Bioreactors with immobilized lipases:
State of the art
Victor M. Baldo, Ana L. Paiva and F. Xavier Malcata
Escola Superior de Biotecnologia, Universidade Catdlica Portuguesa, Porto, Portugal
This review attempts to provide an updated compilation of studies reported in the literature pertaining to reactors containing lipases in immobilizedforms, in a way that helps the reader direct a bibliographic search and develop an integrated perspective of the subject. Highlights are given to industrial applications of lipases (including control and economic considerations), as well as to methods of immobilization and configurations of reactors in which lipases are used. Features associated with immobilized lipase kinetics such as enzyme activities, adsorp- tion properties, optimum operating conditions, and estimates of the lumped parameters in classical kinetic formulations (Michaelis-Menten model for enzyme action and first-order model for enzyme decay) are presented
in the text in a systematic tabular form,
Keywords: Immobilized lipases; activity; stability; optimum operating conditions; reactors
Characteristics and applications of lipases
Lipases, also known as glycerol ester hydrolases (EC 3.1.1.3), belong to the hydrolase enzyme class and were originally employed for the hydrolysis of ester bonds. In addition to plants and animals, in which these enzymes are widespread, many microorganisms (natural or genetically engineered) are also capable of actively producing these enzymes both in endogenous and exogenous forms.
Lipases are unique enzymes in that they require interfa- cial activation for full catalytic performance, a fact that was initially established in 1958 by Sarda and Desnuelle.’ (An interface is hereby considered as the imaginary surface that separates two physically distinct phases, which corresponds on the molecular level to a set of two adjacent layers of ordered molecules, one more hydrophobic and the other more hydrophilic in nature.) Although several theories have been postulated to explain the observed enhancement of lipase activity upon formation of an interface,2 recent re- ports evolving from paneuropean projects on lipases spon- sored by the BRIDGE (EC) and the Nordic (Scandinavian) protein engineering programs have shed, in a dramatic fash-
Address reprint requests to Dr. Malcata, Escola Superior de Biotecnologia, Rua Dr. Ant6nio Bernardino de Almeida, 4200 Porto, Portugal Received 15 August 1994; revised 17 May 1995; accepted 1 June 1995
ion, experimental light over such theoretical interpretations via determination of the three-dimensional structure of 10 lipases and characterization of the kinetic behavior and ste- reoselectivity of 25 different lipases. It is now well estab- lished that: 1) all lipases share primary sequence homolo- gies including significant regions His-X-Y-Gly-Z-Ser- W- Gly or Y-Gly-His-Ser- W-Gly (where X, Y, Z, and W denote generic amino acid residues)3; and 2) the serine residue at the active site is protected by a flap (or o-helical lid), which opens upon contact of the lipase with an interface and thus leads to restructuring of the lipase by creating an electro- philic region (the oxyanion hole) around the aforementioned serine residue, by exposing hydrophobic residues, and by burying hydrophilic ones, all of which increase the affinity of the complex for lipid substrates and help stabilize the transition state intermediate during catalysisU The re- quirement for an interface (irrespective of its nature) is criti- cal; even if one uses a hydrophobic solvent, there are small local pools of water remaining within the folded structure of the lipase, and those water pools in the vicinity of the active site may provide the local interface necessary for enzyme activation.
Lipases catalyze a series of different reactions. In fact, although they were designed by nature to cleave ester bonds of triacylglycerols with the concomitant consumption of water molecules (hydrolysis), lipases are also able to cata- lyze the reverse reaction under microaqueous conditions,
Enzyme and Microbial Technology 18:392-416, 1996 CI 1996 by Elsevier Science Inc.
655 Avenue of the Americas, New York, NY 10010
0141-0229/96/$15.00 SSDI 0141-0229(95)00125-O
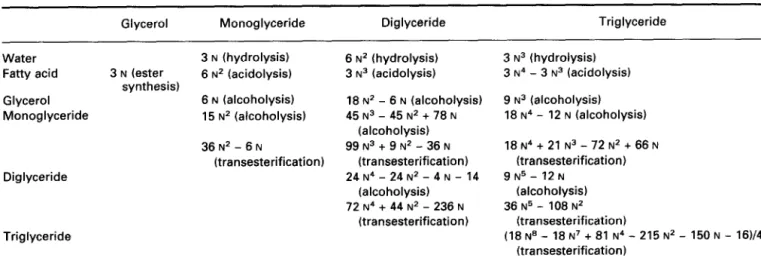
viz. the formation of ester bonds between alcohol and car- boxylic acid moieties (ester synthesis). These two basic pro- cesses can then be combined in a sequential fashion to give rise to a set of reactions generally termed interesterifica- tions. Depending on the particular starting point in terms of substrates, one may have acidolysis (where an acyl moiety is displaced between an acylglycerol and a carboxylic acid), alcoholysis (where an acyl moiety is displaced between an acylglycerol and an alcohol), and transesterification (where two acyl moieties are exchanged between two acylglycer- 01s). All these processes are depicted in Figure 1.
Although lipase-catalyzed reactions can also be carried out using inorganic, metal-derived catalysts, the interest generated by these enzymes as biotechnological vectors for the achievement of various types of reactions in both macro- and microaqueous systems has expanded formidably during the last decade. Indeed, lipase-catalyzed reactions resemble closely the pathways designed by nature for the metabolism of live beings, and so the reaction mechanisms and pro- cesses associated therewith may be viewed as more envi- ronment friendly than bulk syntheses. Furthermore, lipases are capable of producing a wide variety of products with potential high purity (and consequently high added value) resulting from their substrate specificity and stereospecific- ity. Because of the low activation energies involved, further advantages of lipases include mild reaction conditions of temperature and pH, which lead to reduced energy con- sumption and less extensive thermal damage to reactants and products.
Although technical staff are well aware of their versatil- ity and possible applications in industry, the use of lipases is not yet as significant as that associated with such other enzymes as proteases and carbohydrases. This may be be- cause of, first, most detailed studies involving lipases are recent and the usual time lag prior to full commercial ex- ploitation has not yet elapsed, and, second, their cost is still
R, COO% R, COOR, 1 : Hydrolysis 2 : Ester synthesis 1 + 2 : Alcoholysis 1 + 3 : Acidolysis 1 + 2 + 3 + 4 : Transesterification
Figure 1 Schematic representation of the reactions catalyzed by lipases, where Ri (i = 1,2,3,4) denotes an acyl moiety
Reactors with immobilized lipases: V. M. Balcao et al.
relatively high, a constraint that will eventually be over- come in the coming years as a result of evolution encom- passing their extraction and purification, as well as their production via genetic engineering.
The manufacture of household detergents remains the biggest market for industrial enzymes in general, but only now is this market opening up for lipases after genetic de- sign aiming at sufficient high stability and activity under the alkaline conditions prevailing in washing processes. Cur- rently, one of the major application of lipases is in the dairy industry for the controlled hydrolysis of milk fat, a process which is useful for acceleration of cheese ripening, flavor enhancement of butter, manufacture of cheeselike products, and preparation of enzyme-modified cheeses for use as in- gredients in dressings, soups, and sauces.
Lipases possess high potential for hydrolysis, glyceroly- sis, and alcoholysis of bulk fats and oils because of their high specificity and the relative purity of the products de- rived therefrom. The use of such enzymes avoids the need for the high temperatures required by nonenzymatic split- ting, which leads to thermal degradation of the products (e.g., generation of off-flavors and off-colors) and so to the need for postprocessing purification and refining. The up- grading of fats by specific syntheses and the randomization of structured triglycerides is also possible by the use of appropriate lipases, thus allowing the tailoring of fats for desired functional and nutritional properties. Another appli- cation pertains to the production of w3-polyunsaturated fatty acid (o3-PUFA) concentrates from fish liver oils (which have been claimed to provide beneficial health ef- fects via prevention of coronary heart diseases) for use as nutropharmaceutical food supplements, and sequential li- pase-catalyzed chemical incorporation in triglycerides.
Another industrial application with a promising future is the lipase-mediated production of optically pure compounds (rather than racemic mixtures) for pharmaceutical and fine- chemical uses. Lipase-catalyzed resolution of racemic aque- ous mixtures can occur through asymmetric hydrolysis of the corresponding esters, whereas in nonaqueous media, this approach can be extended to stereo- and regiospecific (trans)esterification reactions. The synthesis of optically ac- tive polymers can also be accomplished by this route.
Further applications of lipases in industry include (but are not limited to) the production of emulsifiers for personal care products (skin and suntan creams and bath oils), esters of fatty acids and fatty alcohols (wax esters), and oil waste treatment.
Immobilization of lipases
Lipases are spontaneously soluble in aqueous solutions (as a result of their globular protein nature), but their natural substrates (i.e., lipids) are not. Although use of a proper organic solvent or an emulsifier helps overcoming the prob- lem of intimate contact between substrate and enzyme, the practical use of lipases in such pseudohomogeneous reac- tion systems poses technological difficulties (viz. contami- nation of the products with residual enzymatic activity) and economic difficulties (viz. use of the enzyme for a single
Review
reactor pass). The former leads to constraints on the product level, because the final characteristics of the product depend on such postprocessing conditions as storage time and tem- perature. The latter leads to constraints on the process level, because the useful life of the enzyme is restricted to the space time of the reactor (on the assumption that the space time is small compared with the time scale associated with deactivation of the enzyme). In both cases, part of the over- all potential enzymatic activity is lost. If the lipase is im- mobilized, then it becomes an independent phase within the reaction system, which may be easily retained in the reactor via mechanical means with concomitant advantages in pre- venting contamination of the products and extending its useful active life.
Immobilized lipases are considered hereafter as lipases which are localized in a defined region of space, which is enclosed by an imaginary or material barrier which allows for physical separation of the enzyme from the bulk reaction medium, and which is at the same time permeable to reac- tant and product molecules. Rending a lipase immobilized may therefore be achieved by engineering the enzyme mi- croenvironment or macroenvironment. Examples of engi- neering on the level of the microenvironment of the enzyme encompass immobilization by attachment to a carrier (e.g., covalent attachment, hydrophobic and ion exchange adsorp- tion, and cross-linking) and immobilization by containment in a barrier (e.g., microencapsulation using lipid vesicles, containment in reversed micelles, entrapment in polymeric matrices, and confinement in ultrafiltration hollow fibers). Engineering on the level of the macroenvironment of the enzyme may proceed via modification of the reaction me- dium (e.g., precipitation in an organic solvent).
In addition to the lipase, immobilization also requires an immobilizing agent (i.e., a force that keeps the enzyme as a separate phase from the bulk reaction medium). Such a force can be established between two enzyme molecules, E-E (e.g., cross-linking, intermolecular cystein and/or salt bridges, and intermolecular hydrogen bonds), or between one enzyme molecule and another catalytically inert mol- ecule, E-C (e.g., covalent attachment, ion exchange, and hydrophobic interaction), or between two catalytically inert molecules, C-C (e.g., entrapment and mechanical contain- ment). Virtually every immobilization protocol employed to date for lipases encompasses immobilizing agent(s) at one, two, or all three levels of interaction considered (i.e., E-E, E-C, and C-C, respectively). The immobilizing agent thus comprises: 1) covalent forces as in covalent attachment, cross-linking, entrapment via polymerization, containment within porous membranes of polymeric nature, and inter- molecular cysteine bridges during precipitation; 2) ionic forces as in ion exchange, containment within porous mem- branes of mineral nature, and intermolecular salt bridges during precipitation in apolar solvents; 3) hydrogen bonds as in intermolecular interaction between hydrogen atoms and electronegative atoms during precipitation in apolar sol- vents; and 4) van der Waals forces as in hydrophobic ad- sorption, reversed micelles, microencapsulation, contain- ment within porous membranes of monomeric and hydro- phobic nature, and precipitation in polar solvents. The immobilizing agent may also include a material ligand; this
is the case of multifunctional molecules in cross-linking and spacer molecules in covalent attachment following prelimi- nary derivatization of the support.
The carrier (or the barrier) is an entity larger in size than the enzyme molecule (which may therefore be considered as a continuum) to which the enzyme is directly bound or within which the enzyme is confined, and has the role of helping in the creation of an immobilized, enzyme-rich phase. This support is absent in the case of cross-linking and plain precipitation of the enzyme in an organic solvent. The support may be a liquid, as in reversed micelles, or a solid, as in most of the commonly employed immobilization pro- tocols.
When carriers (with lipase attached to them) or barriers (with lipase confined to them) are used to immobilize a lipase, considerations on three levels of structure are in the order: macroscopic level, microscopic level, and submicro- scopic level. At the macroscopic level, there may be one dominant dimension (e.g., the length in the case of hollow fibers), two dominant dimensions (e.g., the surface in the case of flat-sheet membranes), or three dominant dimen- sions (e.g., the volume in the case of beads or dried micel- lia). The dominant dimension(s) are determined by the manufacture process and are directly related to the charac- teristics of the flow within the reactor (see below). In terms of microscopic characteristics, two factors are of relevance for solid supports: the thickness, on the one hand, and the
Figure 2 Immobilized lipase reactor pyramid enclosing the re- actor configurations studied to date. The reactor space is char- acterized by an axis describing the ratio of length scales of the hydrodynamic eddies and the reactor itself, and two axes de- scribing the volume fraction of the liquids in the reaction me- dium. The vertical sides of the pyramid correspond to a pure phase
Reactors with immobilized lipases: V. M. BalcBo et al.
molecules and unable to significantly affect the movement of the reacting molecules. Both configurations have, how- ever, been seldom reported to date for the case of lipase reactors.
The other (more frequent) situation consists in the con- finement of the lipase to a given portion of space within the reacting fluid defined by physical boundaries coinciding with interfaces; such a configuration requires the existence of at least two phases which are separable by such purely mechanical means as settling, centrifugation, or filtration. Various possibilities exist here for reactors containing im- mobilized lipases, but most situations fall within one of the following cases: 1) a lipase in solid form precipitated within an organic liquid phase; 2) a lipase in soluble form in an aqueous phase and confined by a solid ultrafiltration mem- brane; 3) a lipase contained in an aqueous phase and con- fined by a surfactant liquid membrane within an organic liquid phase; 4) a lipase entrapped within a three- dimensional polymeric matrix dispersed within an organic liquid phase, or dispersed within an aqueous phase; and 5) a lipase attached to a solid support and dispersed within an organic liquid phase, or dispersed within an aqueous phase. All these cases of technological feasibility and practical interest may be represented in the set of Cartesian axes de- picted in Figure 2 in the portion of space corresponding to the triangular pyramid outlined in bold.
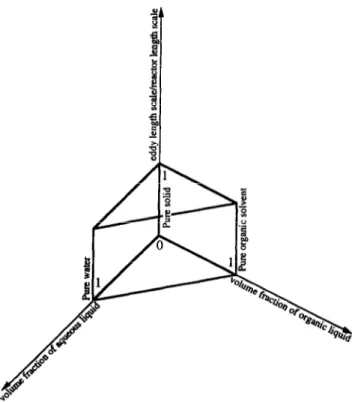
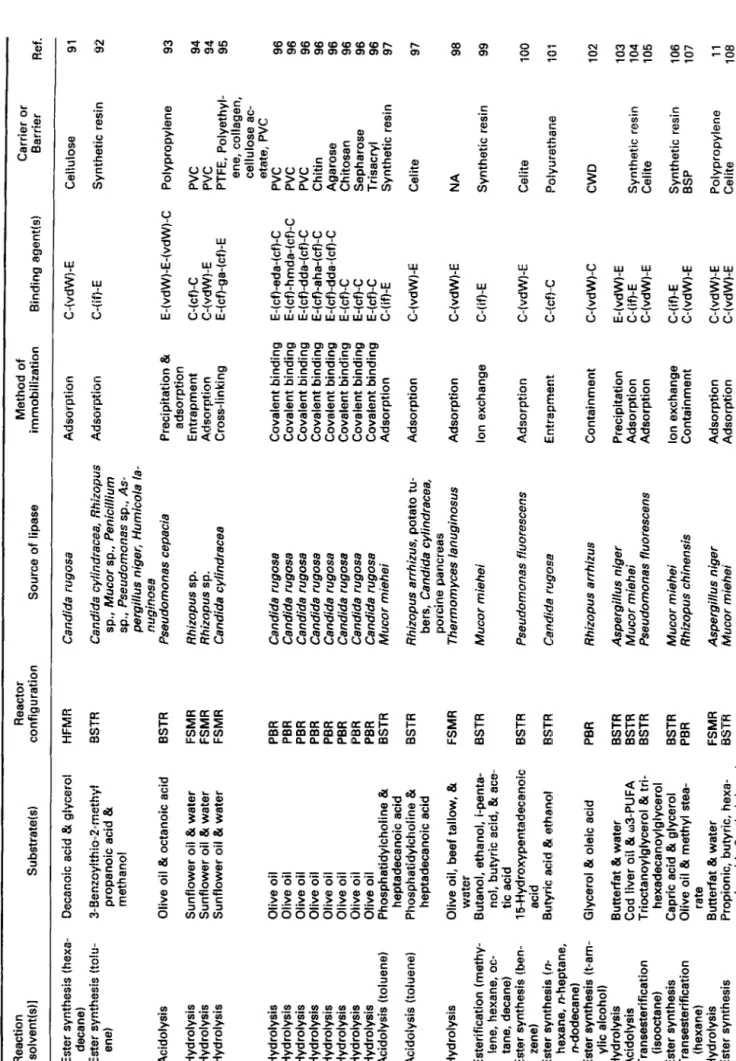
There are several reactor configurations described in the literature which have been used in studies of immobilized lipases, as are depicted in Table 1 (where references are written in chronological order). Important characteristics as type of reaction, solvent (if any), substrate(s), reactor con- figuration, source of lipase, method of immobilization, im- mobilizing agent, and nature of carrier/barrier (if any) are also listed.
As discussed before, use of emulsifiers promotes forma- tion of a pseudouniphase reaction system. However, emul- sion systems have several drawbacks: relatively low pro- ductivity, need of high-speed stirring to prevent clumping, requirement for powerful centrifuging or solvent extraction for separation of product from emulsifier, virtual impossi- bility of reutilization of the enzyme, and difficult control of the water concentration. These difficulties can be circum- vented by immobilizing the lipase, and enclosing it via one of several possibilities, namely, in batch stirred-tank reac- tors (BSTRs), packed-bed reactors (PBRs), fluidized-bed reactors (FBRs), or membrane reactors.
BSTRs are the reactors most commonly used; this reac- tor configuration accounts for more than two thirds of all reported uses (see TubEe I). These reactors operate batch- wise and consist of a vessel in which the reactant fluid mixture is stirred by some mechanical means (e.g., magnetic bars, submerged impellers, reciprocal oscillators, or end- over-end rotators) in a way that avoids the existence of temperature and concentration gradients. The immobilized enzyme is separated from the reaction medium at the end of the reaction by filtration or centrifugation. These reactors are easy to operate (e.g., heat, cool, clean, and maintain) and normally require a very limited set of auxiliary equipment; however, the upper average overall volumetric throughput porous structure, on the other. Small thicknesses and high
porosities are usually sought to minimize diffusional limi- tations upon substrates and maximize available area for li- pase attachment, but they also lead to mechanical weakness; hence, a compromise must be reached. The submicroscopic character is a direct result of the molecular characteristics of the carrier and may range from hydrophilic (i.e., essentially polar) to hydrophobic (i.e., essentially nonpolar). In the case of hydrophobic carriers, the immobilized enzyme is soaked in the organic liquid phase, whereas in hydrophilic carriers the immobilized enzyme is soaked in water; the former situation is useful if one wants deactivation reactions of the enzyme to be maintained as slow as possible, whereas the latter situation should be used when hydrolysis reactions are desired.
An overview of the various reactor configurations em- ploying immobilized lipases in the light of the framework introduced above will be presented in the next section. Later, the characteristic physicochemical features of such reactors (activity, stability, and optimum operating condi- tions) will be listed whenever available followed by control and economic considerations.
Reactors with immobilized lipases
An immobilized lipase reactor, which, as previously ex- plained, is a more cost-effective reactional alternative than a soluble lipase reactor, may be viewed as a portion of space in which the lipase macroscopic movement is restricted to its boundaries. Therefore, the ratio of the length scale asso- ciated with the hydrodynamic eddies of the reaction me- dium to the length scale associated with the reactor may range from zero (a situation that corresponds to pure mo- lecular transport of the reacting species between the reaction medium and the enzyme) to unity (a situation that corre- sponds to pure, long-range convection of the reacting mol- ecules through the reactor). This concept is illustrated in Figure 2.
Generation of hydrodynamic eddies may result solely from Brownian motion (as in reactors operated batchwise under virtually stagnant conditions), or from some extent of mechanical input to the reacting system via shaft work (as in solid rotational agitators) or via non-shaft work (as in bub- bling of a gas within the liquid system or in deliberately disturbing the liquid stream-line pattern following the mo- tionless mixer principle as in reactors operated continuously under essentially turbulent conditions). In general, contact of the reactants with the immobilized lipase is improved by convection relative to plain molecular transport; however, convection is also associated with higher shear rates and/or higher times of exposure to shear (in the case of rotational or motionless agitation), or with higher surface tension and/ or higher interfacial areas (in the case of bubbling agitation), both of which promote disturbances of the elaborate three- dimensional shape of the lipase molecules that lead to faster deactivation.
Confinement of the lipase to a given portion of space within the reacting fluid defined by imaginary boundaries requires either an electromagnetic or a gravitational field able to function as a barrier to the movement of the enzyme
Table 1 Main features of reactors employing immobilized lipases Reaction Reactor [solvent(s)] Substrate(s) configuration Source of lipase Method of immobilization Binding agent(s) Carrier or Barrier Ref.
Hydrolysis Alcoholysis Hydrolysis lnteresterification lnteresterification Alcoholysis
(n-hex- ane, benzene, car- bon tetrachloride, diisopropylether, acetonitrile) Ester synthesis (hex- ane) Acidolysis (petroleum ether) Hydrolysis Acidolysis (hexane) Acidolysis (hexane) Transesterification (isooctane) Transesterification (isooctane) Transesterification (isooctane) Acidolysis (hexane) Ester synthesis (hex- ane)
Transesterification Transesterification Ester
synthesis (Ben- zene, chloroform, l,l,l-trichloroeth- ane, acetone, N,N- dimethyl sulfoxide) Olive oil P-substituted Ethanols (Cl-, Br-, MeO-, BuO-, Me,N-, and E&N- CH,CH,OH) & Ethyl ac- etate BFT I-Stearyl 1,2-linolenyl, 3-oleylglycerol I-Stearyl 1,2-linolenyl, 3-oleylglycerol Tributyrin 61 benzyl alco- hol Oleic acid & mono-, dio- leoylglycerol Olive oil & octanoic, deca- noic, lauric acids Esters of triacylglycerols Soybean oil & lauric, pal- mitic, oleic acids Soybean oil & lauric, pal- mitic, oleic acids
Butterfat Butterfat Butterfat Olive
oil & methyl stearic acid, stearic acid, pal- mitic acid Oleic acid & oleoyl alco- hol; octanoic acid & oc- tanol; myristic acid & propanol Coprah oil 61 methyl stea- rate Coprah oil & methyl stea- rate I-Dodecanol & n-dodeca- noic acid BSTR BSTR Candida rugosa Candida cylindracea & porcine pancreas Covalent binding Precipitation FTMR, PBR, CSTR BSTR BSTR BSTR Candida cyiindracea Mucor miehei Adsorption Ion exchange Adsorption Precipitation BSTR BSTR, CSTR BSTR BSTR, CSTR BSTR, CSTR BSTR BSTR BSTR CSTR Candida deformans, Rhizopus arrhizus Candida cylindracea, C. lipo- Iytica, Pseudomonas fluore- scens, Penicillium cyclopium, P. roquefotii, porcine pan- creas, Humicola lanuginosa, Mucor javanicus, Rhizopus delemar, 13. javanicus, R. ni- veus, Aspergillus niger Oilseed rape Mucor miehei Mucor miehei Mucor miehei Porcine pancreas Aspergillus niger Mucor miehei Candida cylindracea Rhizopus chinensis Precipitation 64 adsorption Precipitation 64 adsorption
Adsorption Adsorption Adsorption Adsorption Adsorption Adsorption Containment
BSTR Mucor miehei Adsorption BSTR BSTR BSTR Candida deformans, Rhizopus arrhizus Mucor miehei Pseudomonas fragi
Adsorption Adsorption Precipitation E-(cf)-E E-(vdW)-E
PEG
C-(vdW)-E C-(if)-E C-(vdW)-E E-(vdW)-E Polypropylene Synthetic
resin
Celite
E-(vdW)-E-(vdW)-C E-(vdW)-E-(vdW)-C C-(if)-E C-(if)-E C-(vdW)-E C-(vdW)-E C-(if)-E C-(vdW)-E C-(vdW)-C C-(if)-E Celite Diatomaceous
earth
Duolite Synthetic
resin
Celite Celite Synthetic
resin Celite CWD Synthetic resin C-(vdW)-E Celite C-(if)-E Synthetic resin E-kf)-peg-(vdW)-E 23 24 24 25 26 27 26 29 29 30 30 31 32 33 34 34 35
Ester synthesis (cy- (R,S)-l-Phenylethanol & clohexane) heptanoic acid BSTR Candida cylindracea Adsorption
C-(vdW)-E C-(if)-E C-(cf)-C C-(vdW)-E C-(cf)-E E-(cf)-ga-(cf)-E C-(if)-E
Glass wool, po- rous glass, BE, phenyl-BE, C,,- BE, lichrosorb RP-18 gel Synthetic resin ENTP, poly- urethane Ca-alginate, ADCPG Amberlite, diaion Octyl-Sepharose Synthetic resin 36 37 38 38 38 38 39 C-(vdW)-E Cellulose 40 C-fvdW)-E Diatomaceous 41 C-(vdW)-E earth Diatomaceous earth 41 E-(vdW)-E 42 E-(vdW)-E 43 E-(vdW)-E-(vdW)-C NA 44 C-(if)-E Synthetic resin NA NA 45 Precipitation E-(vdW)-E Adsorption C-(if)-E Precipitation E-(vdW)-E Synthetic resin E-(vdW)-E 45 2 91 E? 46 3 47,48 49 3. s 49 w. C-(vdW)-C CWD C-(vdW)-E Celite BSTR, CSTR Mucor miehei Adsorption C-(if)-E Synthetic resin BSTR Rhizopus delemar Precipitation E-(vdW)-E C-(vdW)-E Celite C-(vdW)-E Celite C-(vdW)-E Sephadex 51 % r? s z-55 js Ester synthesis Geraniol & lauric acid PBR Hydrolysis Olive oil 84 water Humicola lanuginosa BSTR Humicola lanuginosa Adsorption Entrapment Hydrolysis Olive oil & water BSTR Humicola lanuginosa Adsorption
Hydrolysis Hydrolysis lnteresterification
Olive oil 81 water Olive oil 81 water Cocoa butter stearine, palm stearine & coconut oil Soybean oil 81 water Canola oil & lauric acid BSTR Humicola lanuginosa BSTR Covalent binding Humicola lanuginosa PBR Mucor miehei Cross-linking Adsorption Canola oil & trilaurin, fully hydrogenated HEAR stearin Acylglycerol & water Olive oil 81 water Butanol, ethanol 81 butyric acid Ethyl Dglucopyranoside & Cs-C,s fatty acids Ethyl Dglucopyranoside & Cs-C,s fatty acids
Hydrolysis Acidolysis lnteresterification
HFMR Candida rugosa BSTR Porcine pancreas Adsorption Adsorption BSTR Porcine pancreas Adsorption
Hydrolysis Hydrolysis Ester
synthesis lnteresterification lnteresterification BSTR BSTR BSTR BSTR BSTR Human milk Candida rugosa Precipitation Precipitation Candida cylindracea, Aspergil- Precipitation 81 lus niger, porcine pancreas Mucor miehei adsorption Ion exchange Candida antarctica, Humicola sp., Candida cylindracea, Pseudomonas sp. Chromobacterium viscosum Mucor miehei Candida cylindracea NA BSTR BSTR BSTR BSTR Candida cylindracea Precipitation BSTR Cotton seeds BSTR Adsorption Porcine pancreas Adsorption
lnteresterification lnteresterification Hydrolysis Ester
synthesis (cy- clohexane) Transesterification Acidolysis (hexane) Castor oil & beef tallow Cod liver oil 81 EPA. DHA I-Phenyl ethyl carbbxylic esters & water I-Phenyl ethanol & butyric acid Cottonseed oil & beef fat Groundnut, coconut, mus- tard, soybean oils & lau- ric, oleic, stearic acids Groundnut, coconut, mus- tard, soybean oils & lau- ric, oleic, stearic acids Groundnut, coconut, mus- tard, soybean oils & lau- ric, oleic, stearic acids Soybean oil & lauric acid Butterfat Acidolysis (hexane) Acidolysis (hexane) Acidolysis (hexane) Transesterification (hexane, isooctane) Hydrolysis (n-hexane, n-heptane, n-oc- tane, n-nonane, n- decane, isopropy- lether, isooctane, cyclohexane) BSTR Porcine pancreas BSTR Adsorption Pseudomonas fiuorescens Adsorption Olive oil & water BSTR Candida rugosa Adsorption
Table 1 (continued) 2. Ref. i Reaction [solvent(s)] Substrate(s) Reactor configuration Source of lipase Method of immobilization Binding agent(s) Carrier or Barrier Hydrolysis (isooctane) Esterification Acidolysis (hexane) Transesterification Ester synthesis Transesterification (isooctane) Hydrolysis Ester synthesis Hydrolysis & transes- terification Hydrolysis & transes- terification Transesterification (n-heptane) Olive oil & water Palm olein Refined sand eel oil & EPA, DHA Palm oil & coprah, palm- iste, colza, son de riz, soybean, bourrache oils Acetone glycerol & oleic acid Stearic acid 81 triolein, tripetroselinin, tri-a-lino- lenin; a-linolenic acid, T-linolenic acid, & triole- in; oleic acid & l-y-lino- leoyl-2,3-dioleoylglyc- erol Sunflower oil &water Oleic acid & mono-, di-, trioleoylglycerol Palm oil & stearic acid Palm oil & stearic acid Palm oil mid fraction & ethyl stearate Transesterification (n-heptane) lnteresterification Ester synthesis (n- hexane) Hydrolysis (water) Ester synthesis Ester synthesis
Acidolysis Alcoholysis Alcoholysis Alcoholysis Hydrolysis
(isooctane) Alcoholysis (hexane) Palm oil midfraction & ethyl stearate Cottonseed oil & olive oil Oleic acid & ethanol High erucic acid rapeseed Heptanol & octanoic acid Heptanol & oleic acid Butyl laurate & oleic acid Butyl laurate, ethyl pro- pionate 84 propanol, heptanol, nonanol Menthol & tributyrin; menthol 81 triacetin Menthol & tributyrin; menthol & triacetin Olive oil 84 water Evening primrose oil & butanol; Cod liver oil & butanol BSTR Candida rugosa Adsorption C-(vdW)-E Sephadex 57 BSTR Pseudomonas sp. Adsorption C-(vdW)-E Celite BSTR 58 Mucor miehei Adsorption C-(if)-E Synthetic resin 59 BSTR, PBR Mucor miehei Adsorption C-(if)-E Synthetic resin 60,61 BSTR Mucor miehei Adsorption C-(if)-E BSTR Rhizopus delemar Containment C-(vdW)-E Synthetic resin AOT-RM 62 63 HFMR BSTR BSTR BSTR BSTR Rhizopus sp. Cross-linking Mucor miehei Adsorption Rhizopus sp. Covalent binding Rhizopus sp. Adsorption Porcine pancreas, Rhizopus Adsorption arrhizus, R. javanicus, R. delemar, R. niveus, Aspergil- /us niger, Candida cylindra- E-(cf)-ga-(cf)-E PTFE 64 C-(if)-E Synthetic resin 65 C-(cf)-E TAS 66 C-(vdW)-E Celite 66 C-(vdW)-E Celite 67 BSTR tea Mucor miehei Adsorption C-(if)-E Synthetic resin 67 BSTR Mucor miehei Adsorption C-(if)-E Synthetic resin 68 BSTR Mucor miehei Adsorption C-(if)-E Synthetic resin 69 BSTR Candida rugosa Precipitation BSTR Candida cvlindracea BSTR Candida cilindracee Precipitation Precipitation BSTR Candida cylindracea Precipitation BSTR Candida cylindracea Precipitation E-(vdW)-E 70 E-(vdW)-E 71 E-(vdW)-E 72 E-(vdW)-E 72 E-(vdW)-E 72 BSTR BSTR BSTR BSTR Candida cylindracea, miehei Candida cylindracea Candida rugosa Mucor
Cross-linking Adsorption Containment
Brassica
napus
Ceres
Adsorption
E-(cf)-ga-(cf)-E C-(vdW)-E C-(vdW)-E C-(vdW)-E
Cellulose,
polyeth-
ylene
Polypropylene AOT-RM Celite 73 73 74 75
Alcoholysis (hexane) Evening primrose oil & butanol; Cod liver oil & butanol I-Phenyl ethyl butyrate & l-heptanol
NA Butterfat Butterfat Olive
oil &water Olive oil 84 water Olive oil &water Oleic acid & n-butanol Hexanol 81 oleic, hepta- noic, and lauric acids Acetic, propionic, butyric, valeric, and caproic ac- ids & methanol, etha- nol, butanol, I-pentanol, hexanol, citronellol, and geraniol Acetic, propionic, butyric, valeric, and caproic ac- ids & methanol, etha- nol, butanol, 1-pentanol, hexanol, citronellol, and geraniol Oleic acid 81 n-butanol Olive oil 84 water BSTR Mucor miehei Adsorption C-(if)-E Synthetic resin 75 Alcoholysis (water & cyclohexane)
Transesterification lnteresterification lnteresterification Hydrolysis
(isooctane) Hydrolysis (isooctane) Hydrolysis Ester synthesis Ester synthesis (dec- ane) Ester synthesis (hep- tane) Candida cylindracea Precipitation Mucor miehei Pseudomonas fluorescens Pseudomonas fluorescens Porcine pancreas
Adsorption Adsorption Adsorption Adsorption
Porcine pancreas Covalent binding Porcine pancreas Mucor miehei Rhizopus delemar Covalent binding Adsorption Precipitation Mucor miehei Adsorption
E-(vdW)-E C-(if)-E C-(vdW)-E C-(vdW)-E C-(vdW)-E C-(cf)-E C-(cf)-E C-(if)-E E-(vdW)-E C-(if)-E
76 BSTR BSTR BSTR BSTR BSTR PBR BSTR BSTR Synthetic resin 77 Celite 78 Celite 79 Trityl cellulose 80 Cellulose 80 EPSPS Synthetic resin 83 84 BSTR Synthetic resin Ester synthesis (hep- tane) BSTR Rhizopus arrhizus Containment C-(vdW)-C CWD 84 Mucor miehei Candida cylindracea, Pseudo- monas fluorescens, Rhizopus arrhizus, R. niveus Achromobacter sp., Alcalig- enes sp., Aspergillus niger, Candida cylindracea, C. lipo- lytica, Chromobacterium vis- cosum, Geotrichum can- didum, Humicola lanuginosa, Mucor javanicus, M. miehei, Penicillium cyclopium, P. ro- queforti, Pseudomonas fluo- rescens, Rhizopus arrhizus, R. delemar, R. japonicus, R. niveus Rhizopus arrhizus Candida cylindracea Mucor miehei, Candida antarc- tica Candida cylindracea, Porcine pancreas, Rhizopus arrhizus, Mucor miehei, Candida ant- arctica Candida cylindracea, porcine pancreas, Rhizopus arrhizus, Mucor miehei, Candida ant- arctica Adsorption Containment C-(if)-E C-(vdW)-E Synthetic resin 85 AOT-RM 86 Ester synthesis Hydrolysis 2 (isooctane) Ester synthesis g 0 a P BSTR BSTR Oleic acid & methanol, ethanol, propanol, buta- nol, pentanol, hexanol, decanol BSTR Precipitation E-(vdW)-E 87
is .g
lnteresterification d Hydrolysis (acetoni- trile) ? Hydrolysis (acetoni- .!G trile) Hydrolysis (acetoni- 3 trile) 2 & Alcoholysis AMDG Sepharose =: D 88 $ 89 8 . . Trilaurin and myristic acid Tributyrin & water Tributyrin &water Tributyrin & water PBR BSTR BSTR BSTR Covalent binding Covalent binding Adsorption Precipitation & adsorptionC-(cf)-ga-kf)-E C-(cf)-E C-(if)-E E-(vdW)-E-(vdW)-C
Synthetic resin 89 5 Celite Celite Glycerol & ethyl butyrate BSTR E-(vdW)-E-(vdW)-C Precipitation & adsorption
Table 1 (continued) Reaction [solvent(s)1 Substrate(s) Reactor configuration Source of lipase Method of immobilization Binding agent(s) Carrier or Barrier 9 s. Ref. $ Ester synthesis (hexa- decane) Ester synthesis (tolu- ene) Decanoic acid & glycerol 3-Benzoylthio-Z-methyl propanoic acid & methanol HFMR BSTR Olive oil & octanoic acid BSTR Sunflower oil &water Sunflower oil &water Sunflower oil & water FSMR FSMR FSMR Olive oil Olive oil Olive oil Olive oil Olive oil Olive oil Olive oil Olive oil Phosphatidylcholine & heptadecanoic acid Phosphatidylcholine & heptadecanoic acid PBR PBR PBR PBR PBR PBR PBR PBR BSTR BSTR Olive oil, beef tallow, & water Butanol, ethanol, i-penta- nol, butyric acid, & ace- tic acid 1 L-Hydroxypentadecanoic acid Butyric acid & ethanol FSMR BSTR BSTR BSTR Glycerol & oleic acid PBR Butterfat &water Cod liver oil & o3-PUFA Trioctanoylglycerol & tri- hexadecanoylglycerol Capric acid 81 glycerol Olive oil & methyl stea- rate Butterfat &water Propionic, butyric, hexa- noic acids & ethyl, hexyl alcohols BSTR BSTR BSTR BSTR PBR FSMR BSTR Candida rugosa Candida cylindracea, Rhizopus sp., Mucor sp., Penicillium sp., Pseudomonas sp., As- pergillus niger, Humicola la- nuginosa Pseudomonas cepacia Rhizopus sp. Rhizopus sp. Candida cylindracea Adsorption Adsorption C-(vdW)-E C-(if)-E Cellulose Synthetic resin 91 92
Acidolysis Hydrolysis Hydrolysis Hydrolysis
Precipitation
84
adsorption
Entrapment Adsorption Cross-linking E-(vdW)-E-(vdW)-C C-fcf)-C C-(vdW)-E E-(cf)-ga-(cf)-E Polypropylene PVC PVC PTFE, Polyethyl- ene, collagen, cellulose ac- etate, PVC
PVC PVC PVC Chitin Agarose Chitosan Sepharose Trisacryl Synthetic
resin
Celite
93 94 94 95
Hydrolysis Hydrolysis Hydrolysis Hydrolysis Hydrolysis Hydrolysis Hydrolysis Hydrolysis Acidolysis
(toluene) Acidolysis (toluene) Candida rugosa Candida rugosa Candida rugosa Candida rugosa Candida rugosa Candida rugosa Candida rugosa Candida rugosa Mucor miehei Rhizopus arrhizus, potato tu- bers, Candida cylindracea, porcine pancreas Thermomyces lanuginosus Mucor miehei Covalent binding Covalent binding Covalent binding Covalent binding Covalent binding Covalent binding Covalent binding Covalent binding Adsorption Adsorption E-(cf)-eda-(cf)-C E-(cf)-hmda-(cf)-C E-(cf)-dda-(cf)-C E-fcf)-aha-(cf)-C E-(cf)-dda-(cf)-C E-(cf)-C E-(cf)-C E-(cf)-C C-(if)-E C-(vdW)-E 96 ii 96 96 96 96 96 97 97 Hydrolysis Esterification (methy- lene, hexane, oc- tane, decane) Ester synthesis (ben- zene) Ester synthesis (n- hexane, n-heptane, rt-dodecane) Ester synthesis (t-am- ylic alcohol)
Hydrolysis Acidolysis Transesterification
fisooctane) Ester synthesis Transesterification (hexane) Hydrolysis Ester synthesis Adsorption Ion exchange C-(vdW)-E NA C-(if)-E Synthetic resin 99 98 Pseudomonas fluorescens Candida rugosa Adsorption Entrapment C-tvdW)-E C-(cf)-C Celite Polyurethane 100 101 Rhizopus arrhizus Aspergillus niger Mucor miehei Pseudomonas fluorescens Mucor miehei Rhizopus chinensis Aspergillus niger Mucor miehei
Containment Precipitation Adsorption Adsorption Ion
exchange
Containment Adsorption Adsorption
C-(vdW)-C
CWD
E-(vdW)-E C-(if)-E C-(vdW)-E
Synthetic resin Celite C-(if)-E C-(vdW)-E Synthetic resin 106 BSP 107 C-(vdW)-E C-fvdW)-E Polypropylene 11 Celite 108 102 103 104 105
Ester synthesis Alcoholysis Propionic, butyric, hexa- noic acids, 81 ethyl, hexyl alcohols Glycerol & beef tallow, lard, milk fat, palm oil, palm olein, palm stea- rin, coconut oil, rape- seed oil, olive oil, corn oil, hydrogenated tal- low, hydrogenated lard Glycerol & palm oil, stea- rin BSTR Mucor miehei BSTR Pseudomonas fluorescens Covalent binding C-(cf)-E Precipitation E-(vdW)-E Nylon 108 109 Alcoholysis BSTR BSTR HFMR BSTR Pseudomonas fluorescens, Chromobacterium viscosum, Mucor miehei Candida cylindracea NA Candida cylindracea BSTR Candida cylindracea BSTR Mucor miehei BSTR Mucor miehei BSTR Mucor miehei
Precipitation Precipitation Adsorption Adsorption
E-(vdW)-E 109 Ester synthesis (cy- clohexane) Ester synthesis (hexa- decane) Acidolysis (hexane) Dilaurin & lauric acid Decanoic acid, glycerol Sunflower, safflower, soy bean, linseed oils & oleic acid Sunflower, safflower, soy bean, linseed oils & methyl oleate Sunflower, safflower, soy bean, linseed oils & oleic acid Sunflower, safflower, soy bean, linseed oils & methyl oleate Sal, kokum, mahua, dhupa, mango oils, & methyl palmitate, stea- rate Edible tallow & water Oleic acid & oleic acid stearyl ester Olive oil 61 water Triolein & lauric acid 1-Monovalerin & valeric acid; I-Monoolein & oleic acid I-Monocaprylin & vinyl caprylate l-Monolaurin & vinyl lau- rate 1,3-Divalerin & valeric acid; 1,3-Diolein & oleic acid
E-(vdW)-E C-(vdW)-E C-(vdW)-E 110 111
Celite Celite Synthetic
resin Synthetic resin Synthetic resin 112 Transesterification (hexane) Acidolysis (hexane) Adsorption C-(vdW)-E 112 Adsorption C-(if)-E 112 Transesterification (hexane) Transesterification (hexane) Adsorption C-(if)-E Adsorption C-(if)-E
Hydrolysis Acidolysis Hydrolysis
(isooctane) Acidolysis (petroleum ether) Acidolysis (nhexane, diethyl ether, t-BuOMe) Transesterification (n-hexane, diethyl ether, t-BuOMe) Transesterifrcation (rr-hexane, diethyl ether, t-BuOMel Acidolysis In-hexane, diethyl ether, t-BuOMe) FSMR BSTR BSTR BSTR BSTR BSTR Thermomyces lanuginosos Mucor miehei Candida rugosa Mucor miehei Rhizopus delemar, Chromobac- terium viscosum Rhizopus delemar BSTR Rhizomucor miehei BSTR Rhizopus delemar; Chromobac- terium viscosum Adsorption C-(vdW)-E Ion exchange C-(if)-E Containment C-fvdW)-E Adsorption C-(if)-E Precipitation E-(vdW)-E Precipitation E-(vdW)-E Acrylic Synthetic resin AOT-RM Synthetic resin 114 115 3 3 116 8 3 117 2’ Q. Synthetic resin z 118 . Adsorption C-(if)-E Precipitation E-(vdW)-E ,,, ,, ,,, ,, ,,,/, ,, ),,, ,, ,,,,,, ,, , mm,,, ,,, ,,, 8, 8, ,, 8, mm,, mm,, 887, 8, 8, 8, I, ,, /,,, 8.8, ,, ,,,,” ,,,/ ,,,, ,,,,,
Table 1 (continued Reaction Isolvent Substrate(s) Reactor configuration Source of lipase Method of immobilization Binding agent(s) Carrier or Barrier Ref. p Transesterification (rrhexane, diethyl ether, f-BuOMe) Transesterification (n-hexane, diethyl ether, r-BuOMe) 1,3-Dicaprylin & vinyl cap- rylate BSTR Rhizopos delemar Precipitation E-(vdW)-E 119 1,3-Dicaprin & vinyl capri- BSTR Rhizomucor miehei Adsorption C-(if)-E Synthetic resin nate; 1,3-Dilaurin 81 vi- nvl laurate; 1,3-Dimvris- tin & vinyl.myristate; 1,3-Dipalmitin & vinyl palmitate; 1,SDistearin & vinyl stearate 1,3-Dipentadecanoin & pentadecanoic acid 119 Acidolysis (n-hexane, diethyl ether, t-BuOMe) BSTR Rhizomucor miehei Adsorption C-(if)-E Synthetic resin 119 Ester synthesis (ben- Porcine pancreas Porcine pancreas Adsorption Adsorption C-(vdW)-E zenei Transesterification C-(vdW)-E (benzene) Hydrolysis Hydrolysis Dodecanoic alcohol & do- decanoic acid Butyl octadycylate & do- decanoic alcohol Olive oil, milk fat, 81 water Milk fat & water Containment C-(vdW)-E Entrapment C-(cf)-C Glass, Kieselguhr, Al,Os, agar Glass, Kieselguhr, AI,O,, agar BSP, polyurethane ENT, ENTP Hydrolysis Alcoholysis (n-hex- ane) Acidolysis lnteresterification Olive oil Geraniol 81 methyl, ethyl, propyl, isopropyl, butyl, isobutyl, ferf-butyl, amyl, and octyl acetates Stearic acid & oalm olein Milk fat . BSTR BSTR CSTR BSTR RDRMR BSTR Rhizopus delemar Candida cylindracea, Rhizopus arrhizus Candida rugosa Mucor miehei Containment C-(vdW)-E AOT-RM Adsorption C-(if)-E Synthetic resin 120-121 120-121 122 123 124 125 BSTR Mucor miehei BSTR NA C-(if)-E Synthetic resin 126 E-(vdW)-E-(vdW)-C Celite 127 Deacidification Hyperacid acid oil BSTR Transesterification Tallow & rapeseed oil BSTR Hydrolysis Milk fat & water FSMR Hydrolysis Castor oil &water BSTR Acidolysis Calf fat & isostearic acid BSTR Transesterification Beef tallow & castor oil BSTR Mucor miehei Mucor miehei Candida rugosa Mucor miehei Chromobacterium viscosum, Rhizopus arrhizus, Candida cylindracea Chromobacterium viscosum, Rhizopus arrhizus, Candida cylindracea Rhizopus arrhizus Adsorption Precipitation 81 adsorption Ion exchange
Adsorption Adsorption Adsorption Adsorption
C-(if)-E Synthetic resin 128 C-(if)-E Synthetic resin 129 C-(vdW)-E Polypropylene 130 C-(if)-E Synthetic resin 131 C-(vdW)-E Polypropylene 132 Adsorption C-(vdW)-E Polypropylene, Duolite 132 Acidolysis Calf fat & isostearic acid BSTR Adsorption Transesterification Beef tallow & castor oil BSTR Rhizopus arrhizus Adsorption
Acidolysis Transesterification Ester
synthesis Calf fat 81 isostearic acid Beef tallow 81 castor oil Cod liver fatty acids & glycerol BSTR BSTR BSTR Geotrichum candidum Geotrichum candidum Mucor miehei, Candida antarc- tica Mucor miehei, Candida antarc- tica Rhizopus delemar
Adsorption Adsorption Adsorption C-tvdW)-E C-(vdW)-E C-(if)-E C-(if)-E C-(if)-E
Polypropylene, Duolite Polypropylene, Duolite Synthetic resin Synthetic resin Synthetic resin 132 132 132 132 133 Transesterification Cod liver oil ethyl esters & tributyrin BSTR Adsorption C-(if)-E Synthetic resin Ester synthesis Ethylene glycol 81 lauric (isooctane) acid BSTR Containment C-(vdW)-E AOT-RM 133 134

![Table 1 Main features of reactors employing immobilized lipases Reaction Reactor [solvent(s)] Substrate(s) configuration Source of lipase Method of immobilization Binding agent(s) Carrier or Barrier Ref](https://thumb-eu.123doks.com/thumbv2/123dok_br/14952217.1003653/5.873.80.801.26.1096/features-reactors-employing-immobilized-reaction-substrate-configuration-immobilization.webp)
![Table 1 (continued Reaction [solvent(s)] Substrate(s) Reactor configuration Ester synthesis (hex- Laurie acid & methanol, ane) ethanol, amyl alcohol BSTR](https://thumb-eu.123doks.com/thumbv2/123dok_br/14952217.1003653/13.864.55.819.60.1104/continued-reaction-solvent-substrate-reactor-configuration-synthesis-methanol.webp)
![Table 1 (continued Reaction [solvent(s)] Substrate(s) Reactor configuration Source of lipase Method of immobilization Binding agent(s) Carrier or Barrier Ref](https://thumb-eu.123doks.com/thumbv2/123dok_br/14952217.1003653/15.876.135.785.38.1107/continued-reaction-substrate-reactor-configuration-immobilization-binding-carrier.webp)