Pauliina Ala-Vannesluoma: Moisture absorption of poly(lactide-co-glycolide) and comparison of dehumidifying gases. The purpose of this study was to investigate the water absorption rate of PLGA.
Biodegradable medical device
Polylactide and lactide copolymers
By copolymerizing these two monomers, it is possible to extend the range of homopolymer properties. 22] These thermal properties, which vary with the ratio of lactide and glycolide of the polymer, have an effect on the mechanical properties and the degradation time.
![Figure 1. Polymerization of PLA. [14]](https://thumb-eu.123doks.com/thumbv2/9pdfco/1889862.266028/9.892.185.794.122.281/figure-polymerization-of-pla.webp)
Packing materials
Polypropylene is widely used as a sheath for various medical device applications, as it has excellent transparency and chemical resistance, in addition to good physical properties and printability. However, it is sensitive to photooxidation [32], when sunlight accelerates the rate of oxidation of polymer chains.
Moisture absorption
- Fick’s law
- Hydrolysis
- Effects of moisture on polymer
- Moisture analysis methods
With these equations, the movement of the water molecules in the selected material can be predicted. The by-products from the degradation at the surface quickly dissolve in the surrounding environment and are removed from the bulk polymer.
Drying
Drying parameters
Heat is the most important drying parameter because if there is no heat, the material will not release moisture within the material. Dew point is the temperature at which water vapor in the air condenses to liquid water at the same rate as it evaporates, at constant barometric pressure. Drying time is the time required at a given dew point and temperature to dry the material to a given degree of residual moisture.
Too long a drying time can lead to a degradation of the polymer and a decrease in its physical and other properties and waste of energy. It transports the heat or dry heated air to the material and transports moisture away from the material.
Drying methods
This means that the dew point of the decompressed air is reduced by around 40-50 °C, which offers several advantages, such as allowing the use of compressed air for drying material in a hopper. Compressed air dryers are simple and relatively maintenance-free, but they have some major drawbacks. The energy consumption is also relatively high compared to other tumble dryers, as around 3 times as much compressed air is used as membrane dryers.
However, there is a big difference between a compressed air dryer and a compressed air dryer with a membrane. 63] A membrane improves the compressed air dryer by allowing lower dew point air, which is why its drying ability is better compared to conventional compressed air dryer.
![Figure 12. Basic principle behind the hot air dryer. [60]](https://thumb-eu.123doks.com/thumbv2/9pdfco/1889862.266028/22.892.175.587.104.421/figure-basic-principle-hot-air-dryer.webp)
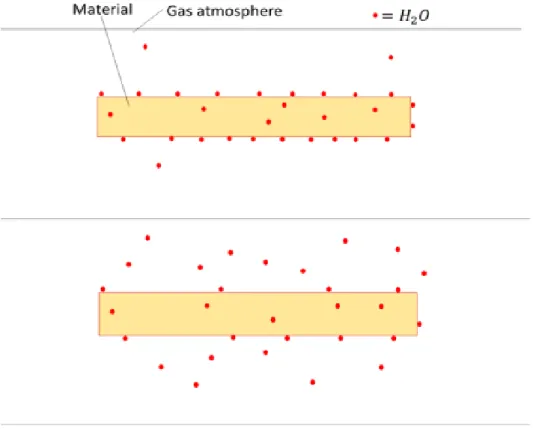
Dehydration gas
Because nitrogen does not react with free water molecules in the atmosphere, this means that it does not draw moisture from the material's surroundings effectively. After the water molecules diffuse into the atmosphere, the nitrogen flow removes these water molecules from the material and the movements of the water molecules towards equilibrium begin again. Due to its chemical structure, moisture wants to react with oxygen and thus the drying process of the material does not rely only on the balance of water molecules.
However, since a requirement of the drying system is gas flow, the amount that gas can hold water molecules is irrelevant since all moist gas is replaced by dry gas. Oxygen can transport the water molecules more efficiently due to its chemical activity as oxygen is highly reactive with the water molecules.
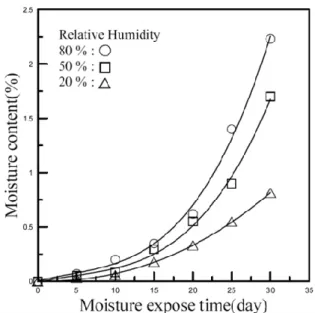
Absorption rate of PLGA in standard conditions
5 METHODS. each sample to prevent any extraction product from being excessively concentrated in water during the tests), while in the second test, the temperature was the same 23℃, but the relative humidity was set to 50.
Effects of the dehumidifying gas on the sample masses
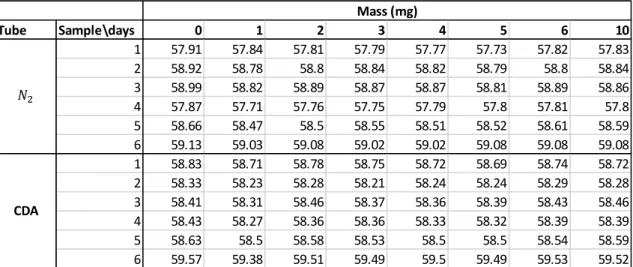
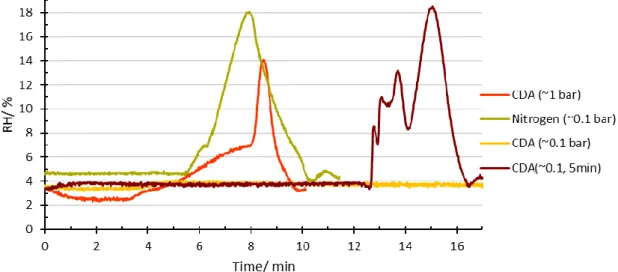
There was a gas flow with a small overpressure ~1 bar CDA and ~0.1 bar nitrogen, pushing the humid gas away from another side of the tube, when the relative humidity inside the tube exceeds 7. When the RH of the tube was greater than 7 %, electrical switch opened and due to the small overpressure moist gas was removed from the tube and replaced by dry gas until the RH is 4 % or smaller.
The recovery time of the gas
Absorption rate in standard conditions
Since it is known how much moisture has an effect on material properties, it can be beneficial to also calculate how long it takes for billets to absorb too much moisture before various heat-demanding processes. So, in addition to the previous results, time, when the moisture content is the critical moisture content 0.02 m-% (the value that should not pass during high temperature processing) and 0.05 m-% (the maximum moisture content value for the product) from the equations of the lines calculated. Moisture content at saturation 𝑐𝑠 is calculated using the Formula 9 for both process phase 1 and process phase 2 samples.
Diffusion coefficient is calculated using the Formula 10 for both process phase 1 and process phase 2 samples. These values may be realistic, as it is stated in ISO 62 that the diffusion coefficient values of the plastic are usually 10−6mm2.
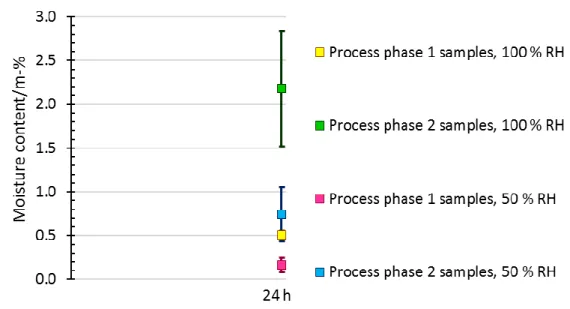
Effects of the dehumidifying gas on the sample masses
The humidity of the containers was monitored every three hours and their ability to recover after opening the tubes was studied. The RH in the CDA tube remained below 4%, while the RH of the nitrogen tube remained below 6.5. Differences were also observed between the minimum RH values in the tubes.
At the end of the test, the wet and dry mass (after the drying process) were measured from both tubes, and from those values, the moisture content of the samples could be calculated using Formula 6. This static electricity could be the result of clothes from the weigher made of synthetic fibers, disposable gloves or any type of friction, especially in a low humidity atmosphere.
The recovery time for the gas
In addition, the error of the scale was relatively large compared to the sample size, which is why the reading of the scale may not have been completely accurate and may partially explain the negative values of the mass differences and the moisture content. However, it can be said that the samples were already practically dry before the vacuum drying. The moisture recovery times for CDA with two pressures and nitrogen after opening the tube.
Because of these results, the 0.1 bar CDA tube was also kept open for 5 minutes to see if there would be a difference. This was a long enough period to see changes in RH values after nearly 13 minutes.
Absorption rate of PLGA in standard conditions
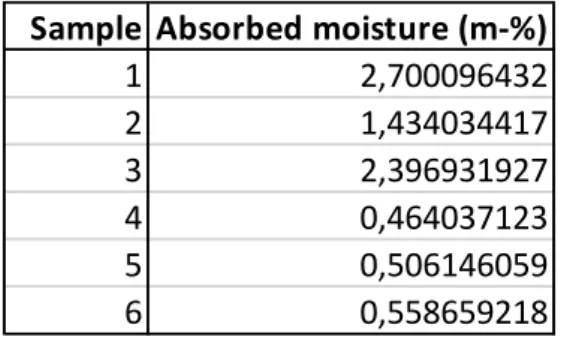
Their unique structure affects the moisture absorption and leads to greater deviation which can be seen from the Figure 23. Greater moisture absorption rate of the process phase 2 samples may also be a result of their oriented nature. During orientation, the polymer chains of the material are reorganized as parallel chains, when the moisture can penetrate the material relatively easily between the polymer chains.
The temperature may therefore be slightly higher than the standard temperature, but still noticeably below the 𝑇𝑔 of the material under consideration, PLGA, so the difference should not make a significant difference. However, humidity can be as low as 15 percent under standard conditions, which could significantly affect the rate of moisture absorption.
Effects of the dehumidifying gas on the sample masses
Both the maximum (CDA at 3.93 and nitrogen at 6.23) and the minimum (CDA at 1.67 and nitrogen at 3.76) values of the RH of the tubes had a significant difference. This may mean that the moisture content of the samples was probably also lower in the tube with CDA. When the moisture content of the samples (see Table 10) was calculated using formula 6, the difference between the samples that were in CDA and those that were in nitrogen was negligible due to the scale error.
According to these results, it can be said that all samples were practically dry. Due to the flow of CDA, moisture can escape from the container, which enables effective moisture transport, since the oxygen of CDA reacts with water molecules relatively easily.
The recovery time for the gas
According to Figure 24, it can be said that CDA worked as assumed in theory (see 2.3.3). According to another theory, there could have been some moisture in the middle of the pipe, where there was no measuring device, and that time just wasn't enough for it to move and mix with the gas at the end of the pipe. During this test, the RH was increased almost to the same scale as in the nitrogen tube with a pressure of 0.1 bar.
However, it took longer for the moisture to diffuse to the other end of the tube (near the moisture logger). In addition, it can be observed that the RH decreases at a faster rate, which supports the theory of more efficient removal of water molecules by CDA.
Future studies
Such as, the more efficient ability to remove water from oxygen molecules, due to the reactivity of oxygen molecules with water molecules. Available: http://what-when-how.com/mems-and-nanotechnology/nano-indentation-studies-of-polyglactin-910-monofilament-sutures-mems-and-. Hoorbin, “Water absorption by polymers: analysis in terms of a simple model,” Transactions of the Faraday Society , vol.
Available: http://www.a-hak-is.com/en/home/what_we_do/services/pipeline_services/drying. Samples were pre-made and cut (means to measure the dimensions of the test samples with an accuracy of ± 0.1 mm) and stored in a container in nitrogen atmosphere. Inside the cabinet, the atmosphere conditions are as follows: In test number one, the temperature is 23℃ and the relative humidity is 100% (6 samples × 300 ml of distilled water).
![Table 1. Physical and chemical properties of PLA. [16]](https://thumb-eu.123doks.com/thumbv2/9pdfco/1889862.266028/9.892.171.806.609.897/table-physical-chemical-properties-pla.webp)
![Figure 2. Chemical structure of PGA. [13]](https://thumb-eu.123doks.com/thumbv2/9pdfco/1889862.266028/10.892.185.704.315.500/figure-chemical-structure-pga.webp)

![Figure 8. Degradation of PLGA via hydrolysis. [40]](https://thumb-eu.123doks.com/thumbv2/9pdfco/1889862.266028/15.892.173.767.110.496/figure-degradation-plga-hydrolysis.webp)
![Figure 9. Bulk degradation. [41]](https://thumb-eu.123doks.com/thumbv2/9pdfco/1889862.266028/15.892.176.791.660.963/figure-bulk-degradation.webp)
![Figure 10. Decreasing properties during polymer degradation. [13]](https://thumb-eu.123doks.com/thumbv2/9pdfco/1889862.266028/16.892.190.586.185.506/figure-decreasing-properties-during-polymer-degradation.webp)
![Table 2. Comparison of coulometric and volumetric Karl Fisher titration. [51]](https://thumb-eu.123doks.com/thumbv2/9pdfco/1889862.266028/19.892.171.805.524.944/table-comparison-coulometric-volumetric-karl-fisher-titration.webp)
![Figure 11. Absorption of the IR radiation. [54]](https://thumb-eu.123doks.com/thumbv2/9pdfco/1889862.266028/20.892.190.523.340.561/figure-absorption-ir-radiation.webp)