This is an Open Access artcle distributed under the terms of the Creatie Commons
Attributon NonCCommercial iicense, hhich permits unrestricted nonCcommercial use, distributon,
and reproducton in any medium, proiided the original hork is properly cited. Fonte:
http://hhh.scielo.br/scielo.phpsscriptssci_aarttext&pidsS2237C
93632015000100028&lngsen&nrmsiso. Acesso em: 20 mar. 2018.
REFERÊNCIA
FORTES, Renata Costa; SOUZA, Jhuly Amado; NOVAES, Maria Rita Carialho Garbi. A doubleCblind,
randomized and placeboCcontrolled clinical trial hith Agaricus syliatcus fungus in anthropometric
profile of homen hith colon cancer. Journal of Coloproctology (Rio de Janeiro), Rio de Janeiro, i.
35, n. 1, p. 28C34, jan./mar. 2015. Disponíiel em: <http://hhh.scielo.br/scielo.phps
scriptssci_aarttext&pidsS2237C93632015000100028&lngsen&nrmsiso>. Acesso em: 20 mar. 2018.
doi: http://dx.doi.org/10.1016/j.jcol.2015.01.001.
w w w . j c o l . o r g . b r
Journal
of
Coloproctology
Original
Article
A
double-blind,
randomized
and
placebo-controlled
clinical
trial
with
Agaricus
sylvaticus
fungus
in
anthropometric
profile
of
women
with
colon
cancer
Renata
Costa
Fortes
a,b,∗,
Jhuly
Amado
Souza
b,
Maria
Rita
Carvalho
Garbi
Novaes
caCursodeNutric¸ão,InstitutodeCiênciasdaSaúde,UniversidadePaulista(UNIP),Brasília,DF,Brazil
bProgramadeResidênciaemNutric¸ãoClínica,HospitalRegionaldaAsaNorte,SecretariadeEstadodeSaúdedoDistritoFederal
(HRAN/SES/DF),Brasília,DF,Brazil
cCursodeMedicina,EscolaSuperiordeCiênciasdaSaúde(ESCS),Fundac¸ãodeEnsinoePesquisaemCiênciasdaSaúde(FEPECS),
SecretariadeEstadodeSaúdedoDistritoFederal(HRAN/SES/DF),UniversidadedeBrasília(UnB),Brasília,DF,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received22February2014 Accepted27July2014
Availableonline28January2015
Keywords:
Anthropometry Colorectalcancer
Agaricussylvaticusfungi
a
b
s
t
r
a
c
t
Introduction:Colorectalcancerisadiseaseinfluencedbygeneticandenvironmentalfactors. Medicinalfungiand/oritsextractshavebeenusedintheadjuvanttherapyofcancerbecause oftheirpharmacological,nutritionalandimmunomodulatoryproperties.
Objective:Toevaluatetheanthropometricprofileofcolorectalcancerwomenafterdietary supplementationwithAgaricussylvaticusfungus.
Methods:Randomized,double-blind,placebo-controlledclinicaltrialwasconductedina publichospitalintheFederalDistrict–Brazilforsixmonths.Sampleof32patientswith colorectalcancer,female,wasseparatedintotwogroups:supplementedwithAgaricus syl-vaticus(30mg/kg/day)andplacebo.Weconductedanthropometry(weight,height,bodymass index,armcircumference,tricepsskinfold,armmusclecircumferenceandfatpercentage) duringthetreatment.Theresultswereanalyzedatthreedifferenttimes(beforethestartof treatment,threemonthsandaftersixmonthssupplementation)usingtheMicrosoftExcel 2007andSPSS19.0,usingStudent’st-testandF,withsignificanceforp≤0.05.
Results:TheAgaricussylvaticusgroupshowedasignificantincreaseinbodymassindex,arm circumference,percentbodyfatandtricepsskinfold,andnon-significantincreaseinarm musclecircumferenceaftersixmonthsofsupplementation.Theseresultswerenotobserved intheplacebogroup.
Conclusion:TheresultssuggestthatdietarysupplementationwithAgaricussylvaticusis capa-bletohavebenefitsinanthropometricparametersofwomenwithcolorectalcancer.
©2015SociedadeBrasileiradeColoproctologia.PublishedbyElsevierEditoraLtda.All rightsreserved.
∗ Correspondingauthorat:CursodeNutric¸ão,InstitutodeCiênciasdaSaúde,UniversidadePaulista(UNIP),Brasília,DF,Brazil.
E-mail:fortes.rc@gmail.com(R.C.Fortes).
http://dx.doi.org/10.1016/j.jcol.2015.01.001
Ensaio
clínico
duplo
cego,
randomizado
e
placebo
controlado
com
fungos
Agaricus
sylvaticus
no
perfil
antropométrico
de
mulheres
com
câncer
colorretal
Palavras-chave:
Antropometria Câncercolorretal FungosAgaricussylvaticus
r
e
s
u
m
o
Introduc¸ão: Ocâncercolorretaléumadoenc¸ainfluenciadaporfatoresgenéticose ambi-entais. Autilizac¸ão de fungos medicinais e/oude seus extratostem sidoutilizada no adjuvantetratamentodocâncerdevidoàssuaspropriedadesfarmacológicas,nutricionais eimunomoduladoras.
Objetivo: Avaliar o perfil antropométrico de mulheres com câncer colorretal após suplementac¸ãodietéticacomfungosAgaricussylvaticus.
Métodos:Ensaioclínicorandomizado,duplo-cego,placebo-controladorealizadoemum hos-pitalpúblicodoDistritoFederalBrasilporseismeses.Amostraconstituídapor32pacientes comcâncercolorretal,sexofeminino,separadosemdoisgrupos:suplementadocom Agar-icussylvaticus(30mg/kg/dia)eplacebo.Realizou-seaantropometria(peso,estatura,índice demassacorporal,circunferênciadobrac¸o,dobracutâneatricipital,circunferência muscu-lardobrac¸oepercentualdegordura)aolongodotratamento.Osresultadosforamanalisados emtrêsmomentosdistintos(antesdoiníciodotratamento,comtrêsmeseseapósseis mesesdesuplementac¸ão),utilizandoosprogramasMicrosoftExcel2007eSPSS19.0,por meiodostestesT-studenteF,comsignificânciaparap≤0,05.
Resultados:OgrupoAgaricussylvaticusapresentouaumentosignificativodeíndicedemassa corporal,circunferênciadobrac¸o,percentualdegorduracorporaledobracutânea tricip-tale,aumentonãosignificativodecircunferênciamusculardobrac¸oapósseismesesde suplementac¸ão.Essesresultadosnãoforamobservadosnogrupoplacebo.
Conclusão: Osresultadossugeremqueasuplementac¸ãodietéticacomAgaricussylvaticus écapazdeexercerbenefíciosnosparâmetrosantropométricosdemulherescomcâncer colorretal.
©2015SociedadeBrasileiradeColoproctologia.PublicadoporElsevierEditoraLtda. Todososdireitosreservados.
Introduction
Nowadays,duetoitsincreasingincidence,cancerhasbecome apublichealthproblemworldwide,1paripassuwiththe
pro-gressive aging of the population, as a consequence of an increasedlifeexpectancy.2
Colorectalcancerisacommonanddeadlydisease, influ-encedbygeneticandenvironmentalfactorsandalsobythe mutualinfluenceofboth.Geneticpredispositionisa predom-inantriskfactorforsomeindividuals;however,environmental factors,includingdiet,physicalactivity,smokingandobesity, arealsoincludedamonghigh-riskfactors.2
Asfortheriskofdevelopingcolorectalcancer,patientscan bedividedasfollows:thoselessthan50yearsandno fam-ilyhistoryofcolorectalcancerareatlowrisk;thoseaged50or moreandwithnootherriskfactorsareincludedintheaverage riskgroup;patientswithpersonalhistoryofpolypsor colorec-talcancer,orwithafamilyhistoryofcolorectalcancerorwith first-degreerelativesdiagnosedwithpolypsareclassifiedas high-riskpeople;andfinally,theveryhigh-riskclassification comprisesthosepatientswithpolypoidsyndromes,orwho aresufferingfrominflammatoryboweldisease.3
Most often, a diagnosis of cancer leads to a phase of muchanxietyand distress, possiblytriggering a picture of depression.Inturn,thedepressioncomesinassociationwith somaticsymptomssuchaslossofappetiteandfatigue,which
mayalsobeassociatedwiththecatabolismand/ortreatment ofthedisease.4
The use of medicinal fungi and/or their extracts as dietary supplements hasincreased considerably, thanksto itsanti-tumor,anticarcinogenic,antiviral,anti-inflammatory, hypoglycemic,hypocholesterolemicandhypotensiveeffects, amongothers,andtheseproductsmayberecommendedas adjuvantsinthetreatmentofmalignantneoplasms.5
Considering the prominence of this theme, this study aimedtoevaluatetheanthropometricprofileofwomenwith colorectalcancerafterdietarysupplementationwiththe fun-gusAgaricussylvaticus.
Methods
Studydesign
Thestudy consistsofarandomized,double-blind, placebo-controlledstudy,whichwasapprovedbytheEthicsCommittee onHumanResearch,StateSecretariatofHealth,Distrito Fed-eral(CEP/SES/DF)underProtocol051/04.Thepatients’freeand informedconsent(FIC)wasobtained,andtheirparticipation was voluntary.The study was conductedatthe Proctology OutpatientClinic,HospitaldeBasedoDistritoFederal,Brazil, betweenNovember2004andJuly2006.
Therandomizationprocedureoccurredthroughsequential numbersrandomlygeneratedbycomputer,whereeach ran-domnumber correspondedtoagroupreceiving thefungus (GroupA)orplacebo(GroupB).Thesenumberswereinserted intoopaque,nottranslucentandclosedenvelopes,withthe generationofthenumbersequenceperformedbyaresearcher blindedtothestudy,afterselectionofpatientswithinclusion andexclusioncriteria.Theenvelopeswere opened sequen-tiallyasthepatientswereconsecutivelyrecruitedforthestudy andcontainedthegrouptowhichthepatientwouldbelong. Onlyafterperformingthestatisticalanalysis,itwasrevealed whichgrouphadreceivedplaceboandwhichreceivedAgaricus sylvaticus.
Patients
The sample consisted of patients with colorectal cancer divided into two groups: those who received placebo and those supplementedwithAgaricussylvaticus. Thefollowing inclusioncriteriashouldbefulfilled:femalepatientswitha confirmed diagnosis of colorectal cancer inthe postopera-tivephase,from threemonthstotwoyearsofsurgery,and olderthan20years.Exclusioncriteriawere:pregnantwomen, breastfeedingmothers,bedriddenindividuals,physically dis-abledpeople,patients usinganalternative therapyor with otherchronicnon-communicablediseases,andinmetastasis process.
Agaricussylvaticusextract
With awidespread geographical distribution and naturally occurringinBrazil,Agaricussylvaticuswasfirstdescribed in Switzerland.ItsidentificationwasconfirmedbytheLondon RoyalBotanicGardens,whosedocumentationwasprovided bytheInstituto deBotânica,EnvironmentStateSecretariat, SãoPaulo,inNovember10,1995.TheAgaricussylvaticusfungus (Family:Agaricaceae),whosepopularnameisSunMushroom, wasobtainedfromaproducerdulyaccreditedbytheEmpresa BrasileiradePesquisaAgropecuária–Embrapa,fromTapiraí, StateofSaoPaulo,Brazil.Thefungusextractwasobtainedby soakingthedehydratedmaterialinhotwaterduring30min; then,the materialwasliquified,sievedand driedina des-iccator.Theanalysis ofAgaricussylvaticus compositionwas performedbytheJapanFoodResearchLaboratoriesCenterand revealedthepresenceofcarbohydrates(18.51g/100g),lipids (0.04g/100g),ergosterol(624mg/100g),proteins(4.99g/100g), amino acids (arginine – 1.14%; lysine – 1.23%, histidine – 0.51%,phenylalanine–0.92%,tyrosine–0.67%,leucine–143% methionine–0.32%,valine–1.03%,alanine–1.28%glycine– 0.94%,proline–0.95%,glutamicacid–3.93%,serine–096%, threonine–0.96%,asparticacid–1.81%,tryptophan–0.32% cysteine–0.25%)andtraceamountsofmicronutrients.
Thedryextractwastransformedintotablets,inaccordance withpharmacotechnicalprocedure.Thedosageofthefungus administeredtopatientsfromthesupplementedgroupwas equivalentto30mg/kg/day,dividedintotwodailydoses(six tabletsaday,threeinthemorningandthreeintheafternoon, inbetweenmeals),consideringthemeanweightofthestudy populationoveraperiodofsixmonths.Asforthegroupof patientswhoreceivedplacebo,thetabletswereadministered
inthesamequantities,withthesameexcipientsandenergy, butwithouttheextractofAgaricussylvaticus(initsplace,the placebogroupreceivedstarch).
Clinicalevolution
Patientswerefollowedforsixmonths.Duringthefirstthree months,the visitswere heldfortnightlyforclinical assess-mentand,inthelastthreemonths,thevisitswereheldevery 30days.
Thefoodanamnesis(semiquantitativeand24-hrecallfood consumptionfrequencyquestionnaire)washeldonthefirst and last days ofconsultation. However, the patients were instructedtoremainwiththeusualdiet,inordernotto inter-ferewith theintervention, althoughduringtreatmentthey havereceivedguidelinesonhowtomaintainahealthydiet. Aftera6-month follow-up, anindividualizeddietwas sug-gested forall patients,who,whennecessary, werereferred tootherhealthprofessionals.
The anthropometric assessment was performed using bodymassindex(BMI),tricepsskinfoldthickness(TSF),arm circumference (AC), arm muscle circumference (AMC) and bodyfatpercent(%BF).However,forstatisticalpurposes,we extractedtheaverageoftheresultsobtainedinthreedifferent times:beforestartingsupplementation,afterthreemonthsof treatmentandaftersixmonthsoftreatment.
Allpatientswerefollowedweeklybyresearcherstoclarify anydoubts,checkontheproperuseofthemushroomandfor confirmationoftheschedule,ensuringgreateradherenceto treatmentandcontrolonthecontinuityofthestudy.
We considered as dropouts those patients who did not attendtheconsultationsduringthefullperiodofsixmonths. Thosepatientswho died beforetheend oftreatment were excludedfromthesample.
Anthropometricassessment
Aspecialformofanthropometricassessment,tobefilledinall theconsultations,wasused.Weightdeterminationwas per-formedwiththepatientbarefooted,wearinglightclothingand withoutjewelleryinterferingwiththemeasurementresults. Thepatientshouldremainstandinginthecenterofthescale, withherbodyweightequallydistributedbetweenbothfeet.6
In ordertoobtainthis variable,aPlenna® –Resolvedigital
scale(MEA-02500model)withbioimpedance(BIA),capacityof 150kg,with0.1kgvariationandproperlycalibratedwasused. For heightmeasurement, the barefooted patient should stayuprightandinanerectposition,withherbodyliftedat maximumextension,headup,lookingforward,inaFrankfurt position,withherbackandthebackofherkneestouchingthe wallandwithfeettogether.6TheFrankfurtanatomicalplane
extendsfromthebottommarginoftheeyesockettothetop borderoftheauditorycanal.7Patients’heightwasmeasured
onlyonce,incentimeters(cm),witha150-cmlonginelastic measuringtapeattachedtoaflatwallwithoutbaseboardand fixedat50cmfromtheground.Awoodsquarewasplacedon topoftheheadofthepatient;withthis,weobtainedameasure with0.1-cmaccuracy.
After data acquisition (weight and height), BMI was obtainedbydividingthepatient’sweightinkilogramsbyher
heightinmeterssquared.BMIvalues<18.5kg/m2would
char-acterizethinness;≥18,5kg/m2and<25kg/m2,normalweight;
≥25kg/m2and<30kg/m2,overweight;and≥30kg/m2,obesity,
accordingtotheclassificationrecommendedbyWorldHealth Organization.8
TSFwas measuredusingaCescorf® quick-reading
com-pass,witharangeupto60mmandaccuracyof±1mm.Three consecutivemeasurementswereobtainedfromTSF,andthe arithmeticaverageofthemeasuredvalueswasconsidered. As for AC measurement, an inextensible-material,150-cm length,1-cmscalemeasuringtapewasused.TheAMCvalue wasobtainedbytheformula:AMC=AC–(0.314×TSF).8
TSF, AC and AMC measures were compared to a Frisancho’s9 referencestandard,and theadequacywas
cal-culatedbydividingthevaluesobtainedbythe50thpercentile andmultiplyingtheresultby100.Astothenutritionalstatus classification,thefollowingvalueswereconsidered:obesity: >120%,overweight:110–120%,normalweight:90–110%,mild malnutrition: 80–90%, moderate malnutrition: 70–80% and severemalnutrition:<70%.10
Thebodyfatpercentage(%BF)wasobtainedalsousingthe Plenna®digitalscale.
Statisticalanalysis
Thepresentedvalueswerecomparedandanalyzedapplying
t-Studentand F statisticaltests,using MicrosoftExcel2007 andSPSS(StatisticalPackagefortheSocialSciences,SPSSInc, Chicago,USA)forWindows,version19.0.Theaccepted statis-ticalsignificanceprobabilitywasp<0.05.
Results
Afterafollow-upofsixmonthsintheProctologyOutpatient Clinic,HospitaldeBasedoDistritoFederal,atotalof40women withcolorectalcancerwhomettheinclusionandexclusion criteriaagreed toparticipateinthe research,and, ofthese patients,twodiedandsixdroppedoutforvarious reasons. Thefinalsampleconsistedof32patientswithameanageof 56.66±14.07years,atstagesI(n=4),II(n=12)andIII(n=16), separatedintogroupsreceivingplacebo(n=16)andAgaricus sylvaticus(n=16).Themeanageswere57.67±13.42yearsand 55.87±15.11 years for placebo and Agaricus sylvaticus sup-plementedgroups,respectively,withnodifferencebetween groups(p=0.39).
As for the body mass index, we observed that the placebogrouphad aninitialBMIof24.25±5.33kg/m2;after
three months, a significant increase in BMI was noted (from 24.25±5.33kg/m2 to 24.40±5.15kg/m2, p=0.01) and
in the sixth month, there was a further increase (from 24.25±5.33kg/m2to24.71±4.73kg/m2,p=0.06),butthislast
resultwasnotstatisticallysignificant(Fig.1).
The supplemented group showed an initial BMI of 24.44±4.59kg/m2;afterthreemonths,asignificantincrease
to 24.91±4.18kg/m2 (p=0.02) was observed, and after six
months a further significant increase to 25.16±3.92kg/m2
occurred,comparedtobaseline(p=0.02)(Fig.1).
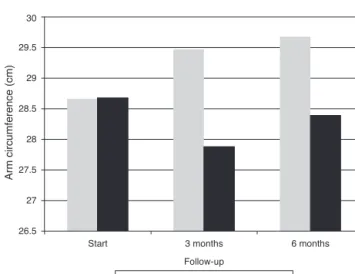
Regardingarmcircumference(AC),intheplacebogroupan initialvalueof28.68±5.80cmforthisvariablewasobserved;
Start 3 months 6 months Follow-up 23.6 23.8 24 24.2 24.4 24.6 24.8 25 25.2 Bmi (kg/m 2)
Agaricus sylvaticus Placebo
Fig.1–Evolutionofbodymassindex(BMI)ofwomenwith colorectalcancerinplacebo(n=16)andAgaricussylvaticus
(n=16)groups,treatedattheProctologyOutpatientClinic, HospitaldeBasedoDistritoFederal,throughouttheclinical follow-up.Placebo:p=0.01andp=0.06andAgaricus sylvaticus:p=0.02andp=0.02,afterthreeandsixmonths, respectively.Student’st-test.
after three months, this value had suffered a significant decline, to 27.88±4.56cm (p=0.05) and, after six months, a non-significant increase, to 28.39±4.39cm (p=0.31), was observed(Fig.2).
The Agaricussylvaticus group presented initialvaluesof 28.66±4.19cmforAC,withasignificantincreaseoverthree (29.47±4.10cm,p=0.01)andsix(29.68±3,74cm; p=0.0001) monthsofsupplementation(Fig.2).
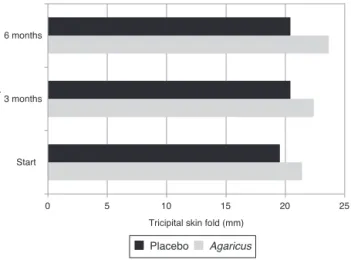
As fortricipitalskinfoldthickness, it was observedthat theplacebogrouphadaninitialmeanof19.53±8.27mmfor
26.5 27 27.5 28 28.5 29 29.5 30
Start 3 months 6 months
Arm circumference (cm)
Follow-up
Agaricus sylvaticus Placebo
Fig.2–Armcircumference(AC)ofwomenwithcolorectal cancerofplacebo(n=16)andAgaricussylvaticus(n=16) groupstreatedattheProctologyOutpatientClinic,Hospital deBasedoDistritoFederal,throughouttheclinical follow-up.Placebo:p=0.05andp=0.31andAgaricus sylvaticus:p=0.01andp=0.0001,afterthreeandsix months,respectively.Student’st-test.
5 0 10 15 20 25 Start 3 months 6 months Follow-up time
Tricipital skin fold (mm)
Placebo Agaricus
Fig.3–Tricipitalskinfold(TSF)ofwomenwithcolorectal cancerofplacebo(n=16)andAgaricussylvaticus(n=16) groupstreatedattheProctologyOutpatientClinic,Hospital deBasedoDistritoFederal,throughouttheclinical follow-up.Placebo:p=0.14andp=0.19andAgaricus sylvaticus:p=0.16andp=0.05,afterthreeandsixmonths, respectively.Student’st-test.
TSF.Afteranintervalofthreemonths,therewasanincrease to20.43±9.14mm(p=0.14)and aftersixmonths,afurther increaseto20.42±8.33mm(p=0.19).Butthesechangeswere notstatisticallysignificant(Fig.3).
Withinthreemonthsofsupplementation,theAgaricus syl-vaticusgroupshowedanon-significantincreaseinTSF(from 21.41±7.44mm to 22.38±5.95mm; p=0.16), followed after sixmonthsbyasignificantincrease(from21.41±7.44mmto 23.66±5.62mm,p=0.05)(Fig.3).
In the placebo group, after arm muscle circumference measurement,anon-significantdecreaseafterthreemonths (from 23.44±4.42cm to 22.82±3.16cm, p=0.14) and a sig-nificant decrease after sixmonths (from 23.44±4.42cm to 22.30±3.29cm,p=0.03)wereobserved(Fig.4).
These findings were not found in the Agaricus syl-vaticus group, which increased its AMC after three (from 21.94±2.61m to 22.45±2.44cm, p=0.10) and six (from 21, 94±2.61cmto22.28±2.65cm,p=0.22)monthsof supplemen-tation,althoughthesechangeswerenotsignificant(Fig.4).
Astothepercentageofbodyfat,the placebogroup pre-sentedinitiallya%BFof36.33±8.44%.Afterthreemonths,this variable had increased(from36.33±8.44%to 36.46±7.25%,
p=0.44)andinthe sixth month,anewincreasewasagain noted(from36.33±8.44%to37.60±8.07%,p=0.19).Butthese changeswerenotstatisticallysignificant(Fig.5).
TheAgaricussylvaticusgrouppresentedaninitial%BFof 36.88±7.33%.Afterthreemonths,anon-significantincrease to37.88±6.60%(p=0.09)wasfound,andaftersixmonthsa significant increase to 39.56±8.68% (p=0.04) was observed (Fig.5).
Discussion
Inthisstudy,thesamplewasconvenientlycomposedof100% ofwomenwithcolorectalcancer.Scientificevidencesuggests
21 21.5 22 22.5 23 23.5 24
Start 3 months 6 months Follow-up
Arm muscle circumference (cm)
Agaricus Placebo
Fig.4–Armmusclecircumference(AMC)ofwomenwith colorectalcancerofplacebo(n=16)andAgaricussylvaticus
(n=16)groupstreatedattheProctologyOutpatientClinic, HospitaldeBasedoDistritoFederal,throughouttheclinical follow-up.Placebo:p=0.14andp=0.03andAgaricus sylvaticus:p=0.10andp=0.22,afterthreeandsixmonths, respectively.Student’st-test.
thatcolorectalcancerismoreprevalentinwomen,affecting moreandmoreoftentheleftcolon.11InBrazil,theInstituto
Nacional do Câncer (INCA) registry for 2012estimated the occurrenceof14,180newcasesofcolorectalcancerinmen andof15,960casesinwomen,correspondingtoanestimated riskof15newcasesper100,000menand16newcasesoutof every100,000women.12
The mean age of our patients was 55 and 57 years for placeboandAgaricussylvaticusgroups,respectively.Inthese groups, the minimum andmaximum ages were 32 and 77 years,respectively.AccordingtoCozerattolinietal.,13more
than90%ofcolonandrectalcancersrelatetoindividualsolder than 50 years,and 75% ofcasesaffect individuals without otherriskfactors,besidesage.
40 39.5 39 38.5 38 37.5 37 36.5 36 35.5 35 34.5
Body fat percentage
Start 3 months 6 months Follow-up
Placebo Agaricus sylvaticus
Fig.5–Bodyfatpercentage(%BF)ofwomenwithcolorectal cancerofplacebo(n=16)andAgaricussylvaticus(n=16) groupstreatedattheProctologyOutpatientClinic,Hospital deBasedoDistritoFederal,throughouttheclinical follow-up.Placebo:p=0.44andp=0.19andAgaricus sylvaticus:p=0.09andp=0.04,afterthreeandsixmonths, respectively.Student’st-test.
Inthisstudy,bothplaceboandAgaricussylvaticusgroups had aninitialBMIwithinthenormalweight range,with a tendencytobeoverweight.Scientificstudieshaveshown a positivecorrelationbetweenoverweight,obesityandriskof developingseveraltypesofcancer,aswellasinmortalityfrom thisdisease.Itisexpectedthatthe probablemechanism is interconnectedwithhyperinsulinemiaandwithahighlevel ofinsulin-dependentgrowthfactor(IGF-1)andofthose pro-teinsthatbindtoIGF-1,aswellaswiththepracticeofdiets characterizedbytoomuchenergyconsumption.13
Thereisahighercorrelationbetweenexcessweightand riskofcolorectalcancer,inwhichtheabdominalorcentral distributionofbodyfatisthemaincomponentofincreasing thisrisk,asthisoccurrenceisrobustlylinkedtoinsulin resis-tanceandhyperinsulinemia.13However,inthisstudy,visceral
fatwasnotevaluated.
Clinicalandexperimentalstudiesshowthatthediet sup-plementedwithAgaricussylvaticusandotherfungipromotes positive effects with respect to nutritional, medicinal and pharmacologicaleffects,andthatthesesupplementscanbe usedasadjuvantsincancertreatment.14Medicinalfungiexert
anaboliceffects,becausetheycontainalltheessentialamino acids,plusimmunonutrientslikearginineandglutaminethat, intimesofmetabolicstress,becomeconditionallyessential, contributingtoimprovementsinnitrogenbalance.1,15
Inthis study,theAgaricussylvaticus groupobtained bet-teranthropometricresults(BMI,AC,TSF,%BF)versusplacebo, includingin relationtoleanbody mass,despiteno signifi-cantfinding.Scientificevidencesuggeststhatmedicinalfungi havebioactivecompoundsabletopreventthemuscleprotein catabolismcommonlypresentinthesepatients,explainingin parttheresultsobserved.15–17
Themechanismsofactionofexistingbioactivecompounds in fungi are not yet fully explained in the literature, but scientificstudies suggestthat thesesubstances can modu-latecarcinogenesis,notonlyintheearlystages,butalsoin advancedphasesintheprogressionofthedisease,especially bystimulatingtheimmunesystem.14
Wefoundnoscientificpapersintheliteraturethat eval-uatedanthropometryand/orthenutritionalstatusofcancer patientsafterdietarysupplementationwithmedicinalfungi, including those of the Agaricaceae family, species Agari-cussylvaticus.However,clinicalstudiesshowthatmedicinal fungiareabletomodulatethemetabolismofcarbohydrates, proteins and lipids, besides exerting beneficial effects on the hematopoietic, immune and gastrointestinal systems, with positive repercussions on the quality of life ofthese patients.15–24
Majormetabolicalterationsinducedbyadvancedtumors include glucose intolerance, decreased insulin secretion, peripheral insulin resistance, increased synthesis and glucose turnover, increased activity of the Cori cycle, increased protein turnover, increased hepatic protein synthesis, increased muscle protein catabolism, reduced plasma concentration of branched chain amino acids, depletion of lipid deposits, increased lipolysis, increased glycerolandfreefattyacidturnover,reducedlipogenesisand hyperlipidemia.4,5,15,16,20,21,23
The depletion of adipose tissue is responsible, in large part,bytheweightlossobservedincancerpatients.Thisis
due tothe different changes infatty acid metabolism and alsototheoccurrenceoflipolysis,increasedlipidoxidation, reductionoflipogenesisandoflipoproteinlipaseactivity,and increased release of lipolytic tumor factors and hormone-sensitivelipase,resultinginhyperlipidemia.15,16,19,20,22
The beneficial effects ofthese fungihave been demon-strated,withinhibitionofanti-tumoractivityandproliferation ofcancercells,expansionofnaturalkillercellfunctionand ofotherimmunologicalparameters,suchasthesecretionof immunoglobulinsIgA,IgMandIgE,andaprogressionof mono-cyteandmacrophagefunctions.5,16,21,22
Itisnoteworthythat,inadditiontohighbiologicalvalue proteinsandofimmunomodulatoraminoacids(suchas argi-nineand glutamine)thathelpinmuscleproteinanabolism of cancer patients, other substances present in medicinal fungistandout:glucans,proteoglucans,lectins,ergosteroland triterpenes– all withthe abilityofmodulatingthe various metabolicandimmuneactionsinthesepatients.5,15,16,22,23
Conclusion
Ourresultssuggestthatdietarysupplementationwith Agar-icus sylvaticus fungus has the ability to bring benefit for anthropometricparametersofwomenwithcolorectalcancer. However,controlledand randomizedclinicaltrials, in addi-tiontothoseperformedinthisstudy,areneededtoelucidate themechanismsofactionofthebioactiveprinciplespresent inAgaricussylvaticus,aswellasothermedicalconditionsthat couldbenefitthroughthissupplementation.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1.ToscanoBAF,CoelhoMS,AbreuHB,LogradoMHG,FortesRC. Câncer:implicac¸õesnutricionais.ComCiênciasSaúde. 2008;19:171–80.
2.ZandonáB,CarvalhoLP,SchimedtJ,KoppeDC,KoshimizuRT, MallmannACM.Prevalênciadeadenomascolorretaisem pacientescomhistóriafamiliarparacâncercolorretal.Rev BrasColoproctol.2011;31:147–54.
3.ValadãoM,LealRA,BarbosaLC,CarneiroM,MuharreRJ.Perfil dospacientesportadoresdecâncercolorretaloperadosem umhospitalgeral:necessitamosdeumprogramade rastreamentoacessíveleefetivo.RevBrasColoproctol. 2010;30:160–6.
4.SilvaMPN.Síndromedaanorexia-caquexiaemportadoresde câncer.RevBrasCancerol.2006;52:59–77.
5.FortesRC,NovaesMRCG.Efeitosdasuplementac¸ãodietética comcogumelosAgaricaleseoutrosfungosmedicinaisna terapiacontraocâncer.RevBrasCancerol.2006;52:363–71.
6.LohmanTG,RocheAF,MartorellR.Anthropometric standardizationreferencemanual.Champaing,IL:Human KineticsBooks;1988.
7.ShilsME,OlsonJA,ShikeM,RossAC.Tratadodenutric¸ão modernanasaúdeenadoenc¸a.9ed.SãoPaulo:Manole;2003.
8.PinhoN,PachecoS,BaluzK,OliveiraA.Manualdenutric¸ão oncológica:basesclínicas.SãoPaulo:Atheneu;2004.
9. FrisanchoAR.Anthropometricstandardsfortheassessment ofgrowthandnutritionalstatus.MI:UniversityofMichigan; 1990.
10.BlackburnGL,ThorntonPA.Nutritionassessmentofthe hospitalizedpatients.MedClinNorthAm.1979;63:1103–15.
11.FonsecaLM,QuitesLV,CabralMMDA,SilvaRG,LuzMMP, FilhoAL.Câncercolorretal–resultadosdaavaliac¸ãopatológica padronizadade521casosoperadosnoHospitaldasClínicas daUFMG.RevBrasColoproctol.2011;31:17–25.
12.Brasil.MinistériodaSaúde.SecretariadeAtenc¸ãoàSaúde. InstitutoNacionaldeCâncer.Estimativasdaincidênciae mortalidadeporcâncernoBrasil.RiodeJaneiro:INCA;2011.
13.CozerattoliniR,GallonCW.Qualidadedevidaeperfil nutricionaldepacientescomcâncercolorretal colostomizados.RevBrasColoproctol.2010;30:289–98.
14.CostaJV,NovaesMRCG,AsquieriER.Chemicaland
antioxidantpotentialofAgaricussylvaticusmushroomgrown inBrazil.JBioanalBiomed.2011;3:49–54.
15.FortesRC,NovaesMRCG.TheeffectsofAgaricussylvaticus
fungidietarysupplementationonthemetabolismandblood pressureofpatientswithcolorectalcancerduringpost surgicalphase.NutrHosp.2011;26:176–86.
16.TaveiraVC,NovaesMRCG,ReisMA,SilvaMF.Hematologic andmetaboliceffectsofdietarysupplementationwith
Agaricussylvaticusfungionratsbearingsolidwalkertumor. ExpBiolMed.2008;233:1341–7.
17.FortesRC,RecôvaVL,MeloAL,NovaesMRCG.Lifequalityof postsurgicalpatientswithcolorectalcancerafter
supplementeddietwithAgaricussylvaticusfungus.NutrHosp. 2010;25:586–96.
18.FortesRC,RecôvaVL,MeloAL,NovaesMRCG.Qualidadede vidadepacientescomcâncercolorretalemusode
suplementac¸ãodietéticacomfungosAgaricussylvaticusapós seismesesdesegmento:ensaioclínicoaleatorizadoe placebo-controlado.RevBrasColoproctol.2007;27:130–8.
19.FortesRC,RecôvaVL,MeloAL,NovaesMRCG.Alterac¸ões gastrointestinaisempacientescomcâncercolorretalem ensaioclínicocomfungosAgaricussylvaticus.RevBras Coloproctol.2010;30:45–54.
20.FortesRC,RecôvaVL,MeloAL,NovaesMRCG.Alterac¸ões lipídicasempacientescomcâncercolorretalemfase pós-operatória:ensaioclínicorandomizadoeduplo-cegocom fungosAgaricussylvaticus.RevBrasColoproctol.2008;28: 281–8.
21.FortesRC,NovaesMRCG,RecôvaVL,MeloAL.Immunological: hematologicalandglycemiaeffectsofdietary
supplementationwithAgaricussylvaticusonpatients colorectalcancer.ExpBiolMed.2009;234:53–62.
22.FortesRC,NovaesMRCG.Terapianutricionalcomfungos medicinaisempacientesoncológicos:umaperspectivano tratamentoadjuvantedocâncer.RevNutr.2010;9:311–9.
23.FortesRC,RecôvaVL,MeloAL,NovaesMRCG.Effectsof dietarysupplementationwithmedicinalfungusinfasting glycemialevelsofpatientswithcolorectalcancer:a randomized,double-blind,placebo-controlledclinicalstudy. NutrHosp.2008;23:591–8.
24.OhnoS,SumiyoshiY,HashineK,ShiratoA,KyoS,InoueM. PhaseIclinicalstudyofthedietarysupplement,Agaricus blazeiMurill,incancerpatientsinremission.EvidBased ComplementAlternMed.2011;2011:192381.