rev bras ortop.2015;50(6):729–738
w w w . r b o . o r g . b r
Original
Article
Quantification
of
platelets
obtained
by
different
centrifugation
protocols
in
SHR
rats
夽
João
Alberto
Yazigi
Junior
∗,
João
Baptista
Gomes
dos
Santos,
Bruno
Rodrigues
Xavier,
Marcela
Fernandes,
Sandra
Gomes
Valente,
Vilnei
Mattiolli
Leite
UniversidadeFederaldeSãoPaulo(Unifesp),SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received28September2014 Accepted16December2014 Availableonline20October2015
Keywords: Plateletcount Centrifugation Platelet-richplasma
a
b
s
t
r
a
c
t
Objective:ToquantifytheplateletconcentrationinthebloodofSHRrats,bymeansof differ-entcentrifugationprotocols,andtoevaluatewhatthemosteffectivemethodforobtaining plateletsis.
Methods:Weused40maleratsoftheisogenicSHRlineage.Theanimalsweredividedinto threegroups:control,usingwholebloodwithoutcentrifugation;singlecentrifugation,using wholebloodsubjectedtoasinglecentrifugationat200×gand400×g;anddouble cen-trifugation,usingwholebloodsubjectedonecentrifugationatdifferentrotations,followed bycollectionofwholeplasmasubjectedtoanothercentrifugationatdifferentrotations: 200×g+200×g;200×g+400×g;200×g+800×g;400×g+400×g;400×g+800×g.Samples of3mlofbloodweredrawnfromeachanimalbymeansofcardiacpuncture.Thebloodwas storedinVacutainercollectiontubescontaining3.2%sodiumcitrate.Thebloodfromthe controlgroupanimalswasanalyzedwithoutbeingsubjectedtocentrifugation.Afterthe bloodfromtheothergroupsofanimalshadbeensubjectedtocentrifugation,thewhole plasmawascollectedandsubjectedtoplateletcountinginthelowerthirdofthesample. Results:Weobtainedgreatestplateletenrichmentinthesubgroupwithtwocentrifugations comprising400×gfor10min+400×gfor10min,inwhichthemeanplateletconcentration was11.30timeshigherthanthatofthecontrolgroup.
Conclusion: Itwaspossibletoobtainahighplateletconcentrationusingviablesimple tech-niques,bymeansofcentrifugationofwholebloodanduseofcommonlyusedmaterials.The mosteffectivemethodforobtainingplateletconcentratewasfoundinsamplessubjected totwocentrifugations.
©2015SociedadeBrasileiradeOrtopediaeTraumatologia.PublishedbyElsevierEditora Ltda.Allrightsreserved.
夽
StudycarriedoutattheLaboratoryofMicrosurgeryoftheDisciplineofHandandUpperLimbSurgery,DepartmentofOrthopedics andTraumatology,UniversidadeFederaldeSãoPaulo(Unifesp),SãoPaulo,SP,Brazil.
∗ Correspondingauthor.
E-mail:junioryazigi73@yahoo.com.br(J.A.YazigiJunior). http://dx.doi.org/10.1016/j.rboe.2015.10.008
Quantificac¸ão
do
número
de
plaquetas
a
partir
de
diferentes
métodos
de
centrifugac¸ão
em
ratos
da
linhagem
SHR
Palavras-chave:
Contagemdeplaquetas Centrifugac¸ão
Plasmaricoemplaquetas
r
e
s
u
m
o
Objetivo: Quantificaraconcentrac¸ãodeplaquetasdosanguederatosSHR,por meiode diferentesprotocolosdecentrifugac¸ão,eavaliarqualométodomaiseficazdeobtenc¸ãode plaquetas.
Métodos: Usamos40ratosmachosdalinhagemisogênicaSHR.Osanimaisforamdivididos emtrêsgrupos:Controle(GCT)-sanguetotalsemcentrifugac¸ão;ÚnicaCentrifugac¸ão(GUC) -sanguetotalsubmetidoaumaúnicacentrifugac¸ão:200ge400g;DuplaCentrifugac¸ão (GDC)-sanguetotalsubmetidoaumacentrifugac¸ão,seguidodecoletadoplasmatotal,e realizadoumacentrifugac¸ão,emdiferentesrotac¸ões:200g+200g;200g+400g;200g+800g; 400g+400g;400g+800g.Foramretirados3mldesanguedecadaanimalpormeiodepunc¸ão cardíaca.Osanguefoiacondicionadoemtubodecoletavacutainercomcitratodesódio3,2%. Osanguedosanimaisdogrupocontrolenãofoisubmetidoàcentrifugac¸ãoefoianalisado. Apósacentrifugac¸ãodosanguedosanimais,submetidoàcentrifugac¸ão,oplasmatotalfoi coletadoesubmetidoàcontagemdeplaquetasnoterc¸oinferiordaamostra.
Resultados: Obtivemos maior enriquecimento de plaquetas no subgrupo de duas centrifugac¸ões (400g por 10 minutos+400g por 10 minutos), no qual ocorreu uma concentrac¸ãomédiadeplaquetas11,30vezessuperioremrelac¸ãoaogrupocontrole. Conclusão: Foipossívelobterumaaltaconcentrac¸ãoplaquetária,comtécnicasimplese viável,pormeiodecentrifugac¸ãodosanguetotaleusodemateriaisdeusocorriqueiro;e métodomaiseficazdeobtenc¸ãodeconcentradodeplaquetasocorreunasamostras sub-metidasaduascentrifugac¸ões.
©2015SociedadeBrasileiradeOrtopediaeTraumatologia.PublicadoporElsevier EditoraLtda.Todososdireitosreservados.
Introduction
Plateletsarecytoplasmicanucleatefragmentsfoundinblood andproducedbymegakaryocytesinthebonemarrow.1,2 Of
the totalnumber of platelets present inthe body,70% are presentinthecirculationand30%inthespleen,remaining inthecirculationforanaverageof10 days,whentheyare removed bythe reticuloendothelial cells ofthe spleenand liver.1–3 Plateletsare directlyinvolved inseveralmajor
dis-eases,asinseverethromboticsyndromesorconditionssuch asarterialthrombosis.4,5
Thehemostaticsystemisinherentlyresponsibleforthe maintenanceofbloodflowandvascularintegrity,asitisable toformaplugoveradamagedsurfaceofthevascular endothe-liumwhenitsuffersaninjury.Thus,hemostasisminimizes bloodlossandpromotestherestorationofthenormalvascular architecture.6–8
Theplatelet-rich plasma(PRP)istheautologousplatelet concentrationinasmallvolumeofplasma,obtainedby cen-trifugationofwholeblood,consideredanimportantsourceof growthfactors.Itsbasicconstitutionisgivenbythree com-ponents:plasma,leukocytesandplatelets.Theplatelet-rich fibrin (PRF) is a platelet concentrate, obtained from fibrin membrane,withhighpotentialfortissueregeneration.Thus, asinPRP,plateletconcentratescontainedinthePRFrelease growthfactors(GFs)thatenhancetheregenerationprocess. Additionally,thefibrinmatrixpromotesangiogenesis, facili-tatestheaccesstotheinjuredsite,withanimportantrolein tissuehealing.9
DescribedforthefirsttimeinFrancein2000,9platelet-rich
fibrin(PRF),anewconceptandstilllittledescribedinthe treat-mentusingfibrin gel,isaplateletconcentrateoverafibrin membranewithahighpotentialforinjuryrepair.The mem-braneisobtainedfromautologousbloodwithouttheaddition ofexternalfactors.10
Itisknownthatplateletsactintheprocessofhemostasis, injuryhealingandreepithelialization,releaseseveralgrowth factors and thatatleastsevendifferent GFwere identified intheinitialphaseofinjuryhealing.Therefore,the release oftheseGFbyplatelet␣-granulesplaysanimportantrolein
the controlandproliferation ofmesenchymalcells, includ-ingfibroblasts.PRPisactivatedbyaddingcalciumions,with thereleaseofGFandtheexocytosisof␣-granulesand
trans-forms fibrinogeninto fibrin. Through this process,wealso obtain autologousfibringlue,alsocalledplateletgel orPRP gel. The PRP gel presentation facilitates its use and han-dlinginseveralsurgicalareasandpromotestheintegration offlapsandgrafts,suchasbone,skin,cartilageorfatcells. Theuse resultsinmorerapidand efficienthealing,dueto adhesioneffectofthegel,whichisproportionaltothepresent fibrinogen.11–13
rev bras ortop.2 0 1 5;50(6):729–738
731
shouldbecollectedinadrytubetoobtainplatelet-rich fib-rin.
ThereareseveralavailablePRPpreparationsystemsusing abenchtop centrifuge, but inpreclinical and clinical stud-ies,theattainedresultsseemcontradictory.Thereisalackof moresystematicandconsistentstudiesonthesubject,which makesPRPpreparationremain anexperimentalprocedure, despiteitspromisingapplicationintissueregeneration.
Calixto,14 whenstudyingthe humanblood,verifiedthat
thebestmethodofobtainingPRPistheoneinwhichasingle centrifugationismade,of200×gfor10min.Pereira,15using
sevenprotocolstoobtainPRPinhorses,verifiedthatthehigher thegforceandthelongerthecentrifugationtime,thehigher wasthetotalplateletconcentration.
Asthe animalsmostoftenusedinresearchlaboratories areratsandduetothelackofstudiesinthisanimalmodel, wedecidedtoperformthisstudytoevaluatethebestmethod to obtain the best platelet concentration, in different pro-tocols, to quantify the concentration of blood platelets in SHRrats,usingdifferent centrifugationprotocolsand eval-uate what the most effective method is toobtain platelet concentrate.
Materials
and
methods
TheworkwasperformedintheLaboratoryofMicrosurgeryof theHandandUpperLimbSurgeryDisciplineofthe Depart-mentofOrthopedicsandTraumatologyandattheLaboratory ofHematologyoftheDepartmentofClinicaland Toxicologi-calAnalysesofourinstitution.Thestudywasapprovedbythe ResearchEthicsCommittee(RECn.0312/12).
Atotalof40malerats oftheisogenic SHRlineagewere usedinthestudy,weighing280–300g,providedbyCentrode Desenvolvimento de ModelosExperimentais para Medicina eBiologia.Theanimalswerekeptincollectivecages inthe vivarium,with5ratsineach,inacontrolledlight-darkcycle environment(12/12h),temperatureof21±2◦C,freeaccessto
waterandfoodthroughouttheentirestudyperiod. Theanimalsweredividedintothreegroups:
(1)ControlGroup(CTG)–wholebloodwithoutcentrifugation (n=5).
(2)SingleCentrifugationGroup(SCG)–wholebloodsubjected toasinglecentrifugation.Thisgroupwasdivided intotwo subgroups:
(a) SCG-I:200×gcentrifugationfor10min(n=5) (b) SCG-II:400×gcentrifugationfor10min(n=5)
(3) Double Centrifugation Group(DCG) – wholeblood sub-jectedtocentrifugation,followedbywholeplasmacollection andsubjectedtoasecondcentrifugationatdifferentrotations. Thisgroupwasdividedintofivesubgroups:
(a) DCG-I:centrifugationat200×gfor10min,followedbya second200×gcentrifugationfor10min(n=5)
(b) DCG-II:200×gcentrifugationfor10min,followedbya sec-ondcentrifugationat400×gfor10min(n=5)
(c) DCG-III200×gcentrifugationfor10min,followedbya sec-ondcentrifugationfor10minat800×g(n=5)
Fig.1–Plasmacollectioninthe1.5mLEppendorftube.
(d) DCG-IV400×gcentrifugationfor10min,followedbya sec-ondcentrifugationfor10minat400×g(n=5)
(e) DCG-V:400×gcentrifugationfor10min,followedbya sec-ondcentrifugationfor10minat800×g(n=5)
Bloodandplasmasamplecollection
Theanimalswereanesthetizedwithanintraperitoneal injec-tionwithanestheticsolutionconsistingofxylazine1U/100g andketamine1U/100g.
After being anesthetized, 3ml of blood was withdrawn from each animalbycardiacpuncture.Thecollectedblood wasplacedinavacutainertubewithsodiumcitrateat3.2%.
Thebloodoftheanimalsfromthecontrolgroupwasnot submittedtocentrifugationandwasanalyzedaftershaking thetubefor2minat4◦Cforhomogenization.
Samplesofwholeanimalbloodwerecentrifuged accord-ingtotheprotocoldescribedaboveforeachgroupinabench centrifuge(Eppendorf5810R)at4◦C.
Aftercentrifugation,theanimals’bloodwassubjectedtoa singlecentrifugationandthewholeplasmawascollectedinto a1.5mLEppendorftubeandsubjectedtoplateletcountingin thelowerthirdofthesample.
For the animals’blood subjected totwocentrifugations, afterthefirstcentrifugationthewholeplasmawascollected ina1.5mLEppendorftubeandsubjectedtoanother centrifu-gation.Theplateletcountwasperformedinthelowerthirdof thesample(Fig.1).
Plateletcount
Theobtainedsampleswere subjectedtoplateletcountina hematologyanalyzer(AnimalBloodCounter–VetABC) cali-bratedforbloodparametersofrats(Fig.2).
Statisticalmethods
r
e
v
b
r
a
s
o
r
t
o
p
.
2
0
1
5;
5
0(6)
:729–738
Table1–Descriptionofresultinghematologicalparameters.
Variable Group Mean SD Median Minimum Maximum N p
Leukocytes CTRL 3060 219.1 3200 2700 3200 5 0.016
200×g-10min 420 465.8 200 100 1200 5
400×g-10min 420 549.5 200 100 1400 5
200×g-10min+200×g-10min 1160 2260.1 100 100 5200 5
200×g-10min+400×g-10min 1860 1105.9 1400 500 3100 5
200×g-10min+800×g-10min 2.700 3755.7 700 300 9200 5
400×g-10min+400×g-10min 3.020 2983.6 1500 400 7800 5
400×g-10min+800×g-10min 3160 3943.7 500 200 8700 5
Erythrocytes CTRL 7,120,000 358,956.8 7,020,000 6.640.000 7,530,000 5 <0.001
200×g-10min 22,000 16,431.7 20,000 10,000 50,000 5
400×g-10min 14,000 11,401.8 10,000 0 30,000 5
200×g-10min+200×g-10min 80,000 134,721.9 20,000 10,000 320,000 5
200×g-10min+400×g-10min 262,000 895,545 240,000 150,000 380,000 5
200×g-10min+800×g-10min 170,000 101,242.3 150,000 60,000 330,000 5
400×g-10min+400×g-10min 120,000 86,313.4 150,000 20,000 220,000 5
400×g-10min+800×g-10min 188,000 199,549.5 70,000 10,000 420,000 5
Platelets CTRL 648,800 30,094.9 648,000 614,000 695,000 5 0.013
200×g-10min 1,632,400 580,962.4 1650.000 682,000 2,122,000 5
400×g-10min 939,600 687,417.1 863,000 110,000 1,764,000 5
200×g-10min+200×g-10min 3,826,000 4920.808.4 456,000 29,000 970.0000 5
200×g-10min+400×g-10min 4,760,000 2254.550.953 530.0000 130.0000 720.0000 5
200×g-10min+800×g-10min 4,080,000 1685.823,241 310.0000 270.0000 6500.000 5
400×g-10min+400×g-10min 7,320,000 2819.929,077 7,300,000 2,800,000 9,700,000 5
400×g-10min+800×g-10min 5,500,000 1813.835,715 5200.000 3400.000 7600.000 5
Plasmavolume(mL) CTRL 0 0 0 0 0 5 <0.001
200×g-10min 0.72 0.11 0.8 0.6 0.8 5
400×g-10min 1.10 0.10 1.1 1 1.2 5
200×g-10min+200×g-10min 1.46 0.11 1.5 1.3 1.6 5
200×g-10min+400×g-10min 1.60 0.10 1.6 1.5 1.7 5
200×g-10min+800×g-10min 1.62 0.04 1.6 1.6 1.7 5
400×g-10min+400×g-10min 1.62 0.08 1.6 1.5 1.7 5
400×g-10min+800×g-10min 1.72 0.08 1.7 1.6 1.8 5
rev bras ortop.2 0 1 5;50(6):729–738
733
Fig.2–Plateletcountinthehematologyanalyzer.
median,minimumandmaximum)werecomparedtothe sub-groupsusingKruskal–WallistestsfollowedbyDunn’smultiple non-parametriccomparisons,whennecessarytocomparethe subgroupstwobytwo.
Results
Theresultsweredepictedusingtablesandbarchartsthat rep-resentthemedianvaluesofeachparameteraccordingtothe subgroups;thetestswereperformedwithasignificancelevel of5%.
Table1showsthatallassessedparametersshowed statis-ticallysignificantdifferencebetweenthegroups(p<0.05).
Figs.3and 4suggesthigher amountsofleukocytes and erythrocytesinthecontrolgroup.
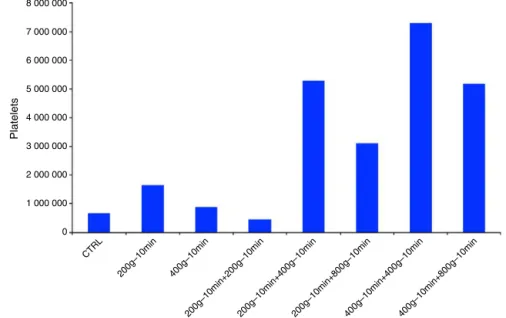
Fig.5showsthatthehighestplateletenrichmentoccurred inthesubgroupoftwo400×g+400×gcentrifugations.
TheplasmavolumevaluesshowninFig.6arehigherwith twocentrifugations(400×gfor10min+800×gfor10min).
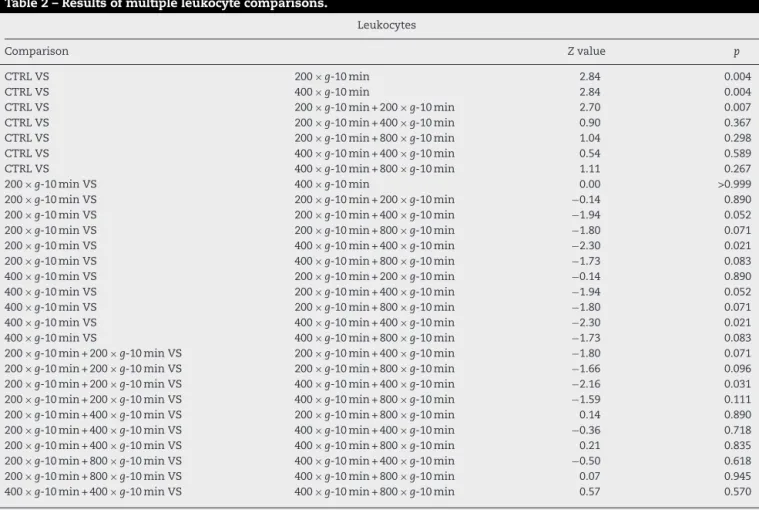
Table2showsthattheresultingamountofleukocyteswas statisticallyhigherinthecontrolgroupwhencomparedtothe subgroupswithasinglecentrifugation(p=0.004andp=0.004) and inrelation tothe subgroupwith two 200×g centrifu-gations and10mineach (p=0.007).Onthe other hand,the subgroupwithtwo 400×g centrifugationsand10mineach showedstatisticallymoreleukocytesthanthesubgroupswith asinglecentrifugation(p<0.05)andthesubgroupwithtwo 200×gcentrifugationsand10mineach(p=0.031).
Table 3 shows that the amount of red blood cells was statistically higher in the control than in the other sub-groups(p<0.05),withtheexceptionofthesubgroupswithtwo centrifugationsof200×g-10min+400×g-10minand200×g -10min+800×g-10min (p=0.192 and p=0.056). These two subgroupsshowedstatisticallymoreredbloodcellsthanthe subgroups ofasinglecentrifugation and subgroupsoftwo 200×gcentrifugationsand10mineach(p<0.05).
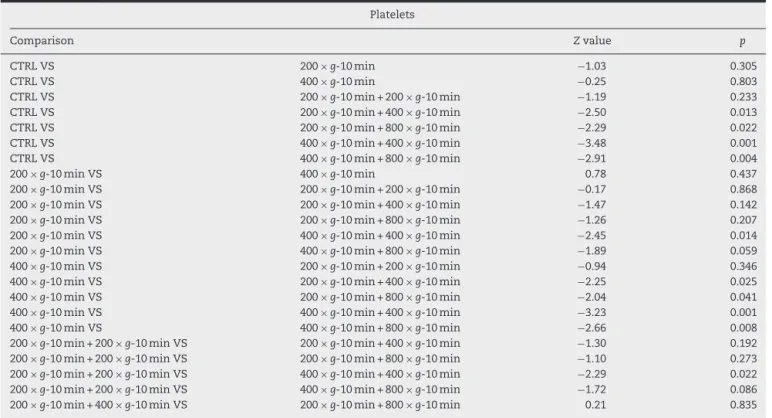
Table4showsthattheamountofplateletswithtwo cen-trifugationswasstatisticallyhigherthanthecontrol,witha singlecentrifugation,andthesubgroupwithtwo centrifuga-tions,with200×gand10mineach(p<0.05).
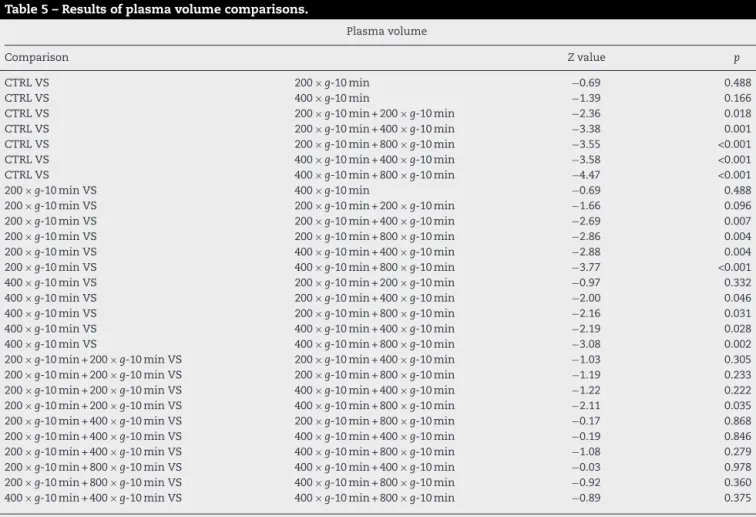
Table5showsthattheobtainedplasmavolumewas statis-ticallysimilartothebehaviorofplatelets.Subgroupswithtwo centrifugationshavehighervolumethantheothersubgroups, exceptforthesubgroupwithtwocentrifugations,with200×g and10mineach.
Thetablesandfiguresshowthattheamountofplatelets with two centrifugations (400×g+400×g subgroup) was statisticallyhigherthanthecontrolwithonlyasingle centrifu-gation,whereasthesubgroupwithtwocentrifugations,with 200×gand10mineach(p<0.05)showedamean concentra-tionofplatelets (400×g+400×g)thatwas11.30-foldhigher comparedtothecontrolgroup.
Discussion
The literature shows techniques to obtain platelet-rich plasma, asthefirst oneused,onlyapplicableinahospital
0
CTRL
200g–10min 400g–10min
200g–10min+200g–10min200g–10min+400g–10min200g–10min+800g–10min400g–10min+400g–10min400g–10min+800g–10min 3500
3000
2500
2000
1500
Leukocytes
1000
500
CTRL
200g–10min 400g–10min
200g–10min+200g –10min200g–10min+400g–10min200g–10min+800g–10min400g–10min+400g–10min400g–10min+800g–10min
8 000 000
7 000 000
6 000 000
5 000 000
4 000 000
3 000 000
2 000 000
1 000 000
0
Erythrocyte
Fig.4–Medianerythrocytevaluesaccordingtosubgroups.
structurewithplasmapheresismachinethatrequiresa tech-niciantomanipulateit.16Theprocedurehadhighermorbidity
andrequiredsophisticatedtechnology.
ThesearchfortechniquestoobtainPRPwithlowercosts hasledtotheuseofsomesimplemethodswithtesttubes and a common centrifuge, which allowthe preparation of platelet-rich plasma at a much lower cost and in an out-patient setting.However, thesemethods are laborious and require learning from those who will perform the proce-dure.
The existing methods in the literature do not reach the same platelet concentrations. Someare insufficient to improvetissueregeneration.Thesefactorsmayexplainmuch ofthe criticismabout theeffectivenessand applicabilityof platelet-richplasma.
Another keyfactor isassociatedwiththe anticoagulant. Usually,sodiumcitrate3.2%isused.Thesodiumcitrate cap-turescalciumionsfrombloodandneutralizedthem,forming a compound named chelate that prevents clot formation. Additionally, thesodium citrate doesnot alter the platelet membranereceptorsand,consequently,thechelationprocess canbereversedbytheadditionofcalciumchloridetoform plateletgel.17
Therecenttechnologyallowsthe useofPRP withsmall bloodvolumes,thusminimizingtheneedforredbloodcell reinfusionandassociatedrisks.However,thereislittle infor-mationabouttheoptimalamountofplateletsforbettertissue repair.
TheliteratureshowsseveralprotocolsforobtainingPRP. Thenumberofcentrifugations(oneortwo)willdependonthe
CTRL
200g–10min 400g–10min
200g–10min+200g–10min200g–10min+400g–10min200g–10min+800g–10min400g–10min+400g–10min400g–10min+800g–10min
8 000 000
7 000 000
6 000 000
5 000 000
4 000 000
3 000 000
2 000 000
1 000 000
0
Platelets
rev bras ortop.2 0 1 5;50(6):729–738
735
CTRL
200g–10min 400g–10min
200g–10min+200g–10min200g–10min+400g–10min200g–10min+800g–10min400g–10min+400g–10min400g–10min+800g–10min 0.0
1.8
1.6
1.4
1.2
1.0
0.8
0.6
Plasma volume (mL)
0.4
0.2
Fig.6–Medianplasmavolumevalues.
methodtobeused.Someauthorssuggestonlyone centrifuga-tion,whileotherreferencesindicateadoublecentrifugation protocol.
AnyprotocoltoobtainthePRPshouldconcentrateplatelets atitsmaximumtocorrespondtotheclinicalresultsreported
intheliterature.Butinadditiontoahighplatelet concentra-tionwithalargereleaseofgrowthfactors,itisveryimportant thattheintegrityofplateletsismaintained.Thegrowth fac-tors must bereleased from viable platelets, as during the actofgranularexocytosis,theplateletcompletesthetertiary
Table2–Resultsofmultipleleukocytecomparisons.
Leukocytes
Comparison Zvalue p
CTRLVS 200×g-10min 2.84 0.004
CTRLVS 400×g-10min 2.84 0.004
CTRLVS 200×g-10min+200×g-10min 2.70 0.007
CTRLVS 200×g-10min+400×g-10min 0.90 0.367
CTRLVS 200×g-10min+800×g-10min 1.04 0.298
CTRLVS 400×g-10min+400×g-10min 0.54 0.589
CTRLVS 400×g-10min+800×g-10min 1.11 0.267
200×g-10minVS 400×g-10min 0.00 >0.999
200×g-10minVS 200×g-10min+200×g-10min −0.14 0.890
200×g-10minVS 200×g-10min+400×g-10min −1.94 0.052
200×g-10minVS 200×g-10min+800×g-10min −1.80 0.071
200×g-10minVS 400×g-10min+400×g-10min −2.30 0.021
200×g-10minVS 400×g-10min+800×g-10min −1.73 0.083
400×g-10minVS 200×g-10min+200×g-10min −0.14 0.890
400×g-10minVS 200×g-10min+400×g-10min −1.94 0.052
400×g-10minVS 200×g-10min+800×g-10min −1.80 0.071
400×g-10minVS 400×g-10min+400×g-10min −2.30 0.021
400×g-10minVS 400×g-10min+800×g-10min −1.73 0.083
200×g-10min+200×g-10minVS 200×g-10min+400×g-10min −1.80 0.071
200×g-10min+200×g-10minVS 200×g-10min+800×g-10min −1.66 0.096
200×g-10min+200×g-10minVS 400×g-10min+400×g-10min −2.16 0.031
200×g-10min+200×g-10minVS 400×g-10min+800×g-10min −1.59 0.111
200×g-10min+400×g-10minVS 200×g-10min+800×g-10min 0.14 0.890
200×g-10min+400×g-10minVS 400×g-10min+400×g-10min −0.36 0.718
200×g-10min+400×g-10minVS 400×g-10min+800×g-10min 0.21 0.835
200×g-10min+800×g-10minVS 400×g-10min+400×g-10min −0.50 0.618
200×g-10min+800×g-10minVS 400×g-10min+800×g-10min 0.07 0.945
400×g-10min+400×g-10minVS 400×g-10min+800×g-10min 0.57 0.570
Table3–Resultsofmultipleerythrocytecomparisons.
Erythrocytes
Comparison Zvalue p
CTRLVS 200×g-10min 3.90 <0.001
CTRLVS 400×g-10min 4.27 <0.001
CTRLVS 200×g-10min+200×g-10min 3.41 0.001
CTRLVS 200×g-10min+400×g-10min 1.30 0.192
CTRLVS 200×g-10min+800×g-10min 1.91 0.056
CTRLVS 400×g-10min+400×g-10min 2.44 0.015
CTRLVS 400×g-10min+800×g-10min 2.18 0.029
200×g-10minVS 400×g-10min 0.37 0.708
200×g-10minVS 200×g-10min+200×g-10min −0.49 0.627
200×g-10minVS 200×g-10min+400×g-10min −2.59 0.010
200×g-10minVS 200×g-10min+800×g-10min −1.98 0.047
200×g-10minVS 400×g-10min+400×g-10min −1.46 0.145
200×g-10minVS 400×g-10min+800×g-10min −1.72 0.086
400×g-10minVS 200×g-10min+200×g-10min −0.86 0.390
400×g-10minVS 200×g-10min+400×g-10min −2.97 0.003
400×g-10minVS 200×g-10min+800×g-10min −2.36 0.018
400×g-10minVS 400×g-10min+400×g-10min −1.83 0.067
400×g-10minVS 400×g-10min+800×g-10min −2.09 0.036
200×g-10min+200×g-10minVS 200×g-10min+400×g-10min −2.11 0.035
200×g-10min+200×g-10minVS 200×g-10min+800×g-10min −1.50 0.134
200×g-10min+200×g-10minVS 400×g-10min+400×g-10min −0.97 0.332
200×g-10min+200×g-10minVS 400×g-10min+800×g-10min −1.23 0.217
200×g-10min+400×g-10minVS 200×g-10min+800×g-10min 0.61 0.542
200×g-10min+400×g-10minVS 400×g-10min+400×g-10min 1.14 0.255
200×g-10min+400×g-10minVS 400×g-10min+800×g-10min 0.87 0.382
200×g-10min+800×g-10minVS 400×g-10min+400×g-10min 0.53 0.598
200×g-10min+800×g-10minVS 400×g-10min+800×g-10min 0.26 0.792
400×g-10min+400×g-10minVS 400×g-10min+800×g-10min −0.26 0.792
Dunn’smultiplecomparisontestresults.
Table4–Resultsofmultipleplateletcomparisons.
Platelets
Comparison Zvalue p
CTRLVS 200×g-10min −1.03 0.305
CTRLVS 400×g-10min −0.25 0.803
CTRLVS 200×g-10min+200×g-10min −1.19 0.233
CTRLVS 200×g-10min+400×g-10min −2.50 0.013
CTRLVS 200×g-10min+800×g-10min −2.29 0.022
CTRLVS 400×g-10min+400×g-10min −3.48 0.001
CTRLVS 400×g-10min+800×g-10min −2.91 0.004
200×g-10minVS 400×g-10min 0.78 0.437
200×g-10minVS 200×g-10min+200×g-10min −0.17 0.868
200×g-10minVS 200×g-10min+400×g-10min −1.47 0.142
200×g-10minVS 200×g-10min+800×g-10min −1.26 0.207
200×g-10minVS 400×g-10min+400×g-10min −2.45 0.014
200×g-10minVS 400×g-10min+800×g-10min −1.89 0.059
400×g-10minVS 200×g-10min+200×g-10min −0.94 0.346
400×g-10minVS 200×g-10min+400×g-10min −2.25 0.025
400×g-10minVS 200×g-10min+800×g-10min −2.04 0.041
400×g-10minVS 400×g-10min+400×g-10min −3.23 0.001
400×g-10minVS 400×g-10min+800×g-10min −2.66 0.008
200×g-10min+200×g-10minVS 200×g-10min+400×g-10min −1.30 0.192
200×g-10min+200×g-10minVS 200×g-10min+800×g-10min −1.10 0.273
200×g-10min+200×g-10minVS 400×g-10min+400×g-10min −2.29 0.022
200×g-10min+200×g-10minVS 400×g-10min+800×g-10min −1.72 0.086
rev bras ortop.2 0 1 5;50(6):729–738
737
Table4–(Continued)
Platelets
Comparison Zvalue p
200×g-10min+400×g-10minVS 400×g-10min+400×g-10min −0.98 0.325
200×g-10min+400×g-10minVS 400×g-10min+800×g-10min −0.42 0.677
200×g-10min+800×g-10minVS 400×g-10min+400×g-10min −1.19 0.233
200×g-10min+800×g-10minVS 400×g-10min+800×g-10min −0.62 0.533
400×g-10min+400×g-10minVS 400×g-10min+800×g-10min 0.57 0.570
Dunn’smultiplecomparisontestresults.
structureofthegrowthfactorproteins.Fragmentedplatelets candegranulate largeamounts ofgrowth factors,but with decreasedeffectiveness.18
In this study, we compared the number of platelets obtainedfromperipheralbloodandsubjectedtooneandtwo centrifugations;thehigherenrichmentoccurredinthegroup withtwocentrifugations.Theresultsaresimilar,when com-paredtotheresultsofsomepublishedstudies,suchastheone byCalixto.14
InthestudybyCalixto,14carriedoutinhumans,testtubes
withtheanticoagulantsodiumcitrateat3.2%andEDTAwere used,which showed adifference inplatelet concentration, higherwhencitratewasused.Basedonthisfact,wechose thecitrateastheonlyanticoagulantinthecollectiontubes.
Vendraminetal.19obtainedplatelet-richplasmaofbetter
qualityusingtwocentrifugationsat400×gfor10min+800×g for10min.Inourstudy,weobtainedahighplatelet concen-tration with 400×g+800×g; however, the highest amount of platelets was obtained with 400×g for 10min+400×g for10min.Thisresultshowsthatthe lysisofplatelets can occurabovethisforce,whichinfluencesitsfinal concentra-tion.
Several studies were performed inhumans, rabbits and horses;however,wedidnotfindcentrifugationprotocolsin rats.WiththeestablishmentofaprotocoltoobtainPRPinrats, whichreachedahighplatelet concentration,weencourage futurestudiestoevaluatetheclinicaleffectivenessof platelet-richplasma.Furtherstudiesmayemerge fromourprotocol
Table5–Resultsofplasmavolumecomparisons.
Plasmavolume
Comparison Zvalue p
CTRLVS 200×g-10min −0.69 0.488
CTRLVS 400×g-10min −1.39 0.166
CTRLVS 200×g-10min+200×g-10min −2.36 0.018
CTRLVS 200×g-10min+400×g-10min −3.38 0.001
CTRLVS 200×g-10min+800×g-10min −3.55 <0.001
CTRLVS 400×g-10min+400×g-10min −3.58 <0.001
CTRLVS 400×g-10min+800×g-10min −4.47 <0.001
200×g-10minVS 400×g-10min −0.69 0.488
200×g-10minVS 200×g-10min+200×g-10min −1.66 0.096
200×g-10minVS 200×g-10min+400×g-10min −2.69 0.007
200×g-10minVS 200×g-10min+800×g-10min −2.86 0.004
200×g-10minVS 400×g-10min+400×g-10min −2.88 0.004
200×g-10minVS 400×g-10min+800×g-10min −3.77 <0.001
400×g-10minVS 200×g-10min+200×g-10min −0.97 0.332
400×g-10minVS 200×g-10min+400×g-10min −2.00 0.046
400×g-10minVS 200×g-10min+800×g-10min −2.16 0.031
400×g-10minVS 400×g-10min+400×g-10min −2.19 0.028
400×g-10minVS 400×g-10min+800×g-10min −3.08 0.002
200×g-10min+200×g-10minVS 200×g-10min+400×g-10min −1.03 0.305
200×g-10min+200×g-10minVS 200×g-10min+800×g-10min −1.19 0.233
200×g-10min+200×g-10minVS 400×g-10min+400×g-10min −1.22 0.222
200×g-10min+200×g-10minVS 400×g-10min+800×g-10min −2.11 0.035
200×g-10min+400×g-10minVS 200×g-10min+800×g-10min −0.17 0.868
200×g-10min+400×g-10minVS 400×g-10min+400×g-10min −0.19 0.846
200×g-10min+400×g-10minVS 400×g-10min+800×g-10min −1.08 0.279
200×g-10min+800×g-10minVS 400×g-10min+400×g-10min −0.03 0.978
200×g-10min+800×g-10minVS 400×g-10min+800×g-10min −0.92 0.360
400×g-10min+400×g-10minVS 400×g-10min+800×g-10min −0.89 0.375
andexpanditsuseintheregenerationofnerves,tissue,bone andligamentgrafts.
Conclusion
Itwaspossibletoobtainahighplateletconcentration,usinga simpleandfeasibletechnique,throughwholeblood centrifu-gationandusingcommonmaterialssuchascollectiontubes, syringesandneedles.Themosteffectivemethodtoobtaina plateletconcentratewasachievedinthesamplessubjected totwocentrifugations(400×gfor10min+400×gfor10min), whichyieldedameanplateletconcentrationthatwas 11.30-foldhigherthanthatinthecontrolgroup.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
ToDr.PrimaveraBorelliGarciaandtechnicianEdsonfromthe HematologyLaboratoryoftheDepartmentofClinicaland Tox-icologicalAnalysis,FaculdadedeCiênciasFarmacêuticasda USP,fortheirhelpandtechnicalsupportfortheresearch.
r
e
f
e
r
e
n
c
e
s
1. ItalianoJE,HartwigJH.Megakaryocytedevelopmentand plateletformation.In:MichelsonAD,editor.Platelets. California:AcademicPress;2002.p.21–36.
2. SchulzeH,ShivdasaniRA.Mechanismsofthrombopoiesis.J ThrombHaemost.2005;3(8):1717–24.
3. AndrewsRK,BerndtMC.Plateletphysiologyandthrombosis. ThrombRes.2004;114(5-6):447–53.
4. GawazM,NeumannFJ,SchomigA.Evaluationofplatelet membraneglycoproteinsincoronaryarterydisease: consequencesfordiagnosisandtherapy.Circulation. 1999;99(1):E1–11.
5.GreggD,Goldschmidt-ClermontPJ.Cardiologypatientpage. Plateletsandcardiovasculardisease.Circulation.
2003;108(13):e88–90.
6.BatesER,LauWC.Controversiesinantiplatelettherapyfor patientswithcardiovasculardisease.Circulation.
2005;111(17):e267–71.
7.ErhardtsenE.Togeneralhaemostasis:theevidence-based route.PathophysiolHaemostThromb.2002;32Suppl.1: 47–52.
8.StassenJM,ArnoutJ,DeckmynH.Thehemostaticsystem. CurrMedChem.2004;11(17):2245–60.
9.ChoukrounJ,DissA,SimonpieriA,etal.Platelet-richfibrin (PRF):asecond-generationplateletconcentrate.PartIV: clinicaleffectsontissuehealing.OralSurgOralMedOral PatholOralRadiolEndod.2006;101(3):e56–60.
10.SunithaRajaV,MunirathnamNaiduE.Platelet-richfibrin: evolutionofasecond-generationplateletconcentrate.Indian JDentRes.2008;19(1):42–6.
11.EppleyBL,WoodellJE,HigginsJ.Plateletquantificationand growthfactoranalysisfromplatelet-richplasma:implications forwoundhealing.PlastReconstrSurg.2004;114(6):1502–8. 12.GreenDM,KlinkB.Plateletgelasanintraoperatively
procuredplatelet-basedalternativetofibringlue.Plast ReconstrSurg.1998;101(4):1161–2.
13.KevySV,JacobsonMS.Comparisonofmethodsforpointof carepreparationofautologousplateletgel.JExtraCorpor Technol.2004;36(1):28–35.
14.CalixtoCA.Plasmaricoemplaquetas(PRP)pormeiode centrífugadebancada[dissertac¸ão].SãoPaulo:Universidade FederaldeSãoPaulo;2011.
15.PereiraRCF.Avaliac¸ãodeseteprotocolosdeobtenc¸ãode plasmaricoemplaquetas(PRP)[dissertac¸ão].SantaMaria: UniversidadeFederaldeSantaMaria,RS;2012.
16.MarxRE,CarlsonER,EichstaedtRM,SchimmeleSR,Strauss JE,GeorgeffKR.Platelet-richplasma:growthfactor
enhancementforbonegrafts.OralSurgOralMedOralPathol OralRadiolEndod.1998;85(6):638–46.
17.AnituaE.Plasmarichingrowthfactors:preliminaryresultsof useinthepreparationoffuturesitesforimplants.IntJOral MaxillofacImplants.1999;14(4):529–35.
18.MarxRE.Platelet-richplasma(PRP):whatisPRPandwhatis notPRP?ImplantDent.2001;10(4):225–8.