online | memorias.ioc.fiocruz.br
Factors associated with seropositivity for anti-Toxoplasma gondii
antibodies in pregnant women of Londrina, Paraná, Brazil
FMR Lopes1, R Mitsuka-Breganó1, DD Gonçalves1, RL Freire1, CJT Karigyo2, GF Wedy2, T Matsuo3,
EMV Reiche4, HK Morimoto4, JD Capobiango5, IT Inoue6, JL Garcia1, IT Navarro1/+
2Graduação em Medicina 3Departamento de Estatística e Matemática Aplicada 4Departamento de Patologia, Analises Clínicas e
Toxicologia 5Departamento de Clínica Médica 6Departamento de Ginecologia e Obstetrícia 1Departamento de Medicina Veterinária
Preventiva, Universidade Estadual de Londrina, Paraná, Brasil
The aim of this study was to evaluate associations between seropositivity for IgG and IgM anti-Toxoplasma gon-dii antibodies and socio-economic and environmental variables in pregnant women of Londrina, state of Paraná, Brazil. We interviewed 492 pregnant women, each of whom answered an epidemiological questionnaire, and col-lected blood samples for measurement of IgG and IgM anti-T. gondii antibodies by chemiluminescence. A confirma-tory diagnosis of acute infection was made by an IgG avidity test. Titres of specific IgG anti-T. gondii were obtained by IFAT. Seropositivity for IgG anti-T. gondii antibodies was observed in 242 women (49.2%) and, of these, six pregnant women (1.2%) showed seropositivity for IgM. Age group, level of education, per capita income, presence of a cat in the house and a habit of eating green vegetables were all factors associated with a greater chance of infection with T. gondii. This study showed that 250 (50.8%) pregnant women were susceptible to T. gondii and con-sidered to be at high risk for toxoplasmosis during pregnancy. Based on the results obtained, is critical to establish a program of health surveillance for toxoplasmosis, in order to contribute to diagnosis and early treatment during the prenatal period. It is also necessary to introduce measures to prevent the Toxoplasma infection in seronegative pregnant women.
Key words: Toxoplasma gondii - congenital toxoplasmosis - pregnant women - epidemiology
Congenital toxoplasmosis occurs when Toxoplasma gondii, an obligatory intracellular protozoan, reaches the foetus transplacentally. More than 90% of pregnant women who acquire a primary infection during gestation are asymptomatic (Montoya & Rosso 2005). T. gondii in-fection can be acquired by ingesting sporulated oocysts found in dirt, sand and food, or ingesting tissue cysts found in raw or rare pork, lamb or sheep (Frenkel 2002).
In general, among all pregnant women who acquire an infection for the first time, 61% will not transmit it to the foetus: 26% of cases will present foetus with sub-clinical infection and 13% will present sub-clinical infection (7% severe infection and 6% mild infection) (Dunn et al. 1999). Identifying the gestational age at primary infec-tion is crucial for the clinical management of pregnant women, since the severity of toxoplasmosis for the foe-tus decreases and the transmission rate increases with increasing gestational age (Beguetto et al. 2003). In the United State, rates of congenital toxoplasmosis have been estimated between one per 10,000 and 10 per 10,000 live births each year (Lopez et al. 2000).
Toxoplasmosis is usually diagnosed based on the de-tection of specific IgG and IgM antibodies; however, the inclusion of other parameters such as IgG avidity, IgA,
and IgE tests and polymerase chain reaction is funda-mental for a conclusive diagnosis of toxoplasmosis dur-ing pregnancy (Jones et al. 2003). Therefore, laboratory diagnosis poses a challenge for health care professionals not only because of the complexity in the interpretation of these markers, but also because modern laboratory techniques are not always available in the national public health care system (Mozzatto & Procianoy 2003).
The aim of this study was to evaluate associations between seropositivity for IgG and IgM anti-T. gon-dii antibodies and socio-economic and environmental variables in women in the first trimester of pregnancy in the Basic Health Units of Londrina, state of Paraná (PR), Brazil.
SUBJECTS, MATERIALS AND METHODS
This study was approved by the Research Ethical Committee from Londrina State University, under num-ber 047/05. Pregnant women who had pre-natal check-ups in all Basic Health Units and were in the first tri-mester of pregnancy were included following their free and informed consent. Samples were collected between May-December 2006 (with a gap between August-No-vember due to a strike in the public health service of the city). A total of 492 blood samples were submitted for the measurement of anti-T. gondii IgG and IgM antibod-ies by the Municipal Central Laboratory.
In order to determine the size of the sample popu-lation, it was estimated that the Basic Health Units of Londrina attend 5,000 pregnant women each year. This number was obtained based on data provided by the Mu-nicipal Health Office of Londrina. Using an expected
Financial support: SETI-PR, SESA-PR, UEL, CNPq + Corresponding author: italmar@uel.br
prevalence of 50%, error of 5% and significance level of 5%, the sample size obtained was 350.
The city of Londrina has 497,833 inhabitants, with 6,872 live births (IPARDES 2009) and 5,000 pregnant women per year in the prenatal of the Basic Health Units. The climate is classified as subtropical humid, with an average annual temperature above 20°C. Agri-culture (e.g., coffee, corn, wheat, vegetables) and live-stock (poultry and cattle) are the main economic activi-ties. The water that supplies the city is captured in the Tibagi and Cafezal Rivers and is distributed to the public according to standards set by the World Health Organi-zation (SANEPAR 2009).
Chemiluminescence’s technology was used for the quantitative determination of specific anti-T. gondii IgG and IgM antibodies (LIAISON, DiaSorin S.p.A., Sal-luggia, Italy).
Samples were considered IgG reactive when the anti-body concentration was greater than or equal to 8.8 UI/ mL, non-reactive when the concentration was less than 7.2 UI/mL, and undetermined when the concentration was between 7.2 and 8.8 UI/mL. Samples were consid-ered reactive for IgM when the antibody concentration was greater than or equal to 8.0 UA/mL, non-reactive when the concentration was less than 6.0 UA/mL, and undetermined when the concentration was between 6.0 and 8.0 UA/mL. In order to confirm the undetermined results, new samples were collected after 15 days.
Samples that were reactive to both anti-T. gondii IgG and IgM were sent to the Clinical Immunology Laboratory of University Hospital of Londrina State University (HU-UEL) in order to confirm acute infec-tion using an IgG avidity test. A semi-quantitative titre of anti-T. gondii IgG antibodies was also determined by IFAT (Camargo 1974).
In order to determine the sociodemographic charac-teristics that could be associated with T. gondii infec-tion, each pregnant woman answered a questionnaire with information about pregnancy, eating and hygiene habits, in addition to social-economic variables. Infor-mation was stored and analyzed in the EpiInfo program (Dean et al. 1994). Chi-squared or Fisher Exact tests, when appropriate, were used to verify the statistical significance and the Odds Ratio (OR) as a measure of the association between toxoplasmosis infection and other variables studied. A significance level of 5% was used in this study. In order to evaluate the independent association of the factors evaluated, a multivariate re-gression analysis of factors with p < 0.20 in the bivari-ate analysis was applied.
RESULTS
The prevalence of specific anti-T. gondii IgG was of 49.2% (CI 95%: 44.7-53.6%) (Table I). In that group, six women (1.2%) were found to be IgM reactive. The aver-age aver-age of all pregnant women was 25.7 ± 6.7 years.
Data from the evaluated sociodemographic charac-teristics that could be associated with the serological re-sults for anti-T. gondii antibodies are presented in Table II. Variables such as age group (p = 0.033), education level (p < 0.001), per capita income (p < 0.001), presence
of cats (p = 0.004) and habit of eating vegetables (p = 0.003) that showed significant association with the pres-ence of IgG antibodies were analyzed in the multivariate logistic regression model (Table III).
DISCUSSION
The prevalence of anti-T. gondii IgG antibodies ob-served in this study (49.2%) is in agreement with pre-vious seropositivity data reported in Brazil, which has varied from 31% in pregnant women from Caxias do Sul, state of Rio Grande do Sul (RS), Southern Brazil, up to 91.6% in pregnant women from Mato Grosso do Sul, Central Western region of Brazil (Detanico & Basso 2006, Figueiró-Filho et al. 2007). According to Buffo-lano (2008), climate plays an indirect role in allowing more (in the case of a moist and hot climate) or less (in the case of a dry and cold climate) survival of oocysts in the environment.
The number of cases of IgM reactivity, observed in 10 women (2.0%), was greater than that (0.4%) found by Figueiró-Filho et al. (2007) in pregnant women from the Central Western region of Brazil, using an enzyme-linked immunosorbent assay (ELISA). On the other hand, our results were similar to those found by Reiche et al. (2000), who observed 1.8% IgM seropositivity us-ing an ELISA method in women at the University Hos-pital in the city of Londrina, PR, during the period from 1996-1998. However, these results are lower than those found by Detanico and Basso (2006) (5.5%) in pregnant women from Caxias do Sul, RS, Southern region of the country, by using enzyme linked fluorescent assay.
Currently, IgM serological tests show high sensitiv-ity and are able to detect very low concentrations of this immunoglobulin. Seropositivity for IgM antibodies can persist for several months or even years after serocon-version; therefore, the IgM titre cannot be used by itself as a reliable marker of acute infection (Liesenfeld et al. 2001). Further testing for an elevated or rising IgG level, low IgG avidity, positive IgA antibodies, or a combina-tion of these is suggested in women showing IgG and
TABLE I
Prevalence of anti-Toxoplasma gondii IgG and IgM antibod-ies obtained in pregnant women from the Basic Health Units
in Londrina, Paraná, 2006
Antibodies Prevalence CI 95%
anti-T. gondiia n (%)
IgG(+) IgM(-) 236 (48.0)
IgG(+) IgM(+) 6 (1.2)
Total IgG(+) 242 (49.2) 44.7-53.6
IgG(-) IgM(+) 4 (0.8)
IgG(-) IgM(-) 246 (50.0)
Total IgG(-) 250 (50.8) 46.3-55.2
IgM seropositivity in their first prenatal test. None of these tests reliably determines the timing of parasitae-mia, and furthermore, sequential use of highly sensitive IgM assays and methods for examining IgG avidity or stage specificity in the first 12 weeks gestational age could reasonably exclude post-conceptional infection in an expectant mother (Buffolano 2008).
Six (1.2%) reactive IgG and IgM samples showed strong avidity, indicating infection in the past four months. Since all pregnant women taking part in this study were in their first trimester of pregnancy when routine serology for toxoplasmosis was performed, this result suggests infection prior to conception and,
there-fore, minimal risk of congenital infection. In one of these patients, though, the IgG titre was 1:16.000 by IFAT, in-dicating a possible recent infection. This was the only pregnant woman directed to specific treatment for toxo-plasmosis in the reference hospital in the region. This result shows the importance of quantifying antibody lev-els, mainly IgG, for the definition of acute cases.
Four other samples (0.8%) were reactive for IgM and non-reactive for IgG. These pregnant women underwent a new serologic evaluation after 15 days, with the same diagnostic tests, and presented the same results, suggest-ing false positive or non-specific IgM antibodies. This demonstrates the importance of confirming previous
se-TABLE II
Analysis of sociodemographic characteristics of pregnant women attended at Basic Health Units in Londrina, Paraná, 2006, according to the seropositivity for anti-Toxoplasma gondii IgG antibodies
Sociodemographic characteristics Reactive anti-T. gondii Odds ratio (CI 95%) p
IgG antibodies/total (%)
Residence
Urban zone 228/468 (48.7) 0.68 (0.30-1.56) 0.358b
Rural zone 14/24 (58.3)
Age group (years)
13-20 53/131 (40.5) 1.0 (reference) 0.024b
21-30 131/255 (51.4) 1.55 (1.01-2.38)
31-48 58/106 (54.7) 1.78 (1.06-2.98)
Education level
complete or incomplete 1st grade 123/205 (60.0) 1.0 (reference) < 0.001b
complete or incomplete 2nd grade 109/255 (42.7) 0.50 (0.34-0.72)
complete or incomplete 3rd grade 10/32 (31.3) 0.30 (0.14-0.67)
Per capita incomea
< R$ 150.00 95/154 (61.7) 1.0 (reference) < 0.001b
R$ 151.00-R$ 350.00 94/198 (47.5) 0.56 (0.37-0.86)
> R$ 351.00 53/137 (38.7) 0.39 (0.24-0.63)
Water consumption from the public system
Yes 234/478 (49.0) 0.72 (0.25-2.10) 0.546c
No 8/14 (57.1) 1.0 (reference)
Presence of vegetable garden at home
Yes 29/65 (44.6) 0.81 (0.48-1.37) 0.429c
No 213/427 (49.9) 1.0 (reference)
Presence of cats in home
Yes 37/55 (67.34) 2.33 (1.28-4.21) 0.004c
No 205/437(46.9) 1.0 (reference)
Presence of dogs in home
Yes 144/299 (48.2) 0.90 (0.63-1.29) 0.571c
No 98/193 (50.8) 1.0 (reference)
Consumption of raw or rare meat
Yes 92/181 (50.8) 1.11 (0.77-1.60) 0.578c
No 150/311 (48.2) 1.0 (reference)
Habit of manipulating dirt or sand
Yes 33/62 (53.2) 1.20 (0.71-2.05) 0.496c
No 209/430 (48.6) 1.0 (reference)
Habit of eating raw vegetables
Yes 235/463 (50.8) 3.24 (1.36-7.73) 0.005c
No 7/29 (24.1) 1.0 (reference)
ropositivity results. A thorough analysis of IgM results is very important to avoid exposing both the mother and the foetus to unnecessary procedures such as treatment and amniocentesis, which can be harmful for both.
When considering age group, the prevalence of IgG an-tibodies in our study population increased with age (from 31-48 years old). Despite regional differences - whether due to environment, cultural and/or eating habits - similar results were found by Spalding et al. (2005) in pregnant women in RS. This can be explained by the increase of exposure to infection sources throughout life.
Pregnant women with a low per capita income (< R$150.00) and a low education level (complete or in-complete 1st grade corresponding to a basic education) had greater risk of infection with T. gondii. Similar find-ings were reported by Avelino et al. (2004), where preg-nant women from Goiânia, state of Goiás (GO), in the Central Western region of Brazil, with a lower education level and low income (less than two minimum wages) showed a greater chance of acquiring toxoplasmosis. Va-rella et al. (2003) reported that, among all variables stud-ied, the one carrying the greatest risk for toxoplasmosis was an education level less than nine years (OR = 2.2). Liu et al. (2009) studied 235 pregnant women in Chang-chun, China, and found that a low education level was a risk factor for toxoplasmosis. These results underscore the importance of investing in education as a means of promoting health among the population.
The presence of cats in the homes of the pregnant women was also associated with a greater chance of infection by T. gondii. Avelino et al. (2004) analyzed groups of pregnant and non-pregnant women in Goiânia, GO, and verified that the presence of a cat was a risk factor only for pregnant women. The authors declared that having a cat at home, by itself, was not enough to increase the risk of acquiring a T. gondii infection. Ac-cording to Dubey (2000), direct contact with cats is
ir-relevant for T. gondii transmission; therefore, that study only evaluated the presence of cats in the environment, which could lead to soil contamination.
A habit of eating raw vegetables was also considered to be a factor associated with a greater chance of T. gon-dii infection. Our findings are in agreement with those reported by Avelino et al. (2004) in pregnant women from Goiânia, GO.
Despite Cook et al. (2000) having reported that the consumption of raw or rare meat was an important risk factor for toxoplasmosis in pregnant women, this vari-able was not significant in the present study. It was also not significant in the study carried out by Avelino et al. (2004). These contradictory results could be explained by the drastic reduction of the prevalence of toxoplasmo-sis in the swine population in the Northern region of PR, one of the most important species in the transmission of toxoplasmosis to the human population in Brazil (Vidot-to et al. 1990, Tsutsui et al. 2003, Olbrich-Ne(Vidot-to & Meira 2004, Carletti et al. 2005, Dias et al. 2005). This reduc-tion likely occurred as a result of increased technology and sanitary improvements adopted on swine farms over the last 15 years.
There was no significant association of T. gondii infection with either the habit of manipulating dirt and sand by pregnant women or the presence of a vegetable garden at home. These data differed from the results of Spalding et al. (2005) in pregnant women in RS, where contact with soil was the greatest risk factor associated with T. gondii infection.
These differences in factors associated with infec-tion underscore the need for regional studies in order to determine epidemiologic and sociodemographic charac-teristics specific for each population, allowing the devel-opment of targeted methods for control and prophylaxis for toxoplasmosis.
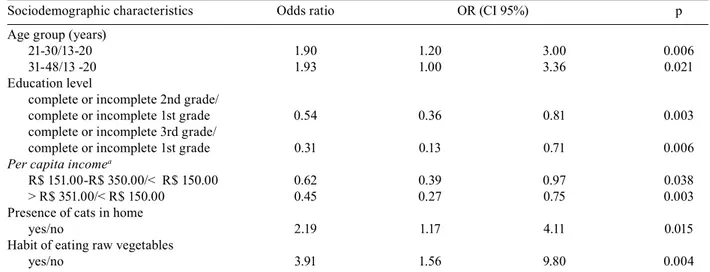
TABLE III
Multi-varied logistic regression for IgG anti-Toxoplasma gondii positive samples obtained from pregnant women attended at the Basic Health Units in Londrina, Paraná, 2006, according to the sociodemographic characteristics evaluated
Sociodemographic characteristics Odds ratio OR (CI 95%) p
Age group (years)
21-30/13-20 1.90 1.20 3.00 0.006
31-48/13 -20 1.93 1.00 3.36 0.021
Education level
complete or incomplete 2nd grade/
complete or incomplete 1st grade 0.54 0.36 0.81 0.003
complete or incomplete 3rd grade/
complete or incomplete 1st grade 0.31 0.13 0.71 0.006
Per capita incomea
R$ 151.00-R$ 350.00/< R$ 150.00 0.62 0.39 0.97 0.038
> R$ 351.00/< R$ 150.00 0.45 0.27 0.75 0.003
Presence of cats in home
yes/no 2.19 1.17 4.11 0.015
Habit of eating raw vegetables
yes/no 3.91 1.56 9.80 0.004
This study revealed high seropositivity for IgG an-tibodies for toxoplasmosis in pregnant women (49.2%); however, 50.8% of women studied were susceptible dur-ing pregnancy. Age, low per capita income, low educa-tion level, the presence of cats in the home and inges-tion of raw vegetables were all factors associated with a greater risk of T. gondii infection.
ACKNOWLEDGEMENTS
To the Municipal Health Office of Londrina (Basic Health Units and CENTROLAB) and to the University Hospital from Londrina State University.
REFERENCES
Avelino MM, Campos-Júnior D, Parada JB, Castro AM 2004. Risk factors for Toxoplasma gondii infection in women of childbear-ing age. Braz J Infect Dis 8: 164-174.
Beguetto E, Buffolano W, Spadoni A, Del Pezzo M, Di Cristina M, Minenkova O, Petersen E, Felici F, Gargano N 2003. Use of an Immunoglobulin G Avidity Assay based on recombinant antigens for diagnosis of primary Toxoplasma gondii infection during pregnancy. J Clin Microbiol41: 5414-5418.
Buffolano W 2008.Congenital toxoplasmosis: The state of the art. Parassitologia 50:37-43.
Camargo ME 1974. Introdução às técnicas de imunofluorescência. Rev Bras Patol Clín 10: 87-107.
Carletti RT, Freire RL, Shimada MT, Ruffolo BB, Begale LP, Lopes FMR, Navarro IT 2005. Prevalência da infecção por Toxoplasma gondii em suínos abatidos no Estado do Paraná, Brasil. Semina, Ciências Agrárias 26: 563-568.
Cook AJC, Gilbert RE, Buffolano W, Zufferey J, Petersen E, Jenum PA, Foulon W, Semprini AE, Dunn DT, Holliman R 2000. Sourc-es of Toxoplasma infection in pregnant women: European multi-centre case-control study. Br Med J 321: 142-147.
Dean AG, Dean JA, Coulomerier D, Brendel KA, Smith DC, Bur-ton AH, Dicker RC, Sulivan KM, Fagan RF, Arner TG 1994. Epi Info, version 6: a word processing, data bases, and statistic pro-gram for epidemiology on microcomputers. Center for Diseases Control and Prevention, Atlanta, Georgia, USA.
Detanico L, Basso RMC 2006. Toxoplasmose: perfil sorológico de mulheres em idade fértil e gestantes. Rev Bras An Clín 38: 15-18.
Dias RAF, Navarro IT, Ruffolo BB, Bugni FM, Castro MV, Freire RL 2005. Toxoplasma gondii em lingüiça de carne suína tipo frescal, com investigação soroepidemiológica em trabalhadores de estabe-lecimentos produtores. Rev Inst Med Trop Sao Paulo 47: 185-189.
Dubey JP 2000. Sources of Toxoplasma gondii infection in pregnan-cy. Br Med J 32: 127-128.
Dunn D, Wallon M, Peyron F, Petersen E, Peckhan C, Gilbert R 1999. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counseling. Lancet353: 1829-1833.
Figueiró-Filho EA, Senefonte FRA, Lopes AHA, Morais OO, Souza Júnior VG, Maia TL, Duarte G 2007. Freqüência das infecções pelo HIV-1, rubéola, sífilis, toxoplasmose, citomegalovírus, her-pes simples, hepatite B, hepatite C, doença de Chagas e HTLV I/
II em gestantes do Estado de Mato Grosso do Sul. Rev Soc Bras Med Trop 40: 181-187.
Frenkel JK 2002. Toxoplasmose.In R Veronesi, Tratado de Infectolo-gia, 2nd ed., Atheneu, São Paulo, p. 1310-1325.
IPARDES - Instituto Paranaense de Desenvolvimento Econômico e Social [homepage on the Internet]. [cited 2009 Feb 05]. Available from: http://www.ipardes.gov.br/perfil_municipal/MontaPerfil. php?Municipio=86000&btOk=ok.
Jones JL, Lopez A, Wilson M, Schulkin J, Gibbs R 2003. Congenital toxoplasmosis: a Review. Obstet Gynecol Surv56: 296-305.
Liesenfeld O, Montoya JG, Kinney S, Press C, Remington JS 2001. Effect of testing for IgG Avidity in the diagnosis of Toxoplasma gondii infection in pregnant women: experience in a referencel. J Infect Dis 183: 1248-1253.
Liu Q, Wei F, Gao S, Jiang L, Lian H, Yuan B, Yuan Z, Xia Z, Liu B, Xu X, Zhu XQ 2009. Toxoplasma gondii infection in pregnant women in China. Trans R Soc Trop Med Hyg 103: 162-166.
Lopez A, Dietz VJ, Wilson M, Navin TR, Jones JL 2000. Preventing congenital toxoplasmosis. MMWR Recomm Rep 49: 59-68.
Montoya JG, Rosso F 2005. Diagnosis and management of Toxoplas-mosis. Clin Perinatol 32: 705-726.
Mozzatto L, Procianoy RS 2003. Incidence of congenital toxoplas-mosis in Southern Brazil: a prospective study. Rev Inst Med Trop Sao Paulo 45: 147-151.
Olbrich-Neto J, Meira DA 2004. Soroprevalência de vírus linfotrópico de células T humanas, vírus da imunodeficiência humana, sífilis e toxoplasmose em gestantes de Botucatu - São Paulo - Brasil. Fatores de risco para vírus linfotrópico de células T humana. Rev Soc Bras Med Trop 37: 28-32.
Sanepar - Companhia de Saneamento do Paraná - Paraná 2009. [cited 2009 Feb 05]. Available from: http://www.sanepar.com.br/sanepar/ calandrakbx/calandra.nsf/0/1335E061E927FB3B8325703300496 D80?OpenDocument&pub=T&proj=InternetSanepar.
Reiche EMV, Morimoto HK, Farias GN, Hisatsugu KR, Geller L, Gomes ACLF, Inoue HY, Rodrigues G, Matsuo T 2000. Prevalên-cia de tripanossomíase americana, sífilis, toxoplasmose, rubéola, hepatite B, hepatite C e da infecção pelo vírus da imunodeficiên-cia humana, avaliada por intermédio de testes sorológicos, em gestantes atendidas no período de 1996 a 1998 no Hospital Uni-versitário Regional Norte do Paraná (Universidade Estadual de Londrina, Paraná, Brasil). Rev Soc Bras Med Trop 33: 519-527.
Spalding SM, Amendoeira MRR, Klein CH, Ribeiro LC 2005. Serolog-ical screening and toxoplasmosis exposure factors among pregnant women in South of Brazil. Rev Soc Bras Med Trop 38: 173-177.
Tsutsui VS, Navarro IT, Freire RL, Freitas JC, Prudêncio LB, Delbem ACB, Marana ERM 2003. Soroepidemiologia e fatores associa-dos à transmissão do Toxoplasma gondii em suínos do norte do Paraná. Arch Vet Sci 8: 27-34.
Varella IS, Wagner MB, Darela AC, Nunes LM, Muller RW 2003. Prevalência de soropositividade para a toxoplasmose em gestant-es. J Pediatr 79: 69-74.