The study could not conclude the involvement of this protein in the developmental polymerization of lignin in Norway spruce, but the regulation of enzyme activity was successfully studied. EV wrote the majority of the manuscript and revised it based on the suggestions of the co-authors.
Introduction
Norway spruce
Nevertheless, the existence of a sequenced genome made it possible to pose gene function research questions in Norway spruce.
Plant cell walls
- Primary cell wall
- Secondary cell wall
SCW formation is a developmental program in which the plant cell synthesizes additional layers of cellulose and hemicellulose on the interior of the PCW and reinforces this structure with lignin (Meents et al., 2018; Coomey et al., 2020). Most lignifying cells undergo programmed cell death (PCD) as part of cell maturation (Bollhöner et al., 2012).
Lignin
- Overview of lignin
- Lignin structure
- Monolignol biosynthesis
- Monolignol glucosylation and deglucosylation
- Monolignol transport
- Oxidation of monolignols
- Source of H 2 O 2 in the developing SCW
- Lignin polymerisation
- Programmed cell death and good neighbours
- Regulation of lignification
Vacuolar H + pyrophosphorylase (V-PPase) directly hydrolyzes cytosolic pyrophosphate (PPi) instead of ATP to transport H + (Gaxiola et al., 2016). In Arabidopsis, two scaffold proteins, membrane steroid-binding proteins (MSBPs), MSBP1 and MSBP2, form hetero- and homodimers at the endoplasmic reticulum (ER) and bind to the cytochrome P450 monooxygenases (C4H, C3'H and F5H) ( Gou et al., 2018 ).
Plant plasma membrane
A master switch for vessel cell differentiation in Arabidopsis, VND7, is regulated by S-nitrosylation (Kawabe et al., 2018). In general, thousands of different lipids are predicted to occur in plant PMs (Yetukuri et al., 2008). Receptor-like kinases (RLKs) of PMs, including wall-associated kinases (WAKs) and receptor-like proteins (RLPs), can detect signals on the apoplastic side of the PM and mediate information between the cell and its environment (Kanneganti and Gupta, 2008; He et al., 2018).
In addition, arabinogalactan proteins are sometimes anchored to the PM on the apoplastic side ( Ellis et al., 2010 ). The only glycolipid at the PM, digalactosyl diacylglycerol, plays a role in replacing some of the phospholipids during phosphorus deprivation ( Russo et al., 2007 ). There is asymmetry in the PM which means that the inner and outer leaflets can have different lipid composition (Gronnier et al., 2018).
Tonoplast
A method called the two-phase separation system (Widell et al. 1982; Larsson et al., 1994) has been used to prepare enriched fractions of PM from plant tissues. PM vesicles from the right side are separated in the upper phase (UP) with PEG while other cell membranes together with the inside-out PM vesicles remain in the lower phase (LP) with dextran due to the surface properties of the vesicles (Widell et al. al., 1982). An optimized two-phase system for Norway spruce tissues of developing xylem, developing phloem and cultured lignin-forming cells produced a membrane fraction containing enriched PM and tonoplast and a small amount of other cell membranes (Kärkönen et al., 2014; Väisänen et al., 2018).
Several sugar transporters belonging to the MFS family, as previously described (Niño-González et al., 2019), are known to be located in the tonoplast. Some primary active pumps of the ABCC type have also been detected in the tonoplast and appear to transport hormones and glutathione conjugates (Martinoia et al., 2018; Borghi et al., 2019). The tonoplast has lipid rafts where V-ATPase specifically localizes, as shown with suspension-cultured Arabidopsis cells (Yoshida et al., 2013).
Aims of the thesis
In addition, intrinsic proteins, glycosidases and proteins involved in stress responses and membrane remodeling, as well as in protein degradation or in cytoskeletal anchoring, have been found in tonoplast proteomic analyzes (Trentman and Haferkamp, 2013). Membrane vesicles prepared from developing xylem and lignin-forming cultured cells were used in biochemical assays. Membrane proteomics adds to the previously reported transcriptomic data from lichen-forming cell cultures of Norway spruce.
MGs are known to accumulate in the developing xylem of gymnosperms as well as in some angiosperms. Since PRXs are known to participate in lignin polymerization in lignin-forming spruce cell culture, the source of the oxidant, H2O2, in SCW is important. Regulation of H2O2 levels in SCW can regulate PRX activity and thus affect lignin polymerization.
Materials and methods
List of methods used in the thesis publications I-III
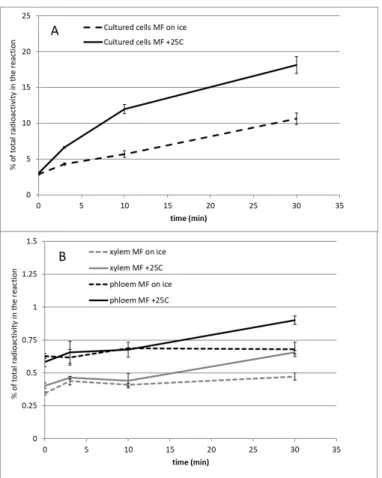
The reaction was then gently mixed, membranes were pelleted by centrifugation (17,000 g , 5 min at 4 °C), and the supernatant was removed.
Results and discussion
Monolignol toxicity
Increasing polymerization (and thus decreasing the concentration of end products and pathway intermediates) by overexpressing LAC4 and LAC7 rescues the growth phenotype in these plants (Perkins et al., 2020). The results of study I are also consistent with the more recent results by Guan et al. 2022) who showed that CA is toxic to Arabidopsis seedlings. Interactions of membrane proteins with surrounding lipids can be influenced by hydrophobic compounds (Sikkema et al., 1995).
On the other hand, an effect on the H + gradient by changing the ion permeability of the membrane (Hossain et al., 2021) would certainly inhibit coniferin-H + antiport. CA, one of the main monolignols, is toxic to plant cells (I), and both lignifying and neighboring cells produce and export it, as has been reported in Norway spruce (Blokhina et al., 2019). A sink in SCW appears to be important for monolignol transport (Perkins et al., 2022) and this would help reduce the monolignol concentration in the cytoplasm.
Monolignol glucoside transporter (MGT)
The pCAg transporter in hybrid poplar had a KM closer to that of coniferin in the developing xylem of Norway spruce: 160–260 μM (Tsuyama et al., 2019). However, this is within the normal range of KM values of tonoplast transporters (Tsuyama et al., 2013). To answer the first hypothesis, Tsuyama et al. 2013) suggested that coniferin is routed to the SCW via endosomal vesicles loaded by a secondarily active coniferin transporter.
In Norway spruce, the lignin structure in the final SCW layer, S3, is relatively rich in dibenzodioxocine structure (Kukkola et al., 2004). It is possible that this structure is formed due to changes in monolinoleic concentrations (Brunow et al., 1998). In vitro, coniferin noncompetitively inhibits the reverse CAD reaction in loblolly pine (Pinus taeda) (O'Malley et al., 1992).
Transport of monolignols and their dimers
The developing xylem in angiosperm species does not contain monolignol glucosides at the same levels as in gymnosperms (Terashima et al., 2016). The loss of a polymerization zinc in lac4 lac17 double mutants of Arabidopsis caused a decrease in lignification and an accumulation of phenols, presumably glucosides, in the vacuole (Perkins et al., 2022). Similarly, in the lignin-forming spruce culture, the polymerization impairs zinc by scavenging H2O2 through KI, thus preventing the function of PRXs, causing accumulation of some glucosides such as coniferin in the cells (Laitinen et al., 2017).
As such, the results are also consistent with the hypothesis of monolignol diffusion where aglycones do not require a transporter, while glucosides do (Vermaas et al., 2019; Perkins et al., 2022). This result can be explained by a similar mechanism that Perkins et al. 2022) created artificially with synthetic liposomes. To my knowledge, the lipid composition of the PM in hardening plant cells is not directly known, and lipid composition affects the diffusion of phenolic compounds through the membrane (Boija et al., 2007).
Candidate transporters
This supports the designation of Jokipii-Lukkari et al. 2018) that these 12 enzymes are involved in monolignol biosynthesis in developing Norway spruce xylem. Members of the ERD6-like family transport monosaccharides to the tonoplast by accelerated diffusion or via the H+ symbol (Yamada et al., 2010; Klemens et al., 2014). However, the closest Arabidopsis homolog for this transporter, AtABCG40, is known to import abscisic acid (ABA) at the PM ( Kang et al., 2010 ).
All were co-expressed with monolignol biosynthesis based on the analysis of the ConGenIE data and the data by Blokhina et al. In addition, a role for phenylpropanoid transporters in the regulation of the phenylpropanoid pathway has been suggested (Biała et al., 2017; Biała and Jasiński, 2018). Even pathway intermediates can be transported, similar to the way ABCG10 transports 4-coumarate and liquiritigenin, which are intermediates of the medicarpin biosynthetic pathway (Biała et al., 2017).
Lignification enzymes associated with the membranes
- Monolignol pathway enzymes in proteomics
- Plastids – or xyloplasts
In the co-expression analyses, 63 genes were co-expressed with the monolignol biosynthesis bait genes in all four datasets, supporting their role in lignification. The membrane proteomes were compared to this small set of co-expressed genes, and twenty of the co-expressed genes were also detected in the proteomics. Proteins corresponding to six of the 12 monolignol biosynthesis genes used as bait in the co-expression experiments were found in the membrane preparations (II).
The detection of the enzymes encoded by the bait genes in proteomic data supports the proposal of Jokipii-Lukkari et al. 2018) that the enzymes encoded by these genes are involved in monolignol production in the developing xylem of Norway spruce. Three C4H enzymes and one C3'H enzyme were found in the membrane proteomic data, and it is tempting to speculate that they may serve as anchors for a metabolon, as suggested for Arabidopsis ( Bassard et al., 2012 ). The shikimate pathway occurs within plastids and uses phosphoenylpyruvate to synthesize phenylalanine and shikimate for use in the monolignol biosynthesis pathway ( Tohge et al., 2013 ; Pascual et al., 2016 ).
PaRBOH1 and its activation by phosphorylation and Ca 2+
In the repeat sequence region, S32, S45, S58, and S71 (serine residues in each of the four repeat units) were phosphorylated at low levels. It is possible that phosphorylation of some serine residues in the PaRBOH1 repeat sequence would have led to increased enzyme activity. As this region of the protein was not clearly responsible for enzyme activation in HEK cell assays, it is possible that the region serves some other regulatory function.
Outside the repeat region, LC-MSE analysis revealed mild phosphorylation at T75, T149, S154, S175, Y342 and S366 with no evidence of enzyme activation. These results suggest that kinases in MF membranes could be interesting candidates for PaRBOH1 activation. A total of 50 kinases were detected in the proteomic analysis (II), of which 46 were found in xylem-developing Norway spruce samples.
Conclusions
In addition, most of the laboratory work was physically carried out at the Ministry of Agriculture and Forestry. The ABCG transporter PEC1/ABCG32 is required for the formation of the developing leaf cuticle in Arabidopsis. Conductor domain-containing protein is part of the machinery required for the formation of the lignin-based Casparian strip in the root.
Direct regulation of NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. The dibenzodioxocin lignin substructure is abundant in the inner part of the secondary wall in Norway spruce and silver birch xylem. Characterization of the UDP-glycosyltransferase UGT72 family in poplar and identification of genes involved in the glycosylation of monolignols.
Upadhyay N, Kar D, Mahajan BD, Nanda S, Rahiman R, Panchakshari N, Bhagavatula L, Datta S. Multitasking capabilities of MATE transporters in plants. Analysis of the b-glucosidase family reveals genes involved in stone cell lignification in Chinese White Pear (Pyrus bretschneideri Rehd.).