAR), 80035-060 – Curitiba, PR, Brasil.
*Corresponding author: < tcdcarva@gmail.com>
Introduction
Sorghum (Sorghum bicolor (L.) Moench) has become a crop choice for farmers because it is drought-resistant
(Habyarimana et al., 2004; Rajarajan and Ganesamurthy, 2011;
Tesso et al., 2005), compared with maize. In Brazil, sorghum is almost exclusively grown as animal feed, in the form of pasture, silage and feed composition (Resende et al., 2011; Von Pinho et al., 2006; Von Pinho et al., 2007).
Within the seed production process, physiological quality
assessment is a key factor in determining its use (Baalbaki et al., 2009). The interest in establishing rapid methods to determine seed viability, especially for storage and commercial purposes, led to the development of the tetrazolium test. As stated by Tunes et al. (2009), this test is important for quality control, because it offers rapid assessment of seed viability, including dormant seeds, in less than 24 hours (Costa et al., 2007), and this is crucial to speed up decision-making at different stages of the seed production process (Bhering et al., 2005). Sorghum seeds may exhibit dormancy when freshly harvested, and using the tetrazolium test is really advantageous in this case because it allows for results of viability over a very short period of time when compared with the germination test, which can take up to 17 days to yield results for dormant seeds (Brasil, 2009).
The test is based on the activity of dehydrogenase enzymes that catalyze respiratory reactions in the mitochondria, resulting in the formation of a stable and non-diffusible red compound, formazan (França-Neto et al., 1998). Thus, it is possible to analyze seed viability by identifying the stained areas in the embryo (ISTA, 2008).
For sorghum seeds, the literature suggests several methodologies for conducting the tetrazolium test. The Rules for Seed Testing (Brasil, 2009) present two ways of pre-conditioning (between paper towels or direct immersion in water), two staining procedures, different concentrations of tetrazolium solution (0.5 to 1.0% ) and several seed residence times in the solution (from three to 24 hours). The International Seed Testing Association - ISTA (ISTA, 2008) indicates direct soaking of seeds in water for 18 hours at 7 °C, followed by longitudinal cut of the embryo and ¼ of the endosperm and staining at 1.0% concentration of the tetrazolium solution for three hours.
Fogaça et al. (2011), working with some of the test variables, recommended removing the testa of sorghum seeds prior to staining. It should be emphasized, however, that this preparation can physicaly damage the tissues of the embryonic axis, and make the test procedure more time consuming.
Despite the importance of the crop, there are different recommendations in the literature for the methodology for conducting the tetrazolium test, and this hinders the choice of the most appropriate procedure. The present study aimed to determine, among the various protocols indicated in the
literature, a practical and efficient method to estimate the
viability of sorghum seeds by the tetrazolium test.
Material and Methods
The research was conducted at the Laboratory of Seed Analysis, Department of Plant Technology and Plant Health,
Federal University of Paraná, Curitiba-PR, from July 2011 to March 2012. Four seed lots of sorghum from simple hybrid Buster (season 2009/10) were used.
The seed samples were homogenized in a centrifugal divider, based on the criteria of Rules for Seed Analysis (Brasil, 2009). During the experimental period, the seeds were stored in Kraft paper bags under controlled environment (17 °C and 55-60% Relative Humidity), to minimize the intensity of deterioration.
The initial quality assessment of the lots was performed by determining water content and germination percentage. Water content was measured by the oven method at 105 ± 3 °C for 24 hours, using two subsamples of 5.0 g of seeds per lot (Brasil, 2009).
The germination test was conducted with four replications of 50 seeds per plot, divided into paper towel rolls moistened with water in amounts equivalent to 2.0 times the weight of the dry substrate and kept in a germination chamber at 25 °C. Seedling assessment was performed on the 10th day after sowing (Brasil, 2009) and the results were expressed as the percentage of normal seedlings.
To study the tetrazolium test (TZ), four subsamples of 50 seeds per lot were used, and the following procedures were tested:
1. Seed pre-conditioning:
a) seeds stored between paper towels moistened with water equivalent to 2.0 times the weight of the paper for 18 hours at 20 °C (Brasil, 2009);
b) direct immersion in 30 mL of water, placed in a glass beaker (capacity = 100 mL) for 18 hours at 7 °C (ISTA, 2008) and 20 °C (Brasil, 2009).
After pre-conditioning, seed water content was determined by the oven method at 105 ± 3 °C (Brasil, 2009), according to the procedure described previously.
2. Preparation: longitudinal bisection along the embryo and endosperm (Figure 1A) by:
a) placing the two halves on a sheet of filter paper
moistened with a solution of tetrazolium equivalent to 2.5 times the weight of the paper.
b) discarding one half and immersing the other half in 5 mL tetrazolium solution in a plastic beaker (capacity = 50 mL).
3. Staining:
a) at 30 °C (Brasil, 2009; ISTA, 2008) and 40 °C (Souza et al., 2009), at concentrations of 0.1% (Dias and Barros, 1995), 0.5% (Brasil, 2009) and 1.0% (ISTA, 2008) for three hours, for preparation a;
After each period of staining, the seeds were kept on fi lter
paper (staining on paper) or immersed in refrigerated water at 5-10 °C (immersion staining) until the time of assessment, which was performed on the same day of staining.
The viability assessment of sorghum seeds was adapted from the methodology used for maize (Dias and Barros, 1995). Figure 1B shows the details of cutting and seed morphology of sorghum, highlighting the vital areas (coleoptile, plumule, mesocotyl, radicle, coleorhiza and scutellum region).
The following criteria were used for distinguishing seed viability by the tetrazolium test:
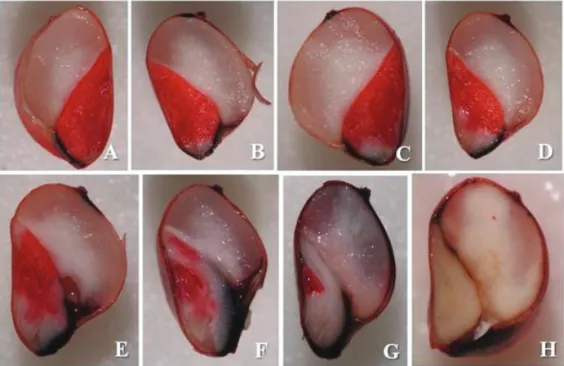
- viable seeds: light carmine, uniform staining, and no damage to the embryo (Figure 2A), or with small damaged areas or dead tissue at the ends of the scutellum, without reaching the vital region (Figure 2B); damage reaching the radicle and part of the scutellum, but with intact mesocotyl (Figure 2C); intense red color of the embryo region, indicating
further deterioration of the injured tissue, damage or discolored
areas on larger regions of the end of the scutellum, as long as they do not reach the vital regions of the embryo (Figure 2D).
- non-viable seeds: intense staining of the embryo with discolored areas on the plumule, the region of the coleoptile and the radicle; presence of discolored areas on the plumule and radicle region, or discolored areas on the coleoptile, the radicle and part of the scutellum; damaged or discolored regions in the mesocotyl and part of the scutellum; damaged or discolored regions in the mesocotyl, radicle region, coleorhiza and part of the scutellum (Figures 2E and 2G); damaged or discolored regions in the central portion of the scutellum or all of the scutellum (Figure 2F), or the fully discolored embryo (Figure 2H).
Figure 1. Cut of sorghum seed (A) and dry sorghum fruit “caryopsis” (B), with emphasis on the scutellum (b.1), coleoptile (b.2), plumule (b.3), mesocotyl (b.4 ), root (b.5) and coleorhiza (b.6).
Figure 2. Sorghum seeds after staining with tetrazolium: viable seeds (A, B, C and D) and non-viable seeds (E, F, G and H).
Data from each test (germination and tetrazolium) were analyzed in a completely randomized design with four replications, and the means were compared by Tukey’s
test (p ≤ 0.05). Data for water content were not statistically
analyzed.
Results and Discussion
After the initial assessment of seed quality performed
by the germination test (Table 1), the lots were classifi ed as
by the tetrazolium test conducted with seed preconditioning between paper towels. It is found that when staining is performed on paper towel at 30 or 40 °C in any of the concentrations of the tested tetrazolium solutions, it was not possible to rank the lots similarly to the germination test (Table 1). In contrast, immersion staining (Table 3, bottom) in the tetrazolium solution of 0.1% at 40 °C
produced the same seed viability classification for the lots
as that of the germination test (Table 1), i.e., lots 1 and 2 were ranked as having the best quality, lot 3 as having intermediate viability, and lot 4 was considered to have the worst performance.
The immersion staining procedure is recommended by ISTA (2008) for sorghum seeds, but at a concentration of 1.0% at 30 °C. The results obtained in the present study revealed the possibility of using the solution with the lowest concentration (0.1%), which not only is more economical, but also allows proper staining of seed tissues, without reducing
the classification of viability. Reducing the concentration
of the tetrazolium solution used for staining seeds has also been suggested for other species, such as 0.075% for cotton (Cervi and Mendonça, 2009) and 0.2% for the castor oil plant (Gaspar-Oliveira et al., 2009).
Table 4 shows the results obtained with the method of seed pre-conditioning by soaking in water at 7 °C. When the two seed halves were stained on paper towel at 30 °C, in all tested tetrazolium solutions, there was no statistical difference in the lots of the experiment.
When conducting the test at a staining temperature of 40 °C, a difference was observed between lots; however, the
classification of the lots at viability levels was not similar to
that of the germination test (Table 1).
When using immersion staining of half seed (Table 4, bottom) with tetrazolium solution of 0.1% at 30 or 40 °C, it was not possible, once again, to rank the lots similarly to the germination test (Table 1).
When using the methodology of pre-conditioning in water at 20 °C with staining on paper towels at 30 °C (Table 5, top), only at the concentration of the tetrazolium solution
of 0.5%, the classification of viability for the lots was the
same as that of the germination test (Table 1). The placement of the two halves allows a better evaluation of seed viability because the two halves of the seed can be analyzed. The estimation of seed viability by the tetrazolium test, through the analysis of the whole embryo, was also recommended for seeds of Brachiaria (Novembre et al., 2006; Dias and Alves, 2008a), guinea grass (Dias and Alves, 2008b), black oat (Souza et al., 2009), triticale (Souza et al., 2010a) and barley (Grzybowski et al., 2012).
Data on initial water content were similar for the four lots under study, and they showed an increase of 0.8 percentage points between the highest and lowest humidity (Table 1). The uniformity of this variable between lots is critical for standardizing assessments and producing consistent results (Marcos-Filho, 2005).
Table 2 shows the water content of the lots after preconditioning by immersion and between paper towels. In preconditioning between paper towels, the seeds reached a lower percentage of water (values ranging from 23.6 to 26.2%) compared with treatment by immersion (range from 28.5% to 36.4%), considering the same hydration period (18 hours).
Table 1. Water content and seed germination of four lots of sorghum.
Seed lots Germination Water content
--- % ---
1 93 a 12.4
2 92 a 11.7
3 88 b 12.0
4 82 c 11.6
C.V. (%) 1,8 -
Means followed by the same lowercase letter in the column do not differ by
Tukey’s test (p ≤ 0.05).
Table 2. Water content of four lots of sorghum seeds after seed pre-conditioning between paper towels (20 °C) and by immersion (7 and 20 °C) for 18 hours.
Seed lots
Water content after preconditioning Between paper towels By immersion
20 °C 7 °C 20 °C
---%---
1 26.2 30.2 36.4
2 24.8 29.5 33.1
3 23.6 29.4 32.7
4 23.6 28.5 32.4
Preconditioning causes softening of seeds (Schabes and Sigstad, 2006), which facilitates the preparation and penetration of the tetrazolium solution, and activates the enzyme systems, thus resulting in a sharper color (Chamma and Novembre, 2007). The seed must achieve a minimum water content during the preconditioning for the correct activation of the enzyme system, ensuring proper development of the stain in the tissue and reliable results (Oliveira et al., 2005).
Table 3. Seed viability of four lots of sorghum by the tetrazolium test, conducted with preconditioning between paper towels at 20 °C with staining on paper and by immersion, and different concentrations of tetrazolium solution.
Seed lots
Preconditioning between paper towels at 20 °C Staining on paper
30 °C 40 °C
Concentrations of the solution Concentrations of the solution
0.1% 0.5% 1.0% 0.1% 0.5% 1.0%
... %...
1 89 a 92 a 90 a 89 a 90 a 91 a
2 83 ab 90 a 85 a 88 ab 85 a 86 ab
3 80 bc 86 a 82 a 71 c 85 a 85 ab
4 75 c 90 a 86 a 77 bc 83 a 78 b
C.V. (%) 3.9 5.6 6.0 6.7 4.5 5.6
Seed lots
Immersion staining
30 °C 40 °C
Concentration of the solution Concentration of the solution
0.1% 0.1%
...%...
1 92 a 92 a
2 85 b 92 a
3 93 a 86 b
4 90 ab 80 c
C.V. (%) 4.0 2.8
Means followed by the same letter in the column do not differ by Tukey’s test (p ≤ 0.05).
Table 4. Seeds viability of four lots of sorghum by the tetrazolium test, conducted with preconditioning by immersion at 7 °C, with staining on paper, and by immersion, adopting different concentrations of tetrazolium solution.
Seed lots
Preconditioning by immersion at 7 °C Staining on paper
30 °C 40 °C
Concentrations of the solution Concentrations of the solution
0.1% 0.5% 1.0% 0.1% 0.5% 1.0%
...%...
1 61 a 81 a 80 a 90 a 86 a 86 a
2 59 a 81 a 74 a 87 ab 85 a 75 b
3 66 a 84 a 71 a 85 ab 68 b 60 c
4 72 a 78 a 71 a 81 b 65 b 70 b
C.V. (%) 11.5 8.3 6.4 5.1 5.0 3.9
Seed lots
Immersion staining
30 °C 40 °C
Concentration of the solution Concentration of the solution
0,1% 0,1%
...%...
1 80 b 68 a
2 86 a 73 a
3 78 b 80 a
4 80 b 78 a
C.V. (%) 3.3 13.1
Means followed by the same letter in the column do not differ by Tukey’s test (p ≤ 0.05).
When preconditioning is performed by immersion in water at 20 °C, and stained by soaking seed halves (Table 5, bottom) in the tetrazolium solution of 0.1% at 30 °C, none of the tested methodologies allowed to classify the viability of
lots similarly to the germination test (Table 1).
tetrazolium test in sorghum. Both pre-conditioning between paper towels (staining of seeds by soaking at 40 °C / 0.1% tetrazolium chloride) or by immersion (seed staining on
paper towel at 30 °C / 0.5% tetrazolium chloride), the distinction of quality of the lots was similar to that of the germination test.
Table 5. Seed viability of four lots by the sorghum tetrazolium test conducted with pre-conditioning by immersion at 20 °C with staining on paper and by immersion, adopting different concentrations of tetrazolium salt.
Seed lots
Preconditioning by immersion at 20 °C Staining on paper
30 °C 40 °C
Concentrations of the solution Concentrations of the solution
0.1% 0.5% 1.0% 0.1% 0.5% 1.0%
...%...
1 91 a 93 a 90 a 72 ab 80 a 92 a
2 81 b 92 a 83 b 79 a 82 a 89 ab
3 80 b 79 b 82 b 67 ab 69 a 84 bc
4 71 c 69 c 81 b 66 b 68 a 80 c
C.V. (%) 4.3 2.8 1.9 8.2 11.2 4.0
Seed lots
Immersion staining
30 °C 40 °C
Concentration of the solution Concentration of the solution
0.1% 0.1%
...%...
1 88 ab 89 a
2 91 a 87 ab
3 82 bc 83 ab
4 78 c 81 b
C.V. (%) 5.0 3.8
Means followed by the same letter in the column do not differ by Tukey’s test (p ≤ 0.05).
This fact shows that these preconditioning procedures, in the tested combinations, provided activation of enzymatic metabolism, which resulted in adequate staining for assessment
and classification of lots in a similar way to that obtained in
the germination test (Table 1). The use of a temperature of 20 °C for a period of 18 hours, in seed pre-conditioning by immersion or between paper towels, is also recommended to black-oat seeds, triticale and white-oat (Souza et al., 2009; Souza et al., 2010a; Souza et al., 2010b).
The preparation of sorghum seeds in the longitudinal bisection along the embryo and endosperm with immersion
staining of half seed was efficient in distinguishing seed
viability, providing greater speed in preparation compared with the staining method of two halves on paper towel. It is noteworthy that staining the two halves of the sorghum seed allows the visualization of the whole seed as well as the characterization of viability. However, more time is needed to prepare the seeds to be placed on the paper towel moistened with the solution; moreover, for conducting this methodology, it is necessary to use a higher tetrazolium solution concentration (0.5%), compared with the preparation by immersion (0.1%).
Figure 2 shows the tissues of sorghum seeds, after staining one half of the seed in tetrazolium solution, as well as viable seeds (Figures 2A, 2B, 2C and 2D) and non-viable seeds (Figures 2E, 2F, 2G and 2H). It was observed that the seeds considered to be viable showed light carmine staining in the vital areas, thus there may have been a small area of dead tissue, although without affecting the embryonic axis. The non-viable seeds showed a part (including the embryonic axis) or all of the embryonic tissue discolored since there was no reduction of tetrazolium salt.
Conclusions
The tetrazolium test can be conducted in a practical and
efficient way in sorghum seeds by preconditioning between
paper towels for 18 hours at 20 °C with immersion staining one half of the seed in the tetrazolium solution 0.1% for three hours at 40 °C.
Acknowledgements
References
BAALBAKI, R.; McDONALD, M.B.; ELIAS, S.G.; MARCOS-FILHO, J.
Seed vigor testing handbook. Ithaca, New York: Association of Official Seed
Analysts, 2009. 346p. (Contribution 32 to the Handbook on Seed Testing).
BHERING, M.C.; DIAS, D.C.F.S.; BARROS, D.I. Adequação da
metodologia do teste de tetrazólio para avaliação da qualidade fisiológica de
sementes de melancia. Revista Brasileira de Sementes, v.27, n.1, p.176-182, 2005. http://www.scielo.br/pdf/rbs/v27n1/25196.pdf
BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para
análise de sementes. Ministério da Agricultura, Pecuária e Abastecimento.
Secretaria de Defesa Agropecuária. Brasília: MAPA/ACS, 2009. 395p. http:// www.bs.cca.ufsc.br/publicacoes/regras%20analise%20sementes.pdf
CERVI, F.; MENDONÇA, E.A.F. Adequação do teste de tetrazólio para sementes de algodoeiro. Revista Brasileira de Sementes, v.31, n.1, p. 177-186, 2009. http://www.scielo.br/pdf/rbs/v31n1/a20v31n1.pdf
CHAMMA, H.M.C.P.; NOVEMBRE, A.D.L.C. Teste de tetrazólio para as sementes de milho: períodos de hidratação e de coloração das sementes.
Revista Brasileira de Sementes, v.29, n.2, p.125-129, 2007. http://www.
scielo.br/pdf/rbs/v29n2/v29n2a17.pdf
COSTA, N. P.; FRANÇA-NETO, J.B.; KRZYZANOWSKI, F.C.; HENNING,
A.A. Metodologia alternativa para o teste de tetrazólio em semente de soja -
Série Sementes. Circular técnica 39, Londrina, Paraná. 2007. 8p. http://www. cnpso.embrapa.br/download/pdf/cirtec39_sementes.pdf
DIAS, M.C.L.L.; ALVES, S.J. Avaliação da viabilidade de sementes de
Brachiaria brizantha (Hochst. Ex A. Rich) Stapf pelo teste de tetrazólio.
Revista Brasileira de Sementes, v.30, n.3, p.145-151, 2008a. http://www.
scielo.br/pdf/rbs/v30n3/19.pdf
DIAS, M.C.L.L.; ALVES, S.J. Avaliação da viabilidade de sementes de
Panicum maximum Jacq pelo teste de tetrazólio. Revista Brasileira de Sementes, v.30, n.3, p.152-158, 2008b. http://www.scielo.br/pdf/rbs/v30n3/20.pdf
DIAS, M.C.L.L.; BARROS, A.S.R. Avaliação da qualidade de sementes de milho. Circular 88, Londrina: IAPAR, 1995. 42p.
FOGAÇA, C.A.; COSTA, R.C.; SIMONI, F.; GEROLINETO, E. Teste de tetrazólio em sementes de Sorghum bicolor L. – Poaceae. Revista de Biologia
e Ciências da Terra, v.11, n.1, p.182-187, 2011. http://redalyc.uaemex.mx/
src/inicio/artpdfred.jsp?icve=50021097020
FRANÇA-NETO, J.B.; KRZYZANOWSKI, F.C.; COSTA, N.P. O teste de
tetrazólio em sementes de soja. Londrina: EMBRAPA-CNPSo, 1998. 72p.
GASPAR-OLIVEIRA, C.M.; MARTINS, C.C.; NAKAGAWA, J. Concentração da solução de tetrazólio e período de coloração do teste para sementes de mamoneira. Revista Brasileira de Sementes, v.31, n.3, p.38-47, 2009. http://www.scielo.br/pdf/rbs/v31n3/a04v31n3.pdf
GRZYBOWSKI, C.R.S.; OHLSON, O.C.; SILVA, R.C.; PANOBIANCO, M. Viability of barley seeds by the tetrazolium test. Revista Brasileira
de Sementes, v.34, n.1, p.47-54, 2012. http://www.scielo.br/scielo.
php?pid=s0101-31222012000100006&script=sciarttext
HABYARIMANA, E.; LAURETI, D.; NINNO, M.; LORENZONI, C. Performances of biomass sorghum [Sorghum bicolor (L.) Moench] under different water regimes in Mediterranean region. Industrial Crops and
Products, v.20, p.23–28, 2004. http://www.sciencedirect.com/science/article/
pii/S0926669004000044
ISTA. INTERNATIONAL SEED TESTING ASSOCIATION. Biochemical test for viability: the topographical tetrazolium test. In: International rules for
seed testing. ed. 2008. Bassersdorf: ISTA, 2008, p.1-30.
MARCOS-FILHO, J. Fisiologia de sementes de plantas cultivadas. Piracicaba: FEALQ, 1.ed. 2005. 495p.
NOVEMBRE, A.D.L.C.; CHAMMA, H.M.C.P.; GOMES, R.B.R. Viabilidade das sementes de braquiária pelo teste de tetrazólio. Revista
Brasileira de Sementes, v.28, n.2, p.147-151, 2006. http://www.scielo.br/pdf/
rbs/v28n2/a20v28n2.pdf
OLIVEIRA, L.M.; CARVALHO, M.L.M.; DAVIDE, A.C. Teste de tetrazólio para avaliação da qualidade de sementes de Peltophorum dubium (Sprengel) Taubert Leguminosae Caesalpinioideae. Cerne, v.11, n.2, p.159-166, 2005. http://
www.dcf.ufla.br/cerne/artigos/11-02-20097975v11_n2_artigo%2006.pdf
RAJARAJAN, K.; GANESAMURTHY, K. Genetic diversity analysis of sorghum [Sorghum bicolor (L.) Moench] genotypes for drought tolerance using SSR markers. Indian Journal of Genetics and Plant
Breeding, v.71, n.1, p.17-24, 2011. http://www.indianjournals.com/ijor.
aspx?target=ijor:ijgpb&volume=71&issue=1&article=003
RESENDE, G.M.; PIRES, D.A.A.; BOTELHO, P.R.F.; ROCHA JÚNIOR, V.R.; SALES, E.C.J.; JAYME, D.G.; REIS, S.T.; PIMENTEL, L.R.; LIMA, L.O.B.; KANEMOTO, E.R.; MOREIRA, P.R. Características agronômicas de cinco genótipos de sorgo [Sorghum bicolor (L.) Moench], cultivados no inverno, para a produção de silagem. Revista Brasileira de Milho e Sorgo,
v.10, n.2, p. 171-179, 2011. http://rbms.cnpms.embrapa.br/index.php/ojs/
article/viewArticle/316
SCHABES, F.I.; SIGSTAD, E.E. Optimizing conditions to study seed germination by calorimetry using soybean (Glycine max [L.] Merr.) seeds.
Thermochimica Acta, v.450, p.96–101, 2006. http://www.sciencedirect.com/
science/article/pii/S0040603106004503
SOUZA, C.R.; OHLSON, O.C.; PANOBIANCO, M. Avaliação da viabilidade de sementes de aveia preta pelo teste de tetrazólio. Revista
Brasileira de Sementes, v.31, n.3, p.57-62, 2009. http://www.scielo.br/pdf/
rbs/v31n3/a06v31n3.pdf
SOUZA, C.R.; OHLSON, O.C.; GAVAZZA, M.I.A.; PANOBIANCO, M. Tetrazolium test for evaluating triticale seed viability. Revista Brasileira de
Sementes, v.32, n.3, p.163-169, 2010a. http://www.scielo.br/pdf/rbs/v32n3/
v32n3a18.pdf
SOUZA, C.R.; OHLSON, O.C.; PANOBIANCO, M. Avaliação da viabilidade de sementes de aveia branca pelo teste de tetrazólio. Revista
Brasileira de Sementes, v.32, n.4, p. 174-180, 2010b. http://www.scielo.br/
pdf/rbs/v32n4/20.pdf
TESSO, T.T.; CLAFLIN, L.E.; TUINSTRA, M.R. Analysis of stalk rot resistance and genetic diversity among drought tolerant sorghum genotypes.
Crop Science, v.45, n.2, p.645-652, 2005. https://www.soils.org/publications/
cs/abstracts/45/2/0645
TUNES, L.M.; BANDINELLI, P.G.; OLIVO, F.; BARROS, A.C.S.A. Tratamento para superação da dormência em sementes de cevada. Scientia
Agraria, v.10, n.1, p.015-021, 2009. http://dialnet.unirioja.es/servlet/
articulo?codigo=2900677
VON PINHO, R.G.; VASCONCELOS, R.C.; BORGES, I.D.; REZENDE,
A.V. Influência da altura de corte das plantas nas características agronômicas
e valor nutritivo das silagens de milho e de diferentes tipos de sorgo. Revista
Brasileira de Milho e Sorgo, v.5, n.2, p.266-279, 2006. http://rbms.cnpms.
embrapa.br/index.php/ojs/article/viewArticle/189
VON PINHO, R.G.; VASCONCELOS, R.C.; BORGES, I.D.; REZENDE, A.V. Produtividade e qualidade da silagem de milho e sorgo em função da época de semeadura. Bragantia, v.66, n.2, p. 235-245,2007. http://www.