The basic characteristic of a resistor is the resistance R [Ω], which connects voltage and current in terms of the equation: dv=Rdi. It is related to the history of the device and all stresses in the material. When Α≠0, MR operates so that the state of the system is at some point on the graph.
To overcome boundary problems, a window function f(w) or f(x) is added to the derivative of the state variable. Transmission properties depend on the magnetization of the electron spins, which depends on external factors (for example, the applied voltage). First, anodic diffusion of the Cu electrode occurs (oxidation process with a half-reaction): Cu→Cu2++2e-.
They are created by the grain boundaries that exist in the metal oxide semiconductor electrolytic crystal. For the RESET process (switching from low to high resistance) a negative voltage is applied to AE, which leads to the breakdown of the existing nano-metal. There has been high tension and interest in the potential applications of the neurological application circuit of MR.
Investigating exponential synchronization (of MR-based coupled chaotic circuits), global exponential dispersion and stabilization, periodicity and global exponential stability of a class, global exponential synchronization, Mittag-Leffler global stability and synchronization (based on MR partial order) and global uniform asymptotic stability, all, of MR-based operation as recurrent neural networks with time-varying delays and general activation functions are respectively presented in [32–37].
Kinetics of electrochemistry concerning the experimental process [49,50]
At each of the electrodes, the charge separation represents a capacitance element and the difficulty of charge transfer represents a resistance. This leads to an excess of Μν+ ions at the interface, on the electrode side. As a result, the solution side of the interface gains an equal but opposite amount of charge (+Unit of charge QS/Unit of area).
Ions can collect in the solution part of the interface due to the electrostatic field of the electrode ('electrostatic adsorption'). In the electrode-solution interface, the adsorption of these substances is also affected by the influence of the electric field in the double layer on their dipoles. 29 freedom more than a non-polarized perfectly electrode whose potential is determined by the composition of the solution.
Charge σΕ is of course a function of the potential of the ideal polarized electrode and may even be equal to zero. If the equilibrium potential of the electrode (potential in the absence of current) is E and the potential of the same electrode due to current flowing is E(I), then the difference η between these two potentials is the overpotential. All (or maximum possible) of the current's amount must be used to oxidize or reduce the electrolyte.
The state between the WE and the surrounding electrolyte, which means the behavior of the region between the two phases, is the third phase. The amount of electric charge that can accumulate in the layers corresponds to the concentration of adsorbed ions and the surface area of the electrodes. Regarding the contribution of the inner layer to the capacitance, Stern's model is an extension of the previous model.
Applying AC potential and measuring the current through the cell in terms of the phase difference between the two values and the impedance magnitude. Fig.15: Typical examples of Nyquist (a) and Bode Plots with a time constant (b,c) for EIS data presentation (ω=2πf)[80]. So an electrical element, or a network of them connected correctly, is the essence of the equivalent circuit analysis of.
In such a case, the real and the imaginary components of the impedance vector are equal at all frequencies. The Randle's Cell (resistor in series with resistor and capacitor network in parallel) is one elementary equivalent circuit of the situated study.

Experimental
Additionally, the effect of spin speed on the metal deposition rate can also be used to determine whether the electrodeposition process is a diffusion controlled or chemically controlled process. However, if the deposition rate is independent of rotation, the reaction is likely to be chemically controlled. In [58], interest is focused on the influence of the presence of nickel or nickel and chloride on the electrodeposition of copper via copper sulfate in sulfuric acid or sodium or ammonium sulfate.
The Cu layer on the Au substrate inherits the <111> texture of the Au grains within the first few micrometers, depending on the current density it evolves to a <110> texture for thinner layers (probably as the authors mention by a multiple twinning mechanism). resulted from XRD analysis. Increasing the potential amplitude reaching a maximum at the deposition rate also resulted in rough, spongy, or porous electrodeposited Cu. The nature of the mechanisms at work in such systems needs further study for better insight.
There are two ion layers, one at the interface and the other at the boundaries of the active diffusion layer. This means that the system, as properly described, requires two capacitance factors (CPE), the interfacial Helmholtz double layer capacitance and the layer pseudocapacitance factor. Instant glue is applied to the top of the cap from the outside so the electrodes are robust.
Thus, the actual free surface for the Cu metal deposition and the Z-spectra measurements was the cylindrical base of the Carbon Rod. Electrolyte resistance: is the resistance of the electrolyte (of any physical form) between the counter and reference electrodes. It depends on the ion concentration, the type of ions, the temperature and the geometry of the region in which current is passed.
In Equivalent Circuit Analysis, it is most commonly concerned as a resistance R, and its value is a result of appropriate data analysis. Most times, a CPE is intended to describe the double-layer Helmholtz capacitance CH at the interface region connected in parallel with an "interface resistance" - double-layer interface resistance. Its value depends on the dehydrated charge transfer rate and storage of the element (can be an electrodeposited Cu layer) and corresponds to the variation between the differential charge and voltage.
Results
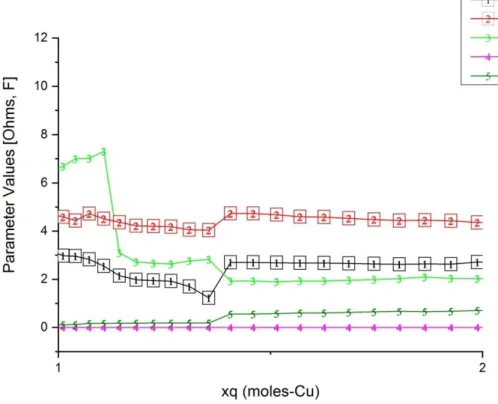
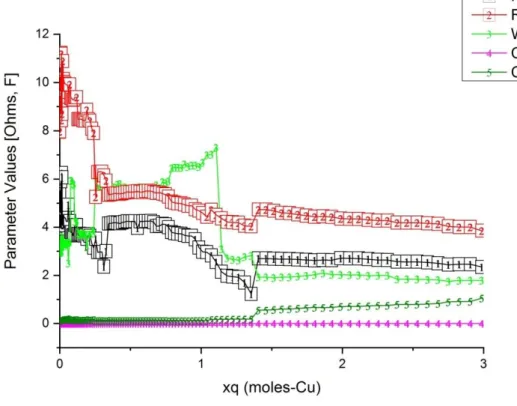
65 Fig.30: EIS values versus the normalized xq deposited DC component charge of the EIS setup (Cu mass transfer q=q(dm)=int[idc x t] ) on the carbon surface in 3D plotted projections. In the background, log-normal x-axis, up to xq=1 with the colors to give a better view of the evolution of the Z spectra and. 66 Fig.31: The parameter values of the equivalent circuit in Fig.27 were the result of the EIS data fitting process versus the normalized xq deposited DC component charge of the EIS setup (Cu mass transfer q=q(dm)=int [idc x t]) on the carbon surface.
Conclusions
Equivalent circuit analysis is one factor that can provide better insight into such a strongly nonlinear system. Electrodeposition kinetics, nucleation process dynamics, and deposition product morphology analysis could really enhance the studies of such systems. The factors that influence the morphology and quality of the deposited product are of crucial importance for such systems to withstand other technologies.
Too many factors influence such system behavior, increasing the knowledge required for the relevance of such studies.
26] Raja T, Mourad S, Digital logic implementation in Memristor-based crossbars, International Conference on Communications, Circuits and Systems, pp 939–943. 32] Guodong Zhang, Yi Shen, Exponential synchronization of delayed Memristor-based chaotic neural networks via periodic intermittent control, Neural Networks. 33] Zhenyuan Guo, Jun Wang, Zheng Yan, Global exponential dissipativeness and stabilization of Memristor-based recurrent neural networks with time-varying delays, Neural Networks.
34] Guodong Zhang, Yi Shen, Quan Yin, Junwei Sun, Mondiale exponential periodicity and stability of a class of multi-delay Memristor-based recurrent neural networks, Information Sciences Shiping Wen, Gang Bao, Zhigang Zeng, Yiran Chen, Tingwen Huang, Mondiale exponential synchronization of Memristor-based recurrent neural networks with time-varying delays, Neural Networks Jiejie Chen, Zhigang Zeng, Ping Jiang, Global Mittag-Leffler stability and synchronization of Memristor-based neural networks of fractional order, Neural Networks. 37] Jin Hu and Jun Wang, Global Uniform Asymptotic Stability of Memristor-based Recurrent Neural Networks with Time Delays, WCCI 2010 IEEE World Congress on Computational Intelligence, July CCIB, Barcelona, Spain. 48] Ádám Rák, György Cserey, Macromodeling of the Memristor in SPICE, IEEE TRANSACTIONS ON COMPUTER-AIDED DESIGN OF INTEGRATED CIRCUITS AND SYSTEMS, VOL.
Wang, Mechanism of copper electrodeposition by pulse current and its relation to current Efficiency, Journal of Applied Electrochemistry. El Kaliea, Effect of organic solvents on the electrodeposition of copper from acidified CuSO4, Abdel Journal of Dispersion Science and Technology. 57] Noura Touabi, Sanja Martinez, Moussa Bounoughaz, Optimization of Electrochemical Copper Recovery Process: Effect of Rotation Speed in Chloride Medium of pH=3 ,Int.
Zangari, Electrochemical nucleation and growth of copper from acid sulfate electrolytes on n-Si(001), Journal of The Electrochemical Society, 154(7) D339-D345 (2007). Durand, In situ X-ray absorption spectroscopy study of copper under potential deposition on Pt(111): role of the anions on the Cu structural arrangement, Electrochimica Acta. Vorontsov, Structure and properties of copper coatings electrolytically deposited under X-ray radiation, Journal of Surface Investigation.
75] Jaishree Mathur, and Manish Gupta, Effect of Electrodeposition Parameters on the Morphology of Copper Thin Films, Vol. Gewirth, Atomic Force Microscopy Examination of Cu Trench Electrodeposition, Journal of The Electrochemical Society, 150(5) C292-C301. Zarrouk, Effect of Environmental Cinnamon Extracts on Copper Electrodeposition in an Acidic Bath, Portugaliae Electrochimica Acta.