This study extracted microalgae and photosynthetic consortia from the local environment and artificially adapted them to mixed, untreated wastewater using a stepwise bioprospecting approach. Natural consortia were sampled from the local environment, studied for use in nutrient removal and wastewater treatment, and artificially adapted to harsh wastewater conditions with the aim of maximizing removal efficiency and biomass production while minimizing energy input. The Tree Bark Consortium (TB) was sampled in February 2019 by removing a section of bark from a Norway spruce (Picea abies), washing the dry algal culture from the bark in approximately 50 mL of tap water and using the liquid to inoculate.
The lake water (LW) consortium was sampled in June 2019 by collecting ~500 mL of lake water from the shoreline and skimming a floating biofilm from the surface. BD was collected from Lehtoniemi municipal wastewater treatment plant (Kuopio, FI) in September 2017 and ACE was obtained from FinnForel Oy (Varkaus, FI) in August 2019. The study was conducted in four experimental phases, with new variables of introduced during each phase experiment, in order to clarify the best culture conditions and consortia for nutrient removal and biomass production from the tested wastewaters.
Colonies were hand-picked from the remaining plates using digital microscopy and resuspended in liquid BBM medium without TC alteration for a second propagation period to increase cell density prior to the experiment. MA inoculum was from the TC-amended agarose BBM plate saved from Phase I, resuspended in 500 ml liquid BBM and allowed to grow for 7 days prior to the experiment. The remaining liquid culture from the MC pots was combined and expanded in 10% BD in ACE prepared as per the beginning of Phase II.
C1 was derived from the MC consortium, acclimated to BD and ACE during Phase II and subsequent propagation in the 10:90 BD:ACE mixture, which artificially selected for the most proficient species to dominate the culture.
Results and discussion
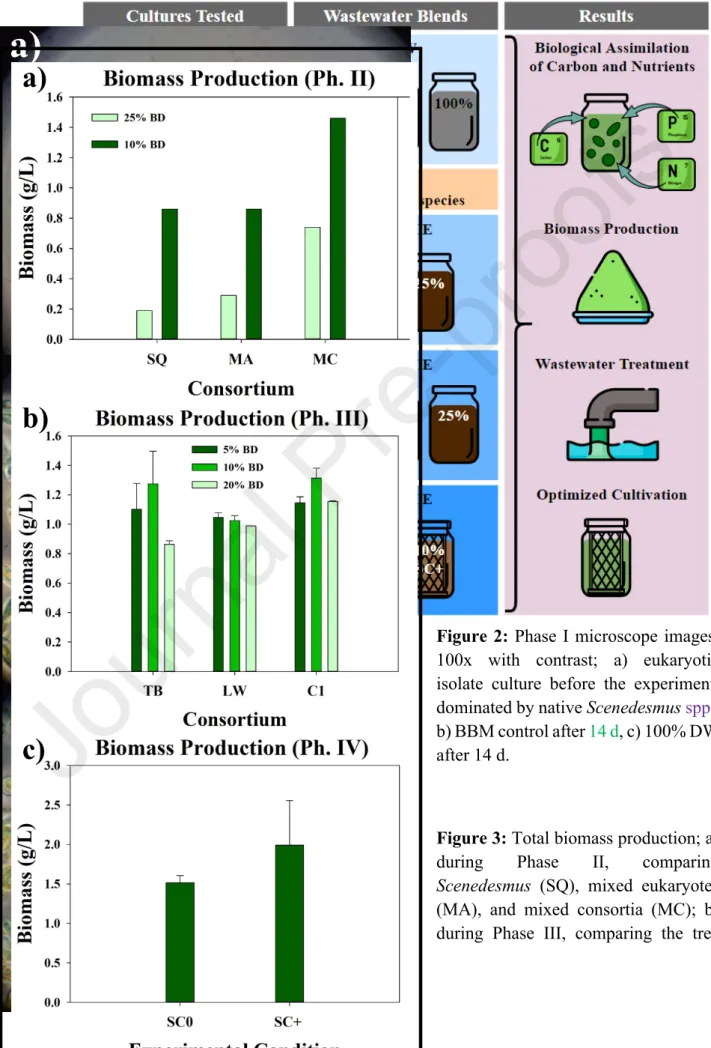
A 10% BD concentration produced the highest biomass overall in phase II (Figure 3a), as well as the highest biomass in TB and C1 consortia in phase III (Figure 3b). During phase III, when comparing three different consortia with different community structure, biomass results were similar between consortia (Fig. 3b). Average biomass production was highest at 10% BD, mirroring the results obtained in Phase II (which compared 10% and 25% BD concentrations) (Fig. 3a).
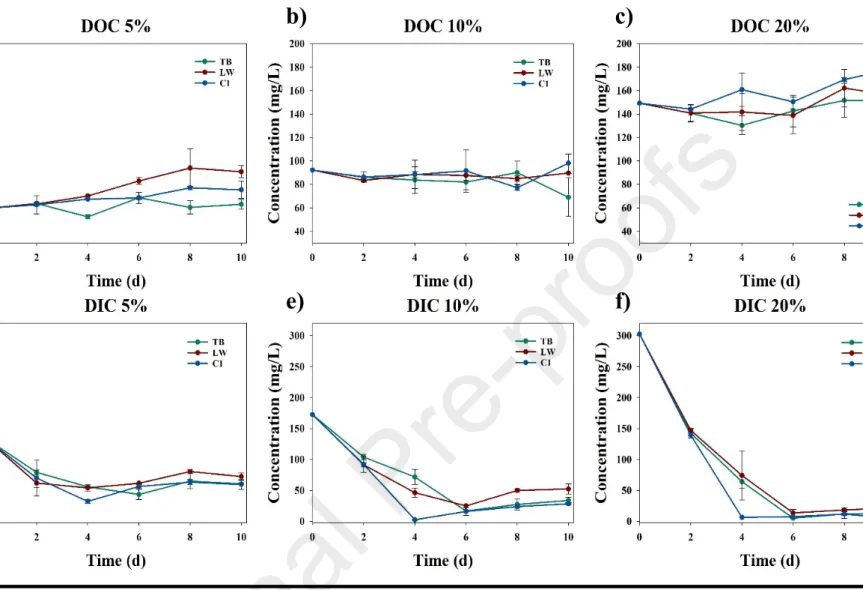
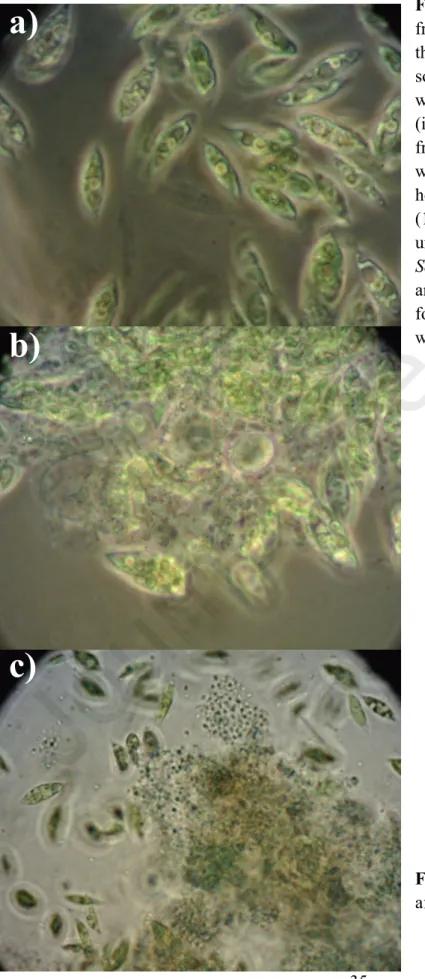
Microscope images from phase II (Fig. 5a) and phase III (Fig. 5b) suggest that normally free-living eukaryotic microalgae were able to thrive immobilized in extracellular polysaccharide matrices secreted by prokaryotes in co-culture; an observation that prompted the scaffolding test performed in Phase IV. During phase I, DOC removal efficiency (Fig. 6) increased steadily with the concentration of DWW, which could be attributed to higher populations of heterotrophic bacteria introduced with greater concentrations of DWW. Across all consortia and BD concentrations tested in phase III, DOC remains stable with respect to initial concentration (Fig. 7a–c), due to rates of organic carbon leaching and cell death roughly matching carbon consumption via algal mixotrophy and bacterial metabolism.
DIC removal efficiency (relative to initial concentration), however, increases significantly with BD concentration (Fig. 7d–f). During Phase III, at 20% BD (highest DIC concentration), DIC removal was >92% under all experimental conditions (Fig. 8b, Table 3). This is attributed to a combination of increased biodiversity (compared to SQ) (Fig. 9a,b) and selection of better light penetration for eukaryotic photosynthesizers (Fig. 10a,b).
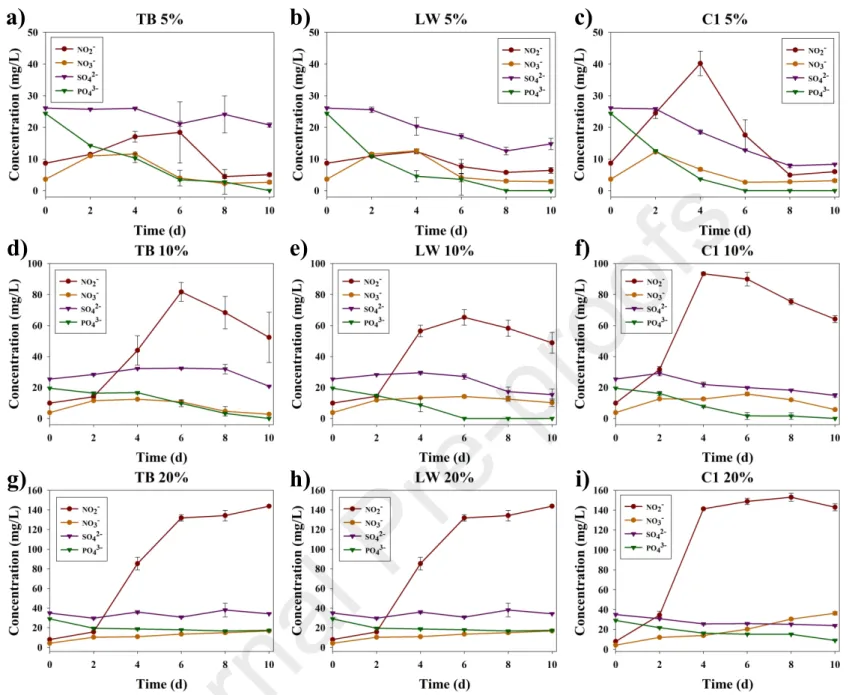
TDN removal efficiency was negatively correlated with BD concentration (with NO2- showing a strong increase at BD concentrations of 10% and higher) (Fig. 11d-i). Total nitrogen showed a net decrease under all conditions; however, plotting the change of nitrite and nitrate over time shows different rates of nitrification (Fig. 11). Although biomass production was lower under 5% BD (Fig. 3b), nutrient removal was more efficient (Fig. 8b), with pre-adapted consortium C1 achieving 100% phosphate removal as early as day 6th (Fig. 11c). due to eukaryotic dominance at low BD concentrations.
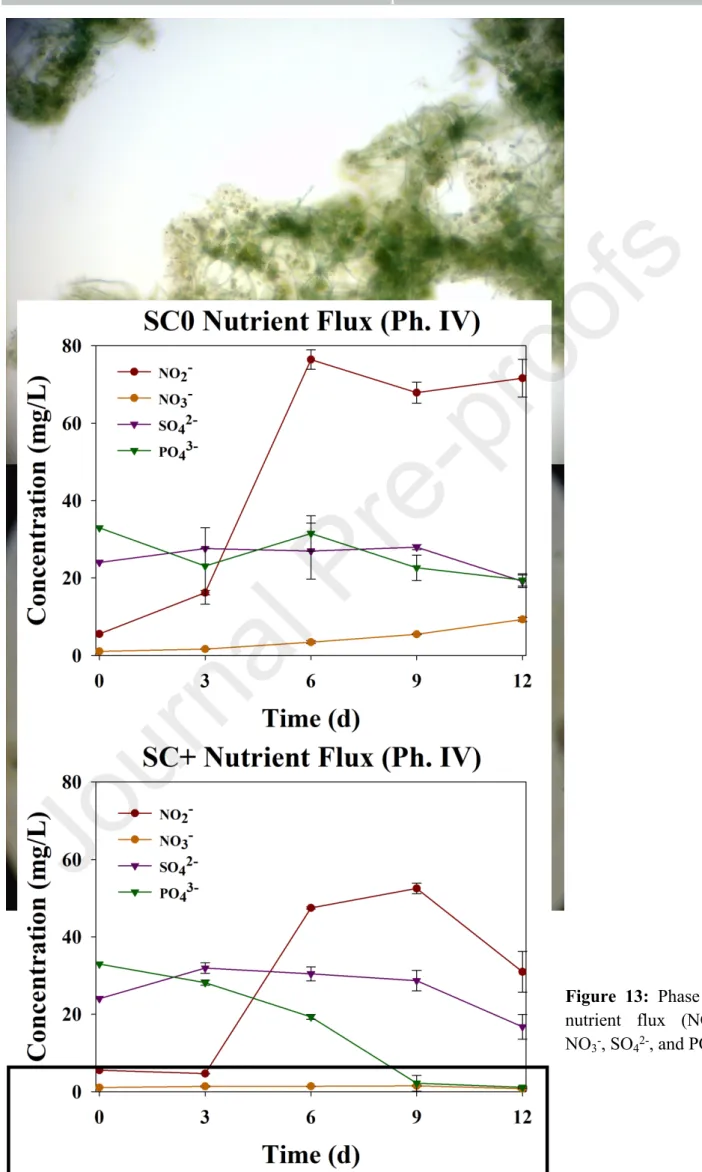
However, total dissolved nitrogen standards (<15 mg/L) [35], were only met under the 5% BD condition tested during phase III (Fig. 11a-c). During phase IV, SC0 (without scaffold) showed almost complete DIC removal (96%, Fig. 8c), while SC+ (with scaffold) showed 66%. While SC+ favored greater overall biodiversity (Fig. 12a), free-living eukaryotic species (such as native Scenedesmus spp.).
Interestingly, a sharp peak in NO2- was observed in both cultures after 6 days, but showed a steady decrease in SC+ after 9 days (Fig. 13). Although net phosphate removal was complete during phase IV, SCO showed a small increase in phosphate at day 6 (Fig. 13), mirroring the results obtained in phase I (Fig. 6).
Conclusions
Allahverdiyeva, Potential of the green alga UHCC00027 grown in municipal wastewater under Nordic conditions for nutrient removal and biodiesel feedstock, Algal Res. Ruan, Cultivation in wastewater of the microalga Auxenochlorella protothecoides UMN280 on concentrated municipal wastewater for simultaneous nutrient removal and energy feedstock production, Appl. Béline, The color of the digested sludge and the light intensity influence the removal of nutrients and the appearance of competition in microalgae-bacteria.
Kornaros, Effect of organic carbon and nutrient supplementation on the digestate-grown microalgae, Parachlorella kessleri, Bioresour. Ergas, Advances in algal-prokaryotic wastewater treatment: A review of nitrogen transformations, reactor configurations and molecular tools, J. Kong, Microalgae cultivation and nutrient removal from sewage sludge after ozonation in algal-bacteria system, Ecotoxicol.
Megahed, Biomonitoring the detoxification efficiency of an algal-bacterial microcosm system for coking wastewater treatment: harmonization between Chlorella vulgaris microalgae and wastewater microbiome, Sci. Adey, High diversity within the periphyton community of an algae scrubber on the Susquehanna River, Ecol. Bhatnagar, Sequential cultivation of microalgae in raw and recycled dairy wastewater : growth of microalgae, wastewater treatment and biochemical composition, Bioresour.
Sibi, Sodium bicarbonate as an inorganic carbon source for higher biomass and lipid production Integrated carbon capture in Chlorella vulgaris, Achiev. Lu, Effects of dissolved inorganic carbon and mixing on autotrophic growth of Chlorella vulgaris, Biochem. Zhong, Adsorption removal of ammonium and phosphate from water by fertilizer controlled release agent made from wheat straw, Chem.
Lovitt, Strategies for the recovery of nutrients and metals from anaerobically digested dairy farm sludge using cross-flow microfiltration, Water Res. Frear, Evaluation of an integrated ammonia stripping, recovery and biogas scrubber system for use with anaerobically digested dairy manure, Biosyst. Ohtake, New technique for phosphorus recovery from aqueous solutions using amorphous calcium silicate hydrates (A-CSHs), Water Res.
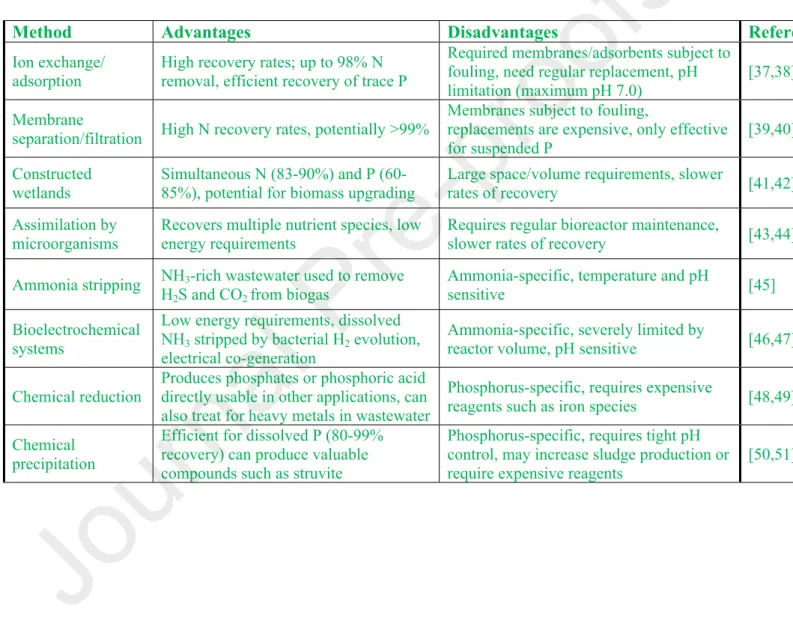
Bashan, Recent advances in the removal of phosphorus from wastewater and its future use as a fertilizer Water Res. Abbreviations: dairy wastewater (DWW), biogas decay (BD), aquaculture wastewater (ACE), commercial Scenedesmus quadricauda (SQ), mixed local eukaryotes (MA), mixed local consortia (MC), lake water consortium (LW), tree bark consortium (TB), pre -customized consortium (C1). BD, biogas digestate; ACE, aquaculture effluent; DIC, dissolved inorganic carbon; DOC, dissolved organic carbon; TDN, total dissolved nitrogen; NO2-, nitrite; NO3-, nitrate; SO42-, sulfate;.
BD, biogas digestate; ACE, aquaculture wastewater; DIC, dissolved inorganic carbon; DOC, dissolved organic carbon; TDN, total dissolved nitrogen; and PO43-, phosphate (n/a, not measured).


![Table 1: Comparison of current technologies used for nutrient (nitrogen, N; phosphorus, P) recovery from wastewater [42].](https://thumb-eu.123doks.com/thumbv2/9pdfco/1891028.267194/40.892.101.876.335.999/comparison-current-technologies-nutrient-nitrogen-phosphorus-recovery-wastewater.webp)