HAL Id: jpa-00248063
https://hal.archives-ouvertes.fr/jpa-00248063
Submitted on 1 Jan 1994
HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci- entific research documents, whether they are pub- lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Vesicles as an equilibrium structure of a simple surfactant-water system
L. Cantù, M. Corti, E. del Favero, A. Raudino
To cite this version:
L. Cantù, M. Corti, E. del Favero, A. Raudino. Vesicles as an equilibrium structure of a sim- ple surfactant-water system. Journal de Physique II, EDP Sciences, 1994, 4 (9), pp.1585-1604.
�10.1051/jp2:1994219�. �jpa-00248063�
Classification
Physic-s
Abstracts82.70 61.25
Vesicles
as anequilibrium structure of
asimple surfactant- water system
L. Cantb
('),
M. Corti(2),
E. Del Favero(')
and A. Raudino(3)
(')
Department ofChemistry
and Biochemistry, Medical School,University
of Milan, via Saldini 50, 20133 Milan,Italy
(2)
Department
of Electronics,University
of Pavia, viaAbbiategrasso
209, 27100 Pavia,Italy
(3) Department ofChemistry, University
of Catania, viale A. Doria 6, 95125 Catania, Italy(Receii,ed J April 1994, receiied in final form 20 May J994, accepted 27 May J994)
Abstract. Unilamellar vesicles are observed to form spontaneously in dilute water solutions of the
ganglioside
GM3, which is a double-tailedbiological amphiphile
with a saccharidicheadgroup. Static and
dynamic,
bothpolarized
and depolarized, laser light scattering measure-ments show that the
population
of vesicles, of about 500h
in diameter, is inequilibrium
with avery small number of much
larger
nonspherical objects,
which can be schematized as discs withan average radius of 5 COO
h. Hydrophobic
chains in the vesiclebilayer
arelikely
to show asignificative degree
ofinterdigitation.
Discs are found to disappearcompletely
when a secondamphiphile,
GM I, with the samehydrophobic
part and a much larger saccharidic headgroup is added.Spontaneous
vesicle formation is associated to the smallrigidity
of theganglioside
bilayer which may derive from the large mismatch in the lateral dimension of the polar heads and hydrocarbon chains.1. Introduction.
Self-association of
amphiphilic
molecules into different structures, likemicelles, bilayers, vesicles,
etc., has been agrowing
field of interest in statisticalphysics [1, 2]. Thermodynamic equilibrium
is animportant
condition forquantitative
theoreticalpredictions.
Unlike otherstructures, vesicles are
generally
believed to be in a metastable state of matter, sincepreparation
methodsnormally
involvesupply
of external energy, like sonication and pressure filtration, or chemical treatments likedetergent depletion
orreversed-phase evaporation.
Spontaneous
formation of small unilamellar vesicles has been neverthelessreported
in ratherspecial
cases, eitherby mixing
two ionic surfactants withoppositely charged
head groups[3, 4],
or for unusualpH
conditions[5, 6],
orby
dilution of spongephases
inmulticomponent
systems
[7, 8]. Recently
the spontaneous formation of vesicles made of asingle amphiphile,
the
ganglioside GM3,
inthermodynamic equilibrium
in water at normalpH
has also beenreported [9].
This last case isquite
different from thosereported
in[3]
and[4]
where the interaction between the two differentamphiphiles gives
rise to a spontaneous curvature of thebiiayer
and hence the vesicle formation is due to a clearenthalpic
effect. Infact,
for thesingle
component system the spontaneous curvature of thebilayer
isstrictly
zero since the twomonolayers,
made of the sameamphiphile,
have spontaneous curvatures of the samemagnitude
butopposite
insign. Therefore, entropic
effects inconjunction
with aquite
lowbending elasticity
of thebilayer play
animportant
role in the GM3 spontaneous vesicle formation.In this paper a more detailed account of the spontaneous formation of
ganglioside
vesicles ispresented.
Careful static anddynamic,
bothpolarized
anddepolarized,
laserlight scatterinj experiments
are used to characterize the system and toverify
theimportant finding
that in solution vesicles are inequilibrium
with a small amount oflarge
aggregates,mostly
of lamellar type.Temperature
affects both the vesicle distribution and the amount oflarge
aggregates.Thermodynamic equilibrium
isquite
evident, sincelarge
aggregates, after removalby microporous
filtration, reformimmediately
at the expense of the vesiclepopulation
present in solution. In addition, if a secondamphiphile,
of the same type of theganglioside
GM3 but witha
larger headgroup,
isadded,
thelarge
aggregatesdisappear completely
andonly
small unilamellar vesicles of the mixed surfactant type are present in solution. This lastexperimental
observation drives to the non trivial conclusion that the addition of a second
amphiphile,
which isexpected
to cover thehydrophobic edges
and therefore stabilize finite lamellarobjects,
has theopposite
effect. This discards thepossibility
that the presence of thelarge
lamellaraggregates in the
single
GM3 surfactant solutions is due to the stabilization effect of eventualtrace
impurities
which are difficult to rule out frombiological amphiphiles.
GM3 vesicles arefound to have a small
bilayer thickness,
which can be ascribed tohydrophobic
chaininterdigitation,
a feature which is notnormally
encountered inphospholipid
vesicles.The behaviour of the pure GM3
ganglioside
in solution isqualitatively
discussed in terms of the lowbending elasticity
of theganglioside bilayer,
which may also come from theappreciable
mismatch in the lateral dimension of thepolar
head and thehydrocarbon
chains of theganglioside
molecule, with a saccharidic head group muchlarger
than, for instance, thecholine of
phospholipids.
Such a mismatch inpacking
areas may alsojustify
some anharmonic contributions to the energy of thebilayers.
In the case of lowbending elasticity,
and hencelarge
thermalfluctuations,
a small anharmonic term may favour finite size lamellar structures,as shown in
appendix.
This canhelp
inunderstanding
the existence of a small lamellarpopulation
inequilibrium
with vesicles in thesingle amphiphile
solution of theganglioside
GM3.
2.
Ganglioside
molecules in solution.Gangliosides
are sialic acidcontaining glycosphingolipids
that arenormally
present in theouter leaflet of
plasma
membranes of vertebrate cells[10]. They
have a markedamphiphilic
character with an
oligosaccharide
chainheadgroup
and a double tailedhydrophobic
partconstituted
mainly by C20 sphingosine
andCj8 fatty
acid. In nature alarge variety
ofgangliosides
is found, which differ in thelength
and conformation of both theoligosaccharide
and
hydrocarbon
chains.As an
example, figure
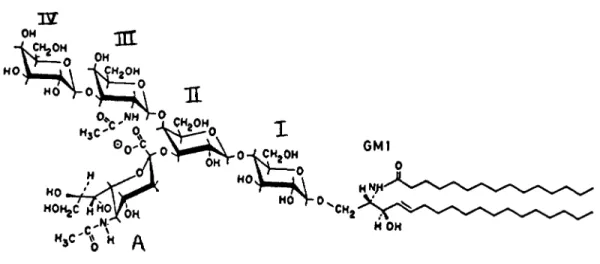
shows the chemical structure of theganglioside
GMI. A sialic acid A is attached to the sugar II in theoligosaccharide
chain I, II, III and IV.Sugar
IV ismissing
in GM2 and sugars IV and III aremissing
in GM3. Recent NMR studies[I I]
show that a net of interactions connects ratherstrongly
sugar III and the sialic acidA,
thusreducing
the overall rotational freedom of the
headgroup.
Differently
fromphospholipids, gangliosides
may form micelles since thelarge
sugarheadgroup
cansupply enough
curvature to formglobular
structures rather than vesicles orW
OH
~z
cHzoH
Ho
~
~fl
O. NH
H>C'~' I
GMT 0 H
~~ ,' HNfi
' '
OH
/~
Fig, I. The ganglioside GMT.
bilayers [I1, 12].
Forinstance,
the GM I and GM2 molecules, of molecularweight
560 and 398respectively,
self assemble in solution to form micelles with anaggregation
number N of 300 and 530. The critical micelle concentration is very low for allgangliosides,
of the order of 10~8 M[13].
For smallerheadgroups,
as in the case of GM3, micelles are no more formed andbilayer
type structures are found[9, 11].
Thegangliosides
used in the presentexperiments
areextremely
pure, as obtained after carefulchromatographic procedures [10].
GM3 and GM I areprepared
as sodium salts.They
dissolve in water at normalpH.
GM3 solutions reach anequilibrium
afterhaving
beenkept
at room temperature for acouple
ofdays.
From that time on,they
arehighly stable,
as shownby
the exactreproducibility
of measurementsperformed
on the same
samples
over several months.3. Static and
dynamic
laserlight scattering [14].
The laser
light scattering technique
is not intrusiveand, therefore, quite
suitable tostudy equilibrium properties,
but it is often believed difficult to beapplied
tocomplex
distributions of aggregates. Thetechnique
is nevertheless verypowerful
if static anddynamic
measure-ments, both on the
polarized
anddepolarized
components, are combinedtogether
for a modelfitting
of the aggregate distribution[15].
Somegeneral expressions
for incidentpolarized light
normal to the
scattering plane
arereported
below, in the case of noninteracting particles.
3.I POLARIZED SCATTERING. The excess average scattered
intensity l~ l~
relative to theintensity
scatteredby
the solventl~
can begiven
in terms of thephysical properties
of theparticles
if (k ))
=
(l~ 1~ )/l~
=
A
(dn/dc
)~ c (r)
M(r)
P(k,
r) dt.(1)
The instrumental constant A is 5.83
g/cm~
for water as a solvent and dn/dc is the refractive index increment of the solution.Polydispersity
is accounted forby
the termc(t.),
which is theweight-concentration
ofparticles
of characteristic dimension r such thatc(r)
dr= c~, the
total concentration in
g/cm3
present in solution.M(r)
is the molecularweight,
P(k, r)
theparticle
form factor[16]
and k=
(4
wn/A ) sin o/2 is the modulus of thescattering
vector, with o thescattering angle,
A the laserwavelength
and n the refractive index of the solution.For a
polydisperse
solution ofparticles
in Brownianmotion,
the modulus of the field correlation function gi jr(, describing
thetemporal
behaviour of theintensity
fluctuations of the scatteredlight,
isexpressed
as aweighted
sum ofexponentials [14, 16]
(1) (gi(r)(
=
lG(r)exp(- rr)dr (2)
where r
=Dk~,
with D theparticle
translational diffusion coefficient(interactions
areneglected).
G(r ),
theintensity
of the scatteredlight
at agiven
k-value due toparticles having
diffusion coefficient
D,
isgiven by
G
(r (k, I))
~ A(dn/dc
)2 c(r)
M(r)
P(k, 1) (3)
where the connection between r and the
geometrical
parameters, here summarized with thecharacteristic dimension r, is
given by
theparticle hydrodynamics.
It isimportant
to stress thatboth statics and
dynamics depend
onpolydispersity
via the concentration distributionc(r) weighted by
theproduct M(t.)P (k, r).
Furthermore,particle shape
effects come intodynamics
notonly by
the term P(k, r),
as instatics,
but also from theparticle hydrodynamics.
For instance, the Stokes Einstein relation for
spheres
D
=
k~ T/(6 w1~Ru), (4)
with
k~
the Boltzmann constant, T the absolutetemperature
and 1~ the solventviscosity,
is extended toellipsoids
with thehydrodynamic
radiusRu given
in terms of the Perrin factor[17]
f
and the radius of thesphere
ofequivalent
volume r~ asRu
=
fr~.
The Perrin factor isshape dependent.
It isobviously
I for asphere
and increases with the axial ratio with two differentfunctional
dependences
for oblate andprolate ellipsoids.
The translational diffusion coefficient D of an oblateellipsoid
of rotation was calculatedby
Perrin[18]
in terms of itsminor,
b, andmajor,
a, dimension and axial ratio p= bla as
D
=
(kB
T/6 w ~Jb) pF (p (5)
with
F
(p ),= (p
2)-
'/2 tan- 'j(p
2)"2 (6)
From a measurement of the
intensity
correlationfunction,
the function gj (r)(
is obtainedon a set of
delays.
Inprinciple,
it is thenpossible
to solve theintegral equation (2)
for theweighting
function G(r
and hence the desired distribution c(r
)[14].
Inpractice
statistical andsystematic
errors in the collected data make the inversion process rather difficult. Ifshape polydispersity
is also present, direct inversion becomeshopeless.
In these cases it is useful to characterizepolydispersity by
the cumulant timeexpansion
of thelogarithm
of the normalized field correlation function[14].
The linear termjrj
=
lrG(r)dr (7)
and the
quadratic
termp =
(r in
)~ G(r)
dr(8)
can be obtained
experimentally
withgood precision,
so thatthey
can be usedprofitably
in a fit of a model distribution.3.2 DEPOLARIZED LIGHT SCATTERING. If aggregates are formed
by optically anisotropic molecules,
like theganglioside
GM3 for instance, the scatteredlight
can have adepolarized component. Depolarized light scattering theory predicts
that for thinspherical vesicles,
even if made up ofanisotropic
molecules, nodepolarization
can occur, due to intemal cancellation effects[19].
On the other hand if theshape
of theaggregate
isaxisymmetric,
theoptical anisotropy
of the moleculegives
rise to adepolarization
effect. This is the case with anamphiphilic cylindrically-symmetric
molecule embedded into acylindrically symmetric aggregate
like a disc.Depolarization
measurements are thereforequite
useful to evidentiatefragments
ofbilayers
in presence of alarge population
ofspherical vesicles,
because vesicles do not contribute.For thin
anisotropic discs,
theoretical calculations[20] predict
theangular dependence
of scatteredlight intensity
in the VHconfiguration,
that is thehorizontally-polarized
scatteredintensity lvu,
due to incidentlight polarized vertically (perpendicular
to thescattering plane)
:lvu
=
A
(9 6~/k~)[l~ l~
+sin~ o/2(1.25 l~ l~)] (9)
with 6 the
optical anisotropy,
A a normalization constant[18],
h=
(4
wR/A sin o/2, R thedisc radius and
l~
=
lJ((h
sin y)(sin
y )~~dy (10)
Moreover, alsodynamic light scattering
measurements can be made in the VHconfiguration
to obtain information on the rotational diffusion coefficient & of the
nonspherical particles,
which, asalready
stated, are theonly
ones which contribute to thedepolarized
scatteredintensity.
For an oblateellipsoid
ofrotation,
the rotational diffusion coefficient isgiven by [16]
:&
=
(3 k~
T/167rl~b~) p~[(2
p ~) F(p
I]/(I p~) (I I)
where F
(p )
is definedby equation (6).
The modulus of the field correlation function in VH
configuration
is thenexpressed
in terms of the translational and rotational diffusion coefficients as[16]
gj (r
(~~
÷ exp[- (Dk~
+ 6&)
r(12)
4. Results and
analysis.
After the
preliminary
observationby
electronmicroscopy
that GM3 forms vesicles in water solution[21],
extensive laserlight scattering
measurements, bothpolarized
anddepolarized,
have been
performed.
The
ganglioside GM3, prepared
as sodiumsalt,
was dissolved in 30mM Nacl water solution at a concentration of 0. ImM,
which is very dilute(0.119 mg/cm3).
Nacl was added to shield Coulomb interactions among vesicles[22].
Vesicles formspontaneously
also in purewater or at different salt concentrations. As a test of the fact that GM3 solutions reach
thermodynamic equilibrium spontaneously,
it has been checked that sonicated and unsonicated solutionsgive exactly
the same results. All measurementsreported
in this paper have beenperformed
with unsonicated solutions. Solutions were filtered on 0.4 ~Lmpolycarbonate
filtersprior
to measurements.Aggregates
in solutions were studied as a function oftemperature,
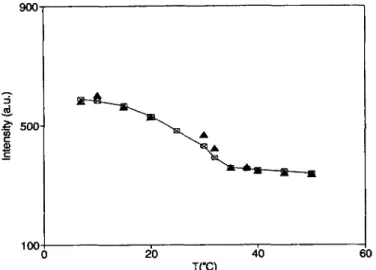
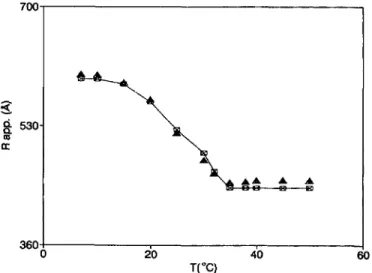
between 7 °C and 50 °C. The temperature
dependence
of the average scatteredintensity
(I)
and theapparent
radius R~~~, measured at thescattering angle
of90°,
arereported
infigure
2 andfigure
3respectively.
The apparent radius is calculated withequation (4)
from thed d
./l
fl A
© ,
g
20
T(°C>
Fig. 2. -Temperature dependence of the average scattered
intensity
(full symbols) from the GM3 solution, measured at anangle
of 90°.Open
symbols represent the values obtained with the fit (the full line is drawn toguide
the eye).o
T(°C>
Fig. 3. Temperature
dependence
of the apparent average hydrodynamic radius (full squares) of the aggregates in the GM3 solution, as directly obtained from the cumulant fit of the correlation functions measured at an angle of 90°. Open symbols represent the values obtained with the fit (the full line is drawn to guide the eye).average diffusion coefficient
(D)
obtaineddirectly
from the measured first cumulant(r) (Eq. (7)).
Of course,R~~~
acquires
a realmeaning
of an average radiusonly
if all aggregates in solutions havespherical shape,
which is not the case for the GM3solution,
as it will be shown later. At variance with the case ofphospholipids
vesicles, measured data do notshow any
hysteresis
effects inheating
andcooling
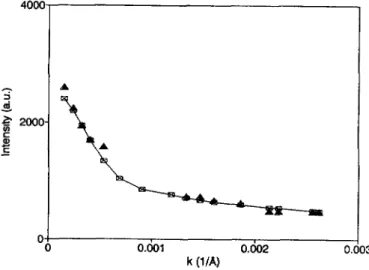
runs at different scan rates from 200 to000 s/°C. The average scattered
intensity
as a function ofangle
isreported
infigure
4 for T=
7 °C and in
figure
5 for T=
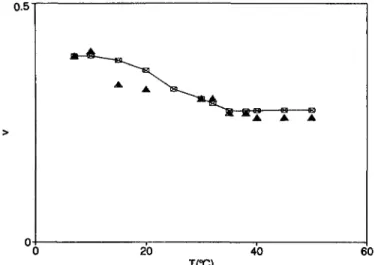
50 °C. The variance v =
p/ (r)
2, obtained from the measured secondcumulant, equation (8),
at o= 90° as a function of temperature is
reported
infigure
6.A first
glance
at theangular intensity
distributions offigures
4 and 5 indicates that alarge polydispersity
isaffecting
the system. A steep curve at low k values istypical
forlarge
aggregates, while a flatter one is present athigh
kvalues,
morelikely
to be due to much smallerparticles.
This firstimpression
issupported by
the fact that(r),
the first cumulant, does not scale as k~ and that v ishigh
andkeeps growing
whilereducing
the observationangle.
This canbe the observed behaviour if very different P
(k,
t-l's weight
theparticle
distribution at differentA 5
d 27
Iw
f
k(IIA>
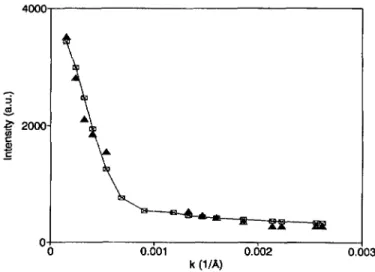
Fig.
4.-Average
scattered intensity (full squares) measured as a function ofangle
(in units ofmomentum transfer) for the GM3 solution at a temperature of 7 °C. Open symbols represent the values
obtained with the fit (the full line is drawn to
guide
the eye).k(iIA>
Fig.
5. The same as figure 4 at a temperature of 50 °C.JOURNAL DE PHYS<QUE H T 4 N'9 SEPTEMBER 1994
> ,
4 4 4
>
Tm>
Fig.
6. Temperaturedependence
of the variance (full symbols) of the GM3 aggregate distribution, measured at an angle of 90°. Open symbols represent the values obtained with the fit (the full line is drawn to guide the eye).k's. Cumulant
analysis
was observed togive quite reproducible
results onrepeated
measurements, while direct numerical inversion
procedures
of the correlation function were not.Therefore,
a model distribution of aggregates which gave the best agreement on absolute scale with all the measured data was searchedby giudicious
trials.Vesicles are known to be present in solution
[21] by preliminary
electronmicroscope
observations. The vesicle molecular
weight M~
can be related to its radius t., asM~
= m
[2 wit(3 V) ](6 r~
+i~/2 ),
with m=
195 Dalton the GM3 monomer molecular
weight,
V 965h~
themonomer
hydrophobic
volume[23],
I thehydrophobic layer
thicknessand r the vesicle radius taken at the middle of the
hydrophobic layer.
The form factorP~(k, r)
of a vesicle is[22]
:P~(k,
r=
(3 [sin
k(r
+i12
+s
)
sin k(r i12
s
k(r
+i12
+s cos
k(r
+i12
+s +
+
k(r i12
s cos
k(r i12
s
)i/ i(k~ (r
+i12
+s
)(3 r~
+(i12
+s)~
))ij
~with s
=
8
h
the thickness of thehydrophilic layer [21].
Since alarge
range of dimensions has to becovered,
a first trial is a Shultz or alognormal
vesicle distribution W~(r).
The result is that it isimpossible
toreproduce
theexperimental if (k,
r))
with such monomodal distributions,regardless
of thefitting
parameters involved. This means that an additional distributionW~(r)
oflarge
aggregates is present in solution. There is no way to get rid of theselarge
aggregates, sincethey
are indynamic equilibrium
with vesicles. Infact,
whenthey
are filtered out,they
reform ratherquickly.
Notbeing
constrained any more toassign
the observedlarge
polydispersity
to the vesicle distributiononly,
it isquite
reasonable to assume that the vesicle distribution is the onepredicted theoretically by
Helfrich[25]
for vesicle inthermodynamic equilibrium [26]
W~
(r
=
N
(2/ (N )
)~ exp(-
N/(N ) (13)
where N
=
M~/m
is the number of monomers in a vesicle. Theonly
free parameter is theaverage vesicle
aggregation
number(N).
The total aggregate distributionW(r)
is then :w(r)
=
w~i>.)
+w~(r). (14)
The
shape
and relative concentration of the aggregatesbelonging
to W~(r
is searched in such a way toreproduce
the whole set ofexperimental
data,equations (1), (7)
and(8).
Of course, anychange regarding W~(r drags
back achange
also inW~(r).
Forexample
theexplicit
form of the first cumulant isjr(k)j
=
(k~
T/6 gry~ )l~ drjc~ w~(r) M~(r) P~(r, k) k2/(r
+i12
+s
)j
++ dr ic~ W~ (r
M~(r
P~
(r,
kk~/R~ (15 )
where c~ + c~ = c~, the total concentration in the
solution,
c~being
the concentration of GM3which goes into vesicles and c~ the one which goes into the
large
aggregates. Thehydrodynamic
radiusRu depends
on the geometry of the aggregate. A constraint to the fitcomes from the conservation of the total concentration of GM3 monomers in solution and in
particular
c~ =
c(r)
dr=
I[c~
W~ (r + c~W~(r)]
dt-(16)
The
general
feature of thefitting procedure
is that the set of parameters obtained from thestatics does not leave any more freedom to the
dynamics,
as can be seenby comparing
equations (I)
and(3).
Therefore thedynamics provides
astringent
test of the fit. If a distribution ofspheres
is assumed forW~(r),
agood
fit is never reached. Infact,
there is no way to obtain asatisfactory
fit unless somelarge
flatobjects,
schematized as very thin oblateellipsoids,
are considered. The final fit is then obtained with an Helfrich distribution of vesiclesW~(r)
and aW~(r)
made up of the sum of two &functions, one of flatbilayers W~(r)
and one ofmultilayer liposomes Wj(r),
with c~= c~ + c-j.
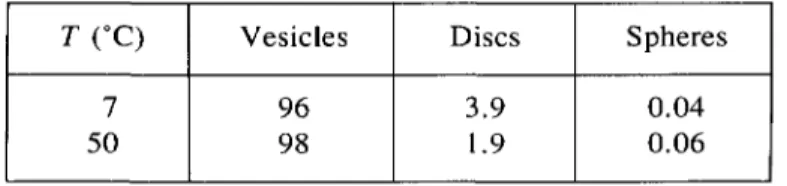
Table I. Fraction
of GM3,
in fbby weight, for
thedifferent
typesof
aggregates at the twoextreme temperatures, as obtained
fi.om
theglobal fit of
thelight scattering dita.
T
(°C)
Vesicles DiscsSpheres
7 96 3.9 0.04
50 98 1.9 0.06
The parameters of the
fit,
concentrations anddimensions,
arereported
in table I for the twoextreme temperatures. Good
consistency
in the fit of the absolute scatteredintensity
data isobtained with a value of 19
h
for thehydrophobic layer
thicknessI.
The full lines infigures
4 and 5 represent the result of the fit for theangular
distribution of the scatteredintensity
at thetwo extreme temperatures, 7 °C and 50 °C. If a linear variation of the fractional concentrations
is assumed for intermediate temperatures, the full lines of
figures
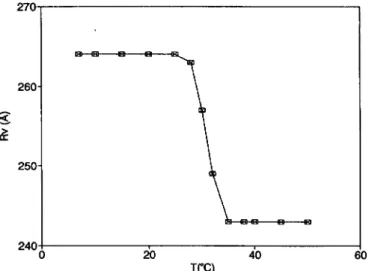
2, 3 and 6 are obtained. As aconsequence, the fit
gives
a variation of the average vesicle radius(i~)
asgiven by figure
7, which shows a transition around 30 °C. Of course, theextremely simple
distribution oflarge
aggregates, made of two &functions, may not be too realistic, but it issurely
indicative of the presence oflarge
flatobjects
in solution. A strong verification of this last conclusion isgiven by
the existence of adepolarized
component of the scatteredlight,
which cannot be due tocentrosymmetrical
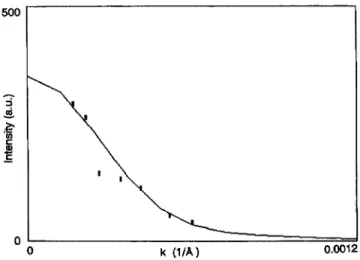
aggregates, like vesicles andliposomes. Figure
8 shows the average40 T(C)
Fig. 7. -Temperature dependence of the average vesicle radius resulting from the
global
fit of thepolarized
scattering data.500
'
O k (i IA
Fig. 8. Angular dependence (in units of momentum transfer) of the
depolarized
VH average intensity scatteredby
the GM3 solution (full squares) the full line represents the expected value for thin discs of 5 0001
radius.depolarized
scatteredintensity
in the VHconfiguration
as a function ofangle
for a I mM GM3 solution at a temperature of 25 °C. The full line in thefigure
is the theoreticalprediction,
calculated with
equation (9),
of the VHdepolarized
scatteredintensity
of thin discs of 50001
in radius.
Fitting
with the behaviour for thedepolarized intensity predicted
for rods[27]
is notacceptable.
From the ratio between the horizontal and vertical components of the scatteredlight,
the value of the intrinsicanisotropy
3 can be found to beequal
to0.02,
a very low valuewhich is an index of the
optical anisotropy
of thesingle
GM3 monomer.Also correlation measurements of the
intensity
fluctuations of thedepolarized
component in the VHconfiguration
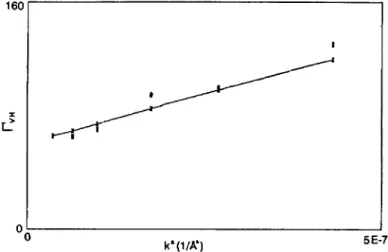
confirms the existence of lamellar type aggregates.Figure
9 shows the k-C
x_a
O
~ k*(i/A') 5E-7
Fig.
9. k-squaredependence
of thedecay
constant (full squares) of the field correlation function of the VHdepolarized
scatteredintensity
for the GM3 solution the full line presents the expected behaviour fora
polydisperse
solution of thin discs (simulated forsimplicity
with 70 fb of discs with radius of 30001
plus 30 % of discs with radius of 90001).
square
dependence
of thedecay
constant of the field correlation functionFv~
=
Dk~
+ 6 &(Eq. (12)).
Full squares are theexperimental
values.They
do not lie on ameaningful straight
line. Low
angle
data are better fittedby assuming larger
dimensions, whilehigh angle
valuesare
likely
to be due to smaller discs. This is a strong evidence ofpolydispersity
infact, larger objects give
ahigher
contribution to theintensity
in the forward direction with respect to smallerparticles.
The rotational diffusion coefficient measurements are indeedquite
sensitive topolydispersity,
due to their cubicdependence
on theparticle
radius(Eq. II)).
Of course, thissensitivity
islacking
in the measurements of the averageintensity
of thedepolarized
scattered
light,
which couldonly
be used to determine the average disc-dimension of 50001.
The full line in
figure
9 is calculated(Eqs. (5)
and(I I)),
with apolydisperse
solution of thin discs,approximated
as oblateellipsoids
with verylarge
axial ratio. Forsimplic(ty,
the disc distribution has beenapproximated
with 70 fb of discs of 30001radius
and 30 fb of discs of9 ooo
A
radius.It may be
argued
that the existence of a smallpopulation
of discs inequilibrium
with vesicles is due to the presence in solution of someimpurities
ofamphiphilic
nature which could shieldfrom water the unfavourable
edges
of the discs. In order to stabilize thediscs,
these molecules must have a smallerpacking
parameter[2]
thanGM3,
that is alarger
ratio of the head-to-tailcross sections. This
point
has been checkedby adding
to the GM3 solution a secondamphiphile,
theganglioside
GM I, which has the samehydrophobic
part of GM3 but alarger headgroup.
In fact, GM Iby
itself forms micelles in solution with a small radius of curvature[I II.
The effect is that discs are not at allstabilized,
butthey gradually disappear
as the GM I concentration is increased, until a pure vesicle solution is obtained for a GM I to GM3 ratio of 35 to 65. This is shown infigure10,
where theangular
distribution of the scatteredlight intensity
of the mixedganglioside
solution is showntogether
with the pure GM3solution,
forcomparison.
Thelarge
scatteredintensity
at lowangles disappears,
when GMT is added,which is a clear indication of the absence of
large
aggregates.Besides,
the scattereddepolarized light intensity
is observed todrop
downcompletely,
which is a further verification that discs are no more present in solution. Thetemperature dependence
of the mixed vesiclesaverage radius is also
completely
different(Fig.
II).
Absence ofimpurities
in thebiologically-
«
@
= @
~
d
# i
9 .
O.O024 »
k(iIA)
Fig.
10. -Angular distribution (in units of momentum transfer) of thepolarized
average scatteredintensity
of the mixed GM1-GM3 solution in the molar ratio 35 to 65 for a total concentration of I mM (fulltriangles).
The angular distribution for the pure GM3 solution is shown forcomparison
(fullsquares).
<
Ttc)
Fig.
ii.Temperature dependence
of the averagehydrodynamic
radius of the GM3 GMT 63 35 mixedganglioside
vesicles.prepared ganglioside
GM3 is also confirmedby
the fact that testexperiments performed
on asmall
sample
ofsynthetically prepared
GM3[28] gives exactly
the samescattering
curves asthe ones obtained with the
biological sample.
GM3-GMT solutions have beenprepared
first in chloroform-methanol, so thatgangliosides
are dissolved and mixed in monomericform,
dried under vacuum and than dissolved in water.The
light scattering
result that thehydrophobic layer
thickness of GM3 vesicles is small isquite interesting,
as it can be due to chaininterdigitation.
Infact,
the best fit value is close tothe
length
of thehydrophobic
part of asingle
GM3ganglioside
molecule and not twice aslarge
as it would have been
expected
if GM3 vesicles behaved like normalphospholipids
ones.Small-angle X-ray
measurements[29] performed
on the same GM3 vesicle solution seem to confirm such a smallbilayer
thickness.5.
Interpretation
and discussion.The
experimental
datagiven
so far for theganglioside
GM3 solutions presentinteresting
results:
I)
vesicles formspontaneously
in asingle amphiphile
water solution and arethermodynamically stable, it)
thebilayer
thickness is smaller than twice the GM3 molecularlength, meaning
chaininterdigitation, iii)
differentpopulations
of aggregates are found insolution,
thatis,
a few percent of theamphiphilic
molecules does not go intovesicles,
but formslarger
aggregatesmainly
of disc-likeshape, iv)
the addition of a secondamphiphile,
theganglioside
GM I, with alarger headgroup
than GM3 does no stabilizediscs,
as it could beexpected
if GMTprovided
a better coverage of the discedges.
Instead, thelarge
aggregatesprogressively disappear
andfinally, only
vesicles are found to be present in the mixed solution.The rather unusual features of these
experimental findings justified
the effort ofobtaining independent consistency checks,
like the static anddynamic depolarised light scattering
measurements for the existence of
lamellar-type
aggregates, and the smallangle X-ray
measurements for the
bilayer
thickness.The fact that GM3 vesicles form
spontaneously
in solution is a clear indication that the energy cost to form a vesicle is rathersmall,
of the order of the thermal energy. For asymmetric bilayer
thebending
energy per unit area[30],
to theapproximation
of nohigher
terms than second order in the mean curvature H
= cj + c~
contributing
toit,
may be written asg =1/2
KH~+
kK, whereK=cjc~
is the Gaussian curvature of thebilayer,
cj andc~
being
theprincipal
curvatures. There are two elastic moduli, thebending rigidity
K and the modulus of Gaussian curvature, k. Thespontaneous
curvature term isabsent,
forsymmetry
reasons, since the two
monolayers
are made of the sameamphiphile,
theganglioside
GM3.The
bending
energy of asphere (cj
= c~=
I/r is 4 ar(2 K + k
).
It may beregarded
as the minimum energyrequired
to form a vesicle from aplanar bilayer
and it isindependent
of the vesicleradius,
within the aboveapproximation.
Thebending rigidity
modulus K ispositive,
while k may be
positive
ornegative.
When twomonolayers
made of the sameamphiphilic
molecules are stuck
together
to form thebilayer,
eachmonolayer
feels frustrated for any finite value of the spontaneous curvature of themonolayers.
As a consequence of the frustration of themonolayers,
the modulus of Gaussian curvature k of thebilayer
is found ingeneral
finite[31]
and itssign
isjust
theopposite
of that of the molecular asymmetry, definedby
the difference of the mean head and chain areas of theamphiphilic
molecule, as demonstrated in reference[32].
For the GM3ganglioside
molecule the areaoccupied by
the head group, made of three sugarrings
of41each arranged
in a ramified structure, isundoubtly larger
than thehydrophobic
area determinedby
the chainspacing,
of the order of 4.21 [33].
The modulus ofGaussian curvature is therefore
expected
to benegative
for theganglioside bilayer.
There arealso
good
reasons to believe that thebending rigidity
itself is small ascompared
to the onereported normally
forphospholipids [34].
First of all thelarge
head group dimension maycause disorder in the
hydrophobic chains,
then thehydrophobic
thickness is small. Both effectsare known to reduce the
bending rigidity
of thebilayer [35]. Finally, bending rigidity
can also be reducedby protrusion
effects[36].
In fact theganglioside
GM3 does not have asharp
transition from the
hydrophilic
to thehydrophobic
partalong
the molecule, due to the presence of the NH, CO and OH groups in thesphingosine (Fig,
I) which may allow more freedom to itsmotion normal to the
bilayer
surface. A low value of thebending rigidity
modulus, of the orderof
k~ T,
has also been foundexperimentally
forlarge
vesicles ofglucosidic
surfactants[37].
The
bending
energy of the GM3 vesicles can therefore bequite small,
due to the combined effect of a smallrigidity
modulus and anegative
k. It is clear that the vesiclephase
can bethermodynamically
stable in this case, since translational entropy can balance the smallpositive
energyrequired
for the vesicle formation. Renormalization of thebending rigidity
dueto thermal ondulations
[38] gives
aslight dependence
of the vesicle energy from its size. The vesicle size distribution(Eq. (13))
used in the fit of the GM3experimental
data, is calculated with an effectiverigidity
which takes care of this renormalization effect. The average vesicle radius determinedexperimentally
reduces with temperaturegoing
from 2651
to 2451from
7°to 50 °C. This could be due to an increased
fluidity
of thehydrophobic
chains of GM3 or, moregenerally,
to the reduction of the effectivebending rigidity
of thebilayer
with temperature.The value of the average diameter of the
spontaneously-forming
GM3 vesicles should be of the order ofmagnitude
of thepersistence length [39]
of the GM3bilayer,
or,similarly,
of thelength
scale L at which the effectiverigidity
K' becomes very small : K'= K
[a
kBT/(4
ar)]
In (Lla ) « kB T,
(17)
where the factor
a is
predicted
to be either[38]
or 3[40]
and a is thebilayer
thickness. In fact, an ondulatedpiece
of membrane will be «floppy
» and start to make contacts withitself,
andeventually
close up in avesicle,
at effectiverigidities
neark~
T, but stillpositive. By taking
Lequal
to530A,
the GM3 vesicle average diameter, anda = 35
A
itsbilayer
thickness, the effectiverigidity
K' becomes zero forK
=0.22k~T,
with a= I, or
K =
0.65 kB T when a =
3. These values of
K are
small,
but not unreasonable.The average vesicle radius has been
predicted
to be a weak function of the surfactant volumefraction,
for spontaneous vesicle formation[7], although
thisdependence
has been measured to be even weaker. The same behaviour has been observed also for GM3 vesicles, for which noappreciable
radius variation has been detected over two order ofmagnitudes
of concentration values, that is from 10-~ to 10-3 in volume fraction of surfactant. The GM3 situation could beindeed similar to the
multicomponent
system described in reference[7].
If the formalismdeveloped
in reference[7],
which calculates the average vesicle radiusby taking
care ofrenormalization effects both in K and in k, is used, a value of
(2
K + k=
2.7 kB T is found for GM3 vesicles,
which, again,
is a rather reasonable value.GM3 vesicles form
spontaneously
in a situation of zero spontaneous curvature of thebilayer.
In fact the two
monolayers
haveexactly
the samecomposition,
since the solution contains asingle amphiphile.
The small amount of Nacl and of free monomers of GM3(of
the order of 10-SM)
present in solution cannot alter thispicture. Furthermore,
it is found that not allamphiphilic
molecules go into vesicles. A few percent of them formlarger
aggregates(Tab. I)
which are inequilibrium
with vesicles. This should somehow be connected to the frustration of thebilayer
in the vesicles. Thethermodynamic equilibrium
is out ofquestion,
sincelarge
aggregates reform very
rapidly,
after removalby microporous
filtration.It was not
possible
toquantify
the lifetime of this kinetic effect with thelight scattering
apparatus used in theexperiments,
but it issurely
shorter than 20-30 s, the timerequired by
the scatteredintensity
to reach itsasymptotic
value after filtration. It is also out ofquestion
that themajority
of thelarge
aggregates are flat with someaxisymmetric shape.
This isclearly
demonstrated
by
thedepolarized
static anddynamic light scattering experiments.
Forsimplicity,
data are fitted with oblateellipsoids
with themajor
semiaxis of 5 000h
andan
axial ratio p of the order of 100. In order to have a
general good
fit of all dataincluding
thescattering intensity
at very forwardangles,
also aquite
small number ofbulky
aggregates hasto be included.
They
representonly
about 5 x10~~
of the total GM3 concentration(Tab.1),
which in terms of volume fraction of GM3 in solution is an
extremely
smallquantity,
of the order of 10~ ~.Aggregates
should bebulky,
otherwise absolute intensities anddynamic
data donot match
together.
Aspherical shape
with a radius of 5 000A
has been considered. Thedynamic depolarized light scattering
data(Fig. 8),
indeed indicate that the oblateellipsoids
arepolydisperse
and notmonodisperse
as it has been assumed for the fit. Of course thelight scattering picture
for thelarge
aggregates isnecessarily oversimplified,
but it is certain that a smallpopulation
of lamellar aggregates(oblate ellipsoids),
with somemultilayer
stacks(bulky spheres),
are present in solutiontogether
with themajor
vesiclepopulation.
The ther-modynamic understanding
of theseexperimental
observations is not at all clear at this moment.Anyway,
someenergetic
considerations can be done. The existence of membrane bunches in solution seems not to be a theoreticalproblem [41, 42].
Thebulky spheres
could therefore represents onions of GM3bilayers
which are shown to exist if a fourth order term in the mean curvature is added to the usualbending
energy of a closedbilayer
ofspherical shape [42].
The number n of skins in the onion is thenpredicted
to beinversely proportional
to the usual term(2
K + k), containing
the elastic moduli of the GM3layers.
Since it has been shown abovethat this term has to be small in GM3 solutions, onion like
stacking
could be feasible.It is
by
far more difficult to accept the idea that lamellaraggregates
arethermodynamically
stable in solution. The
problem
comes from the unfavourableedge
energy due to Waterexposure of the
hydrophobic
chains. Inprinciple,
if the elastic energyrequired
to close a vesicleequals
theedge
energy of thebilayer
disc ofequivalent
surface, thethermodynamic equilibrium
between discs and vesicles could bepossible [43].
For instance, theedge
energy of the GM3vesicle-equivalent
disc of5001radius
and191
ofhydrophobic layer
thicknessbecomes 10 kB T for an
edge
tension of 2.5 x10~~ erg/cm
which is about a factor of 100smaller than what it is
reported
for lecitine vesicles[43].
Such a low value makes thesingle vesicle-single
disc coexistencequite
unfeasible. If on the other hand one considers the ensemble of discs andvesicles,
the overalledge
energyrequired by
discs for a fixed amount ofamphiphile going
into discs(say,
the 2-4fb of GM3 of the presentcase),
isinversely proportional
to the disc radius. Therefore « infinite » lamellae could be inequilibrium
withvesicles,
at the limit. In otherwords,
the totalpositive
elastic energyrequired by
the GM3 vesicles due to the lack of spontaneous curvature of thebilayer,
may beequal
to the totaledge
energy)hat
is neededby
the lamellaraggregates
in the water solution. Asalready
mentioned before, this unfavourable energy condition could be balancedby
translational entropy.It remains to be discussed however
why
discs of finite dimensions, average radius of5 000
A,
are stable in solution.Again entropic
effects couldplay
a role : of course, there is anentropic gain
if an « infinite » lamella breaks up into smaller discs. It isinteresting
however to consider thepossibility
ofhaving
areally
smalledge
energy due to thepeculiar
features of the GM3bilayer.
Theganglioside
molecule itself has alarge
saccharidicheadgroup
which isnormally
believed to bequite hydrated
and mobile. It is thereforeplausible
that the sugargroups could rearrange themselves in order to better shield the exposure to water of the
hydrophobic
tails at the discedges.
This is even moreplausible considering
the fact that thehydrophobic layer
of GM3bilayers
isalready
found to beliquid
like and thinner than for normalphospholipids
due to chaininterdigitation.
Another
possible
way toimagine
a reduction of theedge
energy is to consider thethermally
excited oscillations of a
piece
of membrane in presence of anharmonic contributions to the energy of thebilayer.
It is shown inAppendix
that in this case theaveraging
over all thethermally
excited oscillationsgives
the real structure of theamphiphilic assembly
somewhat different from the oneexpected
in absence of oscillations. This effect is not found with the usual harmonicapproximation.
The calculation shows that the anharrnonic term introduces acoupling
between the inphase
and out ofphase
thermal oscillations of the twomonolayers
inthe
bilayer
so to inducethinning
of thebilayer
on contour lines determinedby
somespatial
modes. Membranes would therefore break
preferentially
at these linesgiving
a finite average dimension to the discs. The anharmonic term takes into account the energy difference betweena concave and a convex deformation of the flat membrane surface. It may become
important
when
large amplitude
motions are present, like in the case of the looseganglioside
aggregates, and when theamphiphilic
molecule itself presents alarge
mismatch between thehydrophilic
and
hydrophobic
cross sectional areas, like for GM3. It could beinteresting
toperform
neutronscattering experiments
which can be sensitive to suchlarge-amplitude
fluctuations in thehigh-
k
region.
It has been shown in the
experimental
section thatlarge
aggregatesdisappear completely
from the GM3 solution when the second
ganglioside
GM I isadded, leaving
vesiclesonly.
Thiscan be
easily
understood in terms of a theoretical modeldeveloped
for mixedcopolymer bilayer