The first author designed and performed all cell culture experiments and provided the data and analysis criteria for the development of the software. The first author tested and provided the feedback for the software development and compared the analysis efficiency of the software with the manual analysis.
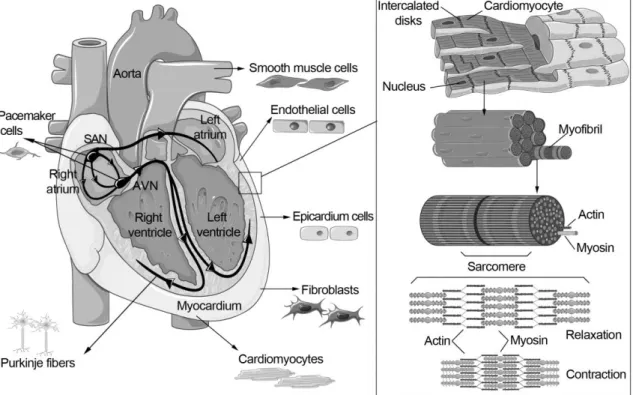
Human heart and cardiomyocytes
Sarcomeres are responsible for the contraction of the CM, and the sarcomeric cytoskeleton consists of thin actin filaments and thick myosin filaments (Figure 1). Actin and myosin filaments form the sarcomere structure, which is responsible for the contraction and relaxation of the entire CM.
Electrophysiology of the heart and cardiomyocytes
- The cardiac action potential
- Calcium cycling
- Cardiac ryanodine receptors and intracellular Ca 2+ release
- Important signaling proteins related to calcium cycling
- Failure of electrical activity and calcium cycling
- Clinical characterization of the electrical activity using ECG and MAP
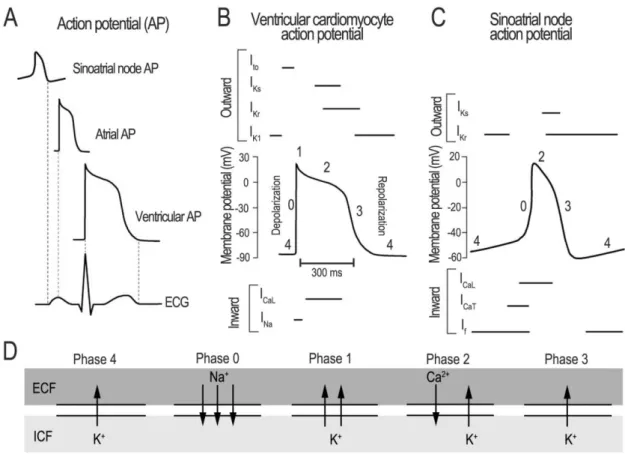
The plateau phase of the AP (phase 2) results from a balance between depolarizing inward L-type Ca2+ current (ICaL) through voltage-gated L-type Ca2+ channels (LTCC) and fast and slow repolarization (IKr). (IKs) delayed rectifier K+ currents (Figure 2). This influx then triggers the opening of the cardiac ryanodine receptor (RyR2) SR Ca2+ release channel and causes a much larger amount of Ca2+ ions to be rapidly released from the SR into the cytosol (Figure 3).
Stem cells
Stem cell renewal and potency
Multipotent or tissue-specific stem cells can only differentiate into a limited number of cell types (Figure 6), usually in the cell types that can be found in the same tissue where the stem cells reside. For example, the bone marrow contains multipotent hematopoietic stem cells that give rise to all the cells of the blood, but not to other types of cells.
Induced pluripotent stem cells
In addition, the cell culture and differentiation methods are similar for both stem cell types (Takahashi et al., 2007). Fusaki et al., 2009 ) Another non-integrating RNA strategy for iPSC generation is to deliver synthetic messenger RNA (mRNA) encoding reprogramming factors directly into the cells.

Characterization of induced pluripotent stem cells
Stem cell-differentiated cardiomyocytes and their characterization
- Cardiac differentiation
- Properties and maturation markers of cardiomyocytes
- Electrical field stimulation of cardiomyocytes
- Methods to study the functionality of cardiomyocytes
- Microelectrode array
- Patch clamp method
- Calcium imaging
- Analysis of the mechanical beating behavior
Differentiation was also successful with END-2 conditioned medium instead of co-culture ( Graichen et al., 2008 ). Direct reprogramming of mouse postnatal cardiac or dermal fibroblasts into functionally induced CMs (iCMs) in vitro was achieved by expressing three transcription factors: GATA-binding protein 4 (Gata4), myocyte-specific enhancer factor 2C (Mef2c), and T- box transcription factor 5 (Tbx5) (GMT factors) (Ieda et al., 2010).

Catecholaminergic polymorphic ventricular tachycardia
- Clinical features of CPVT
- Genetic background of CPVT
- Disease pathophysiology
- Modeling of CPVT
Cerrone et al., 2009 ) Ca2+ leakage is thought to be caused by reduced binding affinity of the protein calstabin2, which stabilizes RyR2. Similarities and differences in the pathophysiological consequences of RyR2 versus CASQ2 mutations were also studied using iPSC-CMs (Novak et al., 2015).
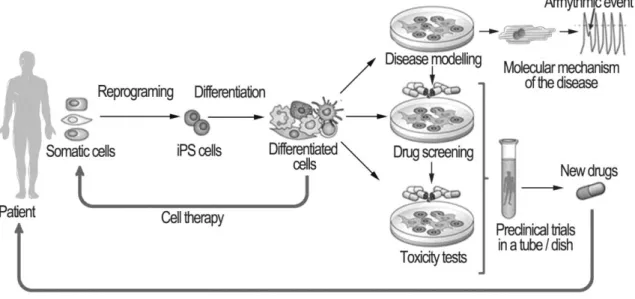
The potential use and challenges of iPSC-derived cardiomyocytes in disease modeling
In addition, stabilization of RyR2 by JTV-519 has been shown to reduce CPVT-triggered arrhythmias at the cellular level ( Sedej et al., 2010 ). Ebert et al., 2012 ) Prolonged QT intervals and other clinically relevant drug responses have been demonstrated from iPSC-derived CMs with different-.
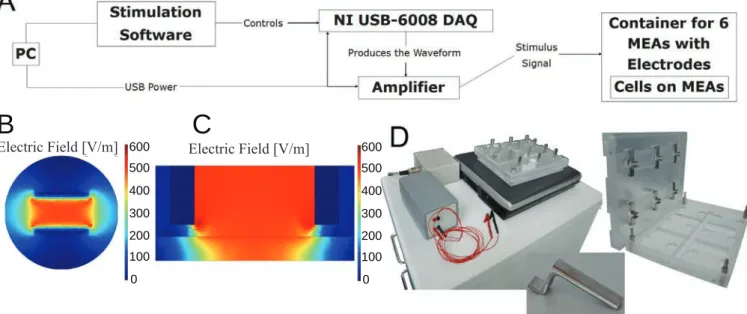
Stimulation experiments with neonatal rat cardiomyocytes (I)
- Neonatal rat cardiomyocytes
- Collagen gel
- Stimulation setup
- Stimulation protocols
- Characterization of stimulated cells
In long-term stimulation tests, the effect of the EFS on cell culture medium pH values was also studied. A Park Systems XE-100 AFM was used to study the surface and electrode wires of the MEA plates.
Human induced pluripotent stem cell-derived cardiomyocytes (II-IV)
- Generation of patient-specific iPSCs (II-IV)
- Characterization of iPSC lines (II, III)
- Differentiation and characterization of cardiomyocytes (II-IV)
- Calcium imaging (II-IV)
- Ca 2+ abnormality analysis software (IV)
- Patch-clamp measurements (II)
The low peak or mid-peak abnormalities are detected if the amplitude of the Ca2+ signal does not reach the normal Ca2+ signal amplitude level. Oscillation of the Ca2+ signal is detected if the signal does not fall low enough before rising to the next peak for at least three peaks.

Clinical data (II, III)
Monophasic action potential and 24-h-ECG recordings (II)
Exercise-stress test and dantrolene infusion (III)
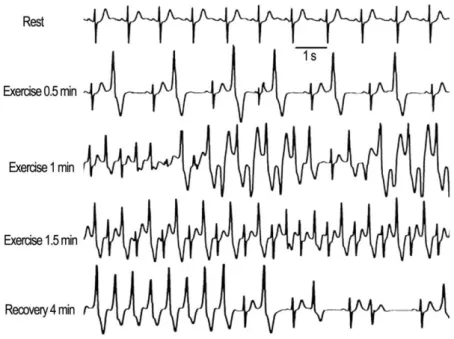
In the final phases of the study, the workload target was the same as in the first study. The first baseline study was performed in the morning and the second exercise test was repeated later in the afternoon after intravenous infusion of dantrolene sodium (Dantrium®, 1.5 mg per kg body weight). The third exercise test was performed on the second day to study the effects after washout of dantrolene.
ECG was recorded continuously throughout the exercise test and the first 8 minutes after the test.
Statistical analysis (I-III)
The patients underwent the exercise stress test with a bicycle ergometer three times with an initial load of 30 W, followed by increasing the load by 15 W every minute. The numbers of PVCs and the maximum number of consecutive PVCs during exercise and in the recovery phase were calculated.
The effect of electrical stimulation on primary cardiomyocytes (I)
The effect of stimulator on cardiomyocytes
Cell viability, morphology and functionality
Gene and protein expressions
Characterization of iPSC and iPSC-derived cardiomyocytes (II, III)
The pluripotency of the cell lines was further confirmed with in vivo teratoma formation and with in vitro EB formation as well as with the expression of all three germ layers. The presence of the RyR2 mutations was verified for all the CPVT-iPSC lines by DNA sequence analysis (Figure 14) or by PCR. iPSC lines were differentiated into spontaneously beating CMs that expressed troponin T and Cx-43 cardiac markers at the protein level. In Study II, expression of troponin T, RyR2, SERCA2a, LTCC, PLB and NCX was confirmed by RT-PCR.
Functionality of iPSC-derived CMs (II-IV)
- Ca 2+ cycling (II, III)
- Analysis of Ca 2+ cycling abnormalities (IV)
- Electrophysiology (II)
- Antiarrhythmic effect of dantrolene on Ca 2+ cycling (III)
No differences in the expression levels of the studied genes or proteins were observed between control and CPVT CMs. The majority of spontaneously beating CMs were categorized as normal in manual and AnomalyExplorer analyses. Comparison of AnomalyExplorer and manual Ca2+ signal analysis of different CPVT cell lines with RyR2 mutation.
In CPVT CMs with the mutation in the N-terminal or central region of the RyR2 protein (clusters 1-3), dantrolene abolished or reduced the majority of Ca2+ cycling abnormalities (Table 4).

Clinical features of CPVT (II, III)
MAP and ECG (II)
Antiarrhythmic effects of dantrolene in patients (III)
The in vivo responder group shows the percentage of the abolished PVCs compared to the baseline. NRCs were exposed to EFS with a new device to study different stimulation protocols and their effects on morphological and functional properties of the cells. CPVT studies have indicated that molecular and cellular fiber research exploiting hiPSC-derived CMs match the clinical phenotypes of the patients in studying both pathophysiological and drug responses of the disease and encourage the continuation of disease modeling using iPSCs even in a mutation- or patient-specific manner.
Summary of findings in Studies I-IV highlighting correspondence of CPVT disease model phenotype with clinical phenotype.

Electrical field stimulation (I, II)
Electrical pacing increased the maturation of Ca2+ cycling and contractile properties, as well as the expression of Ca2+ cycling proteins and T-tubule proteins in hESC-derived CMs (Li et al., 2013;. This decrease in beating rhythm may be due to the tendency of primary cells to dedifferentiate and eventually stop beating in culture (Montessuit et al., 2004) Electrical pacing has also been exploited with disease modeling of iPSC-derived CMs, p e.g., for studying the Ca2+ cycling of CM non-beating CPVT (Jung et al., 2012), as well as for studying the effect of stimulation on nuclear senescence and cellular apoptosis of lamin A/C-associated dilated cardiomyopathy ( Siu et al., 2012).
Pacing has also been used to model atrial fibrillation with primary atrial myocytes (Ji et al., 2013).
The immaturity of iPSC-derived CMs may complicate the analysis of these cells or even cause incorrect phenotypes of the cells. Despite the widespread use of control iPSCs lines generated from healthy individuals, these lines have occasionally been criticized due to possible genetic variations between diseased and control iPSCs, which may cause false conclusions regarding the underlying mechanisms of the disease (Kim et al., 2014). Disease-causing mutations of the iPSCs can be corrected by generating genetically identical (isogenic) sibling iPSC lines in which nucleotides causing the mutation are changed.
Methods based on zinc finger nucleases (ZFNs) (Soldner et al., 2011), transcription activator-like effector nucleases (TALENs) (Ding et al., 2013) or clusters of regularly spaced short palindromic repeats (CRISPRs) (Li et al. , 2015) have been used with iPSCs; however, the generation of corrected isogenic iPSC lines is cumbersome (Kim et al., 2014).
Modeling of CPVT with iPSCs and comparison of the results to the clinical phenotype
- Defects in Ca 2+ cycling and electrophysiology in vitro and correspondence
- RyR2 mutation-specific differences in the Ca 2+ cycling in vitro (III, IV)
- Pharmacological responses of dantrolene (III)
- Antiarrhythmic effects of dantrolene in vitro and in vivo
- New insights into the treatment of CPVT
- Ca 2+ cycling abnormalities and novel Ca 2+ -analysis software (II-IV)
This observation seems natural because CPVT is a disease with a structurally normal heart (Swan et al., 1999). In addition, baseline conditions in HEK293 cells expressing RyR2 mutations showed regular spontaneous Ca 2+ oscillations ( Jiang et al., 2002 ). Furthermore, it is possible that other additional dantrolene binding regions exist in the carboxyl-terminal half or in the N-terminal region of the RyR2 (Kobayashi et al., 2009).
Hayashi et al., 2009; Hayashi et al., 2012) Flecainide along with β-blocker therapy has also been suggested to be beneficial.
Limitations of the study
Furthermore, phenotypic differences may result from DNA variants in the other ∼50% of the genome, rather than the disease-associated mutations. Abnormal Ca2+ cycles were observed in CPVT CMs but rarely in control CMs, which is why at least most of the abnormalities resembled the CPVT disease phenotype. Validation of the in vitro results with the patient data indicated the correct phenotype of our CPVT CMs.
In the clinical recordings in Study II, noise and the acquisition frequency of the ECG signal can be limiting factors in 24-hour recordings and affect the results of the recordings.
Future perspectives
Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-associated defective conformational regulation of the ryanodine receptor. The mowing was more irregular at the end of the experiment than at the beginning (Figures 4A and 4B). Karyotypes of the cell lines were determined using standard G-banding chromosome analysis (Medix laboratories, Espoo, Finland).
Some of the variability in results may stem from differences between types of CM. Antiarrhythmic Effects of Dantrolene in Patients with Catecholaminergic Polymorphic Ventricular Tachycardia and Replication of Responses Using iPSC Models. Karyotypes of cell lines were determined using standard G-banding chromosome analysis (Medix laboratories, Espoo, Finland) or KaryoLite BoBs analysis (Perkin Elmer) based on BACs-on-Beads technology (Molecular and Systems Immunology and Stem Cell Biology , Turku Center for Biotechnology, University of Turku, Finland).
Antiarrhythmic effects of dantrolene in patients with catecholaminergic polymorphic ventricular tachycardia and replication of the responses using iPSC models. Penttinen K, Swan H, Vanninen S, Paavola J, Lahtinen AM, Kontula K, et al. 2015) Antiarrhythmic effects of dantrolene in patients with catecholaminergic polymorphic ventricular tachycardia and replication of the responses using iPSC models.