Introduction
Description of the causative agent of IBR
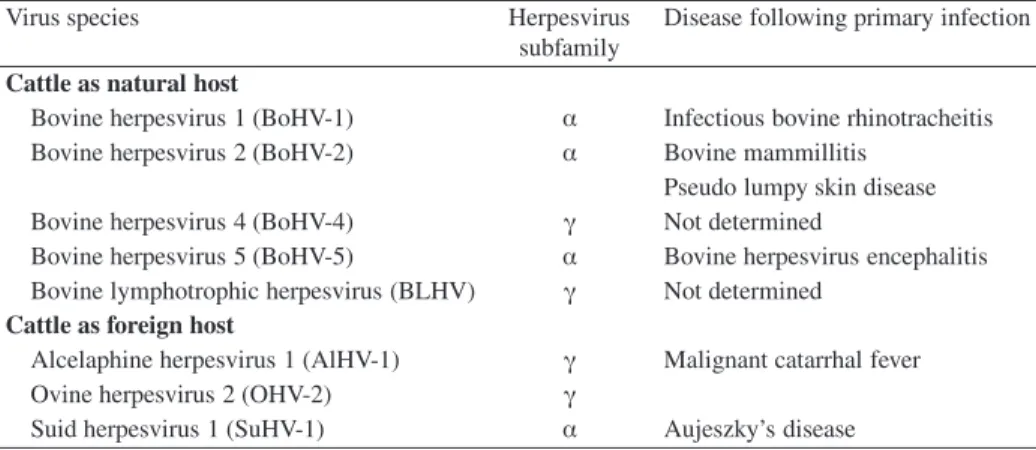
BoHV-1 replication in the cell
After this high-affinity interaction between gD and cell receptors, subsequent virus penetration occurs through fusion of the virion envelope with plasma membrane. As the virus particle is transported to the nucleus, tegument proteins are released into the cytosol of the infected cell, where they may play an important role in early times of virus infection, as they are the first to contact and interact with the intracellular environment. It localizes to the nucleus immediately after infection due to a nuclear localization signal [227].
They encode proteins that are respectively and mainly involved in the regulation of the viral cycle, in the replication of the viral DNA and in the morphogenesis of new virions. Indeed, four possible arrangements of the adjacent UL segment were observed in concatemeric DNA, while the UL of infecting virions is mainly fixed in one direction. Herpesvirus virion assembly is a complex process that proceeds along an ordered morphogenetic pathway.
This development requires a viral kinase activity encoded by US3, because US3 deletion mutants of HSV-1 [164], PrV [93] and MDV [177] accumulate in the perinuclear space.
BoHV-1 pathogenesis
- Entry and tropism
- Replication at the mucosal portal of entry
- Dissemination and spread
- Local dissemination
- Systemic spread by viremia
- Neuroinvasion
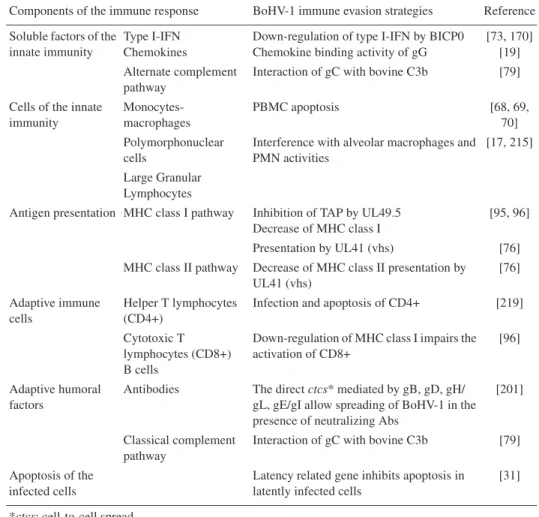
- Immune response and immune evasion strategies
- The role of BoHV-1 in cattle respiratory disease complex
- Latency and reactivation
It was also shown that caspase 3, a key regulatory protein in the apoptotic pathway, is activated late during productive BoHV-1 infection [ 35 , 118 ]. New progeny viruses are shed in the nasal mucus with high excretion titers and are responsible for the rapid spread of the infection within a livestock herd. First, the viruses released into the extracellular medium are fully enveloped particles that can interact with the receptors of sensitive cells.
The rostral parts of the nasal cavity are innervated only by the trigeminal nerve, while the olfactory epithelium in the caudal parts of the nasal cavity contains both olfactory and trigem-. However, there were no significant differences in viral levels found in peripheral organs. ApoE4 appears to facilitate HSV-1 entry and/or spread in the brain much more efficiently than E3.
These effects augment the first antiviral wave by secreting cytokines in the infected epithelium and killing virus-infected cells [ 23 , 24 ]. Therefore, it is controversial to assign any role in the pathogenesis of BoHV-1 to the many mechanisms of immune evasion described so far. By analogy with its homologues of the alphaherpesviruses HSV and PrV, BoHV-1 is thought to penetrate sensitive nerve endings distributed in the infected epithelium [ 47 ].
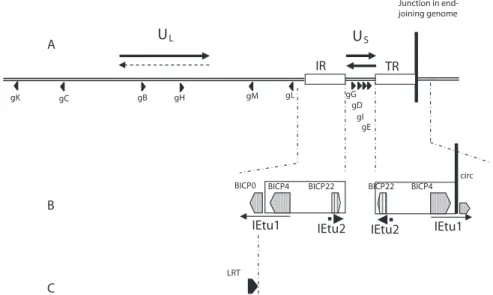
It is then transported along the microtubules of the axons to reach the neuron body in the nerve ganglion. No role in the BoHV-1 latency reactivation cycle has currently been associated with this small ORF. No mechanism currently explains the role of the LR gene in BoHV-1 reactivation and/or re-secretion.
By analogy with the observations obtained with HSV latency and reactivation, the immune system is thought to play a role in the regulation of the latency and reactivation cycle of BoHV-1. However, no data currently support the involvement of any viral protein in the regulation of the immune response during latency. Therefore, a reactivation stimulus that occurs within the first two months after primary infection would not be expected to result in re-shedding of the virus.
However, and in contrast to what is observed in related HSV-1, a thymidine kinase-deficient mutant of BoHV-1 is re-excreted from latency after reactivation treatment [217].
Clinical signs
This result suggests that the progeny virus phenotype may influence the re-shedding properties of BoHV-1 [143]. The names of the diseases affecting cows (infectious pustular vulvovaginitis, IPV) and bulls (infectious pustular balanoposthitis, IPB) clearly describe the clinical pictures observed after primary infection. Although the infection is limited to the genital organs, more severe infection leading to orchitis in the bull and endometritis in the cow has occasionally been reported [59].
Epidemiology
Vaccination and control
The DIVA strategy (aiming at di ff erentiating infected from vaccinated animals)
Several European countries have initiated control programs aimed at eradicating BoHV-1 based on the use of marker vaccines deleted in the gE gene. These marker vaccines, inactivated or live-attenuated, used in conjunction with serological detection of gE-specific antibodies, allow differentiation of infected from vaccinated animals [100, 212]. The effectiveness of DIVA vaccines (distinguishing infected from vaccinated animals) was demonstrated in two field trials.
In the first trial, a significant reduction in the rate of gE seroconversion was observed in herds where the gE-deleted vaccines were used [122]. The second study showed that repeated vaccination protocols using inactivated or live attenuated BoHV-1 vaccines with gE deletion are effective in reducing the incidence of gE seroconversion in dairy cattle and thus the prevalence of gE-positive animals in the herd [39]. . This study indicated the increased effectiveness of repeated vaccination at regular six-month intervals at herd level compared to punctual vaccination of part of the herds [39].
Indeed the strength of the instrument is in full. dependent on the capacity of the diagnostic test to detect BoHV-1 gE-specific antibody. This fairly low level of sensitivity is responsible for 30% false negative responses in individual tests, but it remains sufficient to guarantee a high sensitivity at the level of infected herds. The second disadvantage of this test is due to the poor level of the immune response raised against BoHV-1 gE. Consequently, the time window for the ability of this test to detect gE antibodies can be delayed for up to 42 days [9].
Finally, one can also mention two biosafety concerns about the live attenuated gE deleted vaccine. However, so far there is no indication for a possible persistence of this deletion mutant in the cattle population [121]. A second concern is the potential rise of BoHV-1 recombinants issued from co-infection situations involving a replicative gE-deleted BoHV-1 and a virulent field BoHV-1 strain.
A field observation and two experimental data underlie this concern:. i) isolation of a gE-deleted BoHV-1 vaccine strain in cows vaccinated eight months ago [38]; (ii) frequent growth of BoHV-1 recombinants in coinfected calves [182] and (iii) isolation of a virulent deleted BoHV-1 recombinant under experimental conditions.
The holy grail in BoHV-1 vaccines: the virological protection
A field observation and two experimental data underlie this concern:. i) isolation of a gE-deleted BoHV-1 vaccine strain in cows vaccinated eight months ago [38]; (ii) frequent growth of BoHV-1 recombinants in coinfected calves [ 182 ] and (iii) isolation of a virulent gE deleted BoHV-1 recombinant under experimental conditions [ 142 , 143 ]. Ambiguous results were obtained when both forms (inactivated and live attenuated) of the same marker vaccine were tested. When it was administered twice to seronegative cattle, the labeled attenuated vaccine induced better virological protection than the inactivated vaccines after challenge [11].
However, the inactivated vaccine was more efficient in reducing virus shedding after reactivation of latently infected calves than the live attenuated one [12]. An interesting approach consisted of combining the attenuated vaccine as the initial injection and the inactivated vaccine as a booster injection to complete the primary vaccination. This type of protocol was shown to be the most efficient in reducing virus shedding after challenge [90].
As mentioned above, the immune status of the latent BoHV-1 carrier is a key factor in controlling virus re-shedding upon reactivation stimulus. Therefore, vaccination of latent carriers should be carefully addressed with repeated vaccination schedules at regular six-month intervals to minimize the risk of re-excretion [39].
New trends in BoHV-1 vaccines
Conclusions
6] Baranowski E., Keil G., Lyaku J., Rijsewijk F.A., van Oirschot J.T., Pastoret P.P., Thiry E., Structural and functional analysis of bovine herpesvirus 1 minor glycoproteins, Vet. 9] Beer M., Konig P., Schielke G., Trapp S., Diagnostic markers for the prevention of bovine herpesvirus type 1: opportunities and limitations, Berl. 31] Ciacci-Zanella J., Stone M., Henderson G., Jones C., The latency-related gene of bovine herpesvirus 1 inhibits programmed cell death, J.
38] Dispas M., Schynts F., Lemaire M., Letellier C., Vanopdenbosch E., Thiry E., Kerkhofs P., Isolation of a glycoprotein E-deleted bovine herpesvirus type 1 strain in the field, Vet. [54] Geiser V., Inman M., Zhang Y., Jones C., The latency-related gene of bovine herpesvirus-1 may inhibit the ability of bICP0 to activate productive infection, J. [55] Geiser V., Zhang Y., Jones C., Analysis of a recombinant virus of bovine herpesvirus 1 that does not express the bICP0 protein, J.
69] Hanon E., Meyer G., Vanderplasschen A., Dessy-Doize C., Thiry E., Pastoret P.P., Binding but not penetration of bovine herpesvirus 1 is required to induce apoptosis in target cells, J. 80] Inman M., Lovato L., Doster A., Jones C., A mutation in the latency-associated gene of bovine herpesvirus 1 leads to impaired ocular shedding in acutely infected calves, J. 81] Inman M., Lovato L., Doster A., Jones C., A mutation in the latency-associated gene of bovine herpesvirus 1 disrupts the latency-reactivation cycle in calves, J.
87] Jones C., Geiser V., Henderson G., Jiang Y., Meyer F., Perez S., Zhang Y., Functional analysis of bovine herpesvirus 1 (BHV-1) genes expressed during latency, Vet. . 131] Meyer G., Hanon E., Georlette D., Pastoret P.P., Thiry E., glycoprotein H of bovine herpesvirus type 1 is essential for penetration and propagation in cell culture, J. 150] Nyaga P.N., McKercher D.G. , Pathogenesis of bovine herpesvirus-1 (BHV-1) infections: virus interactions with cellular components of bovine peripheral blood, Comp.
168] Roels S., Charlier G., Letellier C., Meyer G., Schynts F., Kerkhofs P., Thiry E., Vanopdenbosch E., Haala uumamaa dhukkuba herpesvirus loonii 1 meningoencephalitis re’ee ga’eessotaa keessatti, Vet . [ PubMed ] 172] Saydam O., Steiner F., Vogt B., Schwyzer M., Galmoota seelii keessummeessituu pirootiinii battalumatti jalqabu BICP22 herpesvirus loonii 1, Vet. [ PubMed ] 173] Schang L.M., Hossain A., Jones C., Jiiniin latency-walqabatee herpesvirus loonii 1 oomisha guddina marsaa seelii dhorku koodii godha, J. Biol.
182] Schynts F., Meurens F., Detry B., Vanderplasschen A., Thiry E., Rise and survival of bovine herpesvirus 1 recombinants after primary infection and reactivation from latency, J.