The understanding about hPSCs and their differentiation into different cell types has advanced to the point where we can produce most of the human cell types in the laboratory. Some of these properties are fundamental to the use of hPSC-derived cells in modeling adult-onset diseases, drug screening, and replacing cells lost to disease. As one of the largest organs in the body, liver performs several tasks, including some important metabolic, synthetic, immunological and detoxification processes.
In accordance with the functions of the liver, hepatocytes are responsible for the production of albumin, urea, triglycerides, lipoproteins and drug metabolism enzymes. These maturation markers, discussed in the next section, can be used to infer the maturation state of the hepatocytes. As mentioned, traditionally cultured hPSC-derived hepatocytes tend to reach the maturation state of fetal tissue rather than the desired fully matured freshly isolated primary human hepatocytes (PHHs).
While there is much data on the expression levels of specific coding genes, it is essential to note that they do not always correlate with the actual activity levels of the proteins produced. The goal is to be able to produce hPSC-derived hepatocytes with enzymatic activities similar to fully mature ones in vivo, and one way to roughly monitor this is by comparing the expression levels of the respective coding genes.

Morphology
Therefore, it is time to consider some significant differences between these two stages and what kind of structural and functional variance they may entail. protocols, evaluation techniques and functional results vary between different laboratories, the comparison is sometimes quite challenging. When comparing the expression levels of enzymes in hepatocytes under fetal and mature states, most enzymes can be roughly divided into three groups. Enzymes in the second group, such as UGT1A1, present low expression levels during the fetal state, but show a surge at birth and then gradually increase over time to adult levels.
Finally, enzymes of the third group, e.g. CYP1A2 and CYP2B6 have low levels of expression until the neonatal period and progress to mature levels of expression years after birth.
Albumin secretion
Urea production
Triglycerides and lipoproteins
The first three were reported to be expressed at higher levels in hPSC-derived hepatocytes than in PHH, while APOA5 was expressed at lower levels. Of the fatty acid metabolism genes, three were expressed at different levels in hPSC-derived hepatocytes compared to mature ones: acetyl-CoA carboxylase beta (ACACB), biliary acyl-CoA synthetase (SLC27A5), and catalytic subunit AMP-kinase-activated alpha 2. (PRKAA2).
Drug metabolizing enzymes
Other genetic components
Methods of 2D co-culturing
Micropatterning is believed to be one of the most traditional ways to investigate the effect of co-culture on hPSC-derived hepatocytes (Zinchenko et al., 2006a). Other possibilities include using micro-bioreactors and microfluidic biochips for a dynamic culture and thus improved phenotype and stacking of cell sheets to also question 3D cultivation (Kehtari et al., 2018). Since the combination of co- and 3D cultivation will be discussed later in this review, this type of research papers has been omitted from this section.
Some research groups have decided to investigate paracrine signaling between the different cell types and therefore cultured hPSC-derived hepatocytes in the medium of the other cell type (Takagi et al., 2017). On the other hand, other studies have even yielded a ten-fold number of co-culture cells per hPSC-derived hepatocyte (Berger et al., 2015). Attempts have also been reported to mimic the situation in vivo by co-culturing multiple different cell types with the hPSC-derived hepatocytes (G. Wang et al., 2018).
Maturational effects of 2D co-culturing
Unfortunately, none of the papers found had reported on the effect co-cultivation has on triglyceride and lipoprotein levels. Takagi and colleagues stated that CYP3A4 activity was enhanced in hPSC-derived hepatocytes conditioned in mesenchymal stem cell medium (Takagi et al., 2017). Another study found that CYP7A1 expression was more prominent when co-cultured with umbilical vein endothelial cells (Kehtari et al., 2018).
The relative expression levels of AFP and HNF𝛼 in the co-cultures were found to vary by study. Takagi et al found that AFP expression is enhanced in mesenchymal stem cell medium, while Danoy et al. However, indicating an immature state, Javed and colleagues reported that AFP expression was still detectable after 16 days in co-culture with immortalized hepatic stellate cells (Javed et al., 2014).
While 2D culture for hPSC-derived hepatocytes has its own advantages in terms of ease of use, on the other hand, it appears to prevent the cultured cells from organizing naturally in vivo that resembles 3D formation - and thus has a negative effect. effect on the differentiation process and cell function. Since noticing these phenomena, several different 3D culture techniques have been introduced to ensure sufficient cell-cell and cell-matrix. These include forced floating method, agitation based approaches, suspended drop method, use of matrices and scaffolds and use of microfluidic cell culture platforms.
Methods of 3D monoculturing
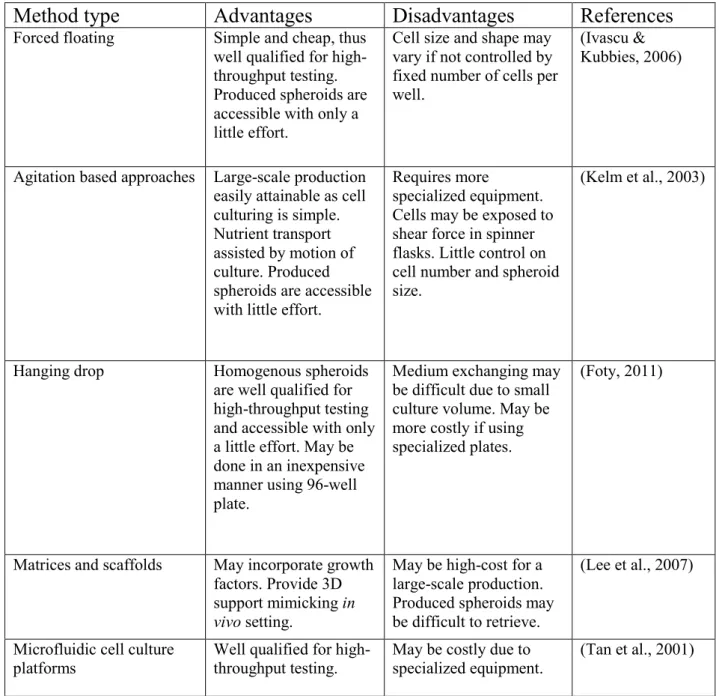
Advantages and disadvantages of different methods of the three-dimensional culture system, an adapted and modified version (Breslin & O'driscoll, 2012).
Maturational effects of 3D monoculturing
The difference, however, was not as significant as with albumin secretion, and one study even reported that the two different cultures produced comparable amounts until their last day of measurement (Freyer et al., 2016). According to Mun and colleagues, expression of genes related to lipid metabolism, including fatty acid uptake and metabolism, triglyceride hydrolysis, beta-oxidation, and cholesterol metabolism and secretion, were upregulated in 3D compared with 2D differentiated hepatocytes (Mun et al., 2019). Therefore, other research groups have also discovered the metabolic process of glycerolipids, the regulation of lipid transport, the regulation of cholesterol transport (for example apolipoprotein F) and finally cholesterol and lipid homeostasis as among the up-regulated genes (Leclerc et al. , 2017; . Ramasamy et al. al., 2013; Sivertsson et al., 2013b).
Consistent with the mRNA findings, Thi and colleagues have reported higher levels of secreted lipoproteins in a 3D environment (Thi et al., 2020). Of particular interest, CYP3A4 and CYP7A1 were found to be upregulated and showed higher activity levels in 3D, except in a study by Freyer and colleagues, where 2D cultures produced insignificantly higher CYP3A4 activities (Freyer et al., 2016). Different studies have reported conflicting results as the research groups of Gieseck, Leclerc, Ardalani and Freyer reported significant, even 20-fold lower transcript amounts in 3D cultures when compared to 2D (Ardalani et al., 2019; Freyer et al. ., 2016; Gieseck et al., 2014; Leclerc et al., 2017) while the groups of Subramanian and Baharvand found the situation to be the opposite (Baharvand et al., 2006; Subramanian et al., 2014).
Protein levels for ASGPR1 and transcript levels for 𝐻𝑁𝐹4𝛼 were found to be elevated in 3D culture, while at least for 𝐻𝑁𝐹4𝛼 they were found to be far behind mature hepatocyte levels (Ardalani et al., 2019; Freyer et al., 2016; Subramanian et al. al., 2014).
Methods of 3D co-culturing
The next step to improve the maturity of hPSC-derived hepatocytes was to combine the two techniques presented and reviewed above, namely co-culture and 3D culture. Subsequently, other studies also concluded that heterotypic cell-cell interactions are critical for self-organization and that paracrine signals secreted by non-parenchymal cells are important for hepatic maturation (Asai et al., 2017; Camp et al. al., 2017). Thus, the technique has become more and more popular, especially during the last few years, while bringing significant progress to the maturity of the acquired hPSC derivative.
Maturational effects of 3D co-culturing
Pettinato and Wang's research groups reported that their organoids, with endothelial cells and mesenchymal cells respectively, produced significantly lower levels of urea than PHHs (Pettinato et al., 2019; S. Wang et al., 2019). Moreover, Wu and colleagues reported that their organoids' CYP3A4 activity at day 45 is more than 80% of that of cryopreserved PHHs (Wu et al., 2019). However, Wang's research group published contradictory results showing significantly lower CYP3A4 activity in organoids than in PHHs (S. Wang et al., 2019).
After induction with omeprazole, rifampicin and phenobarbital, at least CYP 1A1, 1A2, 3A4, 3A7, 2B6 and 2C9 showed significant increases in their activities in organoids compared to monoculture (Pettinato et al., 2019). According to a global transcriptome analysis of liver organoids, their maturity was comparable to PHHs (Takebe et al., 2017). Surprisingly, HNF4𝛼 was found to be expressed at significantly higher levels in organoids versus 3D monocultures (Guan et al., 2017; Pettinato et al., 2019).
One study highlighted that hepatic sinusoidal endothelial cells and hiPS-derived endothelial cells were the best cell type for co-culture, while cholangiocytes and hepatic stellate cells yielded the worst results (Ardalani et al., 2019). Enhancement of functional maturity of induced pluripotent stem cell-derived human hepatocytes by controlled presentation of intercellular interactions in vitro. Characterization of human induced pluripotent stem cell-derived hepatocytes with mature properties and potential for modeling metabolic diseases.
Generation of hepatocyte-like cells from human induced pluripotent stem (iPS) cells by co-cultivation. Artificial cells, nanomedicine and biotechnology Fabrication of a co-culture microbioreactor device for efficient hepatic differentiation of human induced pluripotent stem cells (hiPSC) Fabrication of a co-culture microbioreactor device for efficient. Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with endothelial cells.
Hepatic differentiation and maturation of human embryonic stem cells cultured in a perfused three-dimensional bioreactor. Induction of pluripotent stem cells from cultures of mouse embryos and adult fibroblasts by defined factors. Cultivation in a rotary microgravity bioreactor enhances hepatic differentiation of murine embryonic stem cells on biodegradable polymer scaffolds.