This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except briefly except in connection with reviews or scientific analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is prohibited.
P REFACE TO THE
S ECOND E DITION
Section III, Laboratory Procedures and Protocols, begins with an introduction to the use of the microscope (Chapter 12) and follows with methodologies used to enumerate and identify wine microorganisms (Chapters 13 to 16).
A CKNOWLEDGMENTS
The authors express their gratitude to the wine industry, commercial suppliers and university colleagues around the world. Finally, a special thank you goes to the undergraduate and graduate students, research staff, technicians and other employees with whom the authors have worked.
I NTRODUCTION
Although initially thought of as a major threat, some winemakers have begun to see Brettanomyces as a potential ally in the vintages of the new millennium. Hopefully, further research and experience will provide winemakers with better microbiological control during vinification, which in turn will lead to a continued increase in wine quality.
SECTION I
G RAPE AND W INE M ICROORGANISMS
CHAPTER 1
Y EASTS
INTRODUCTION
REPRODUCTION
- Sexual Reproduction
- Asexual Reproduction
Second, the isolate may be heterothallic and haploid and thus require a compatible mating type that may not be present in the culture. As shown in the electron micrograph of Saccharomyces (Fig. 1.1), the mother cell is left with bud scars after separation from the daughter cells.

TAXONOMY
The anamorphic genus Candida represents a very broad group with a number of species found in wines. Of the yeasts found in grape juice or wine, this genus reproduces uniquely by fission.

NUTRITIONAL REQUIREMENTS
- Nitrogen
- Growth and Survival Factors
Like nitrogen, strains of commercial wine yeast vary in their requirements for oxygen (Julien et al., 2000). Sterols also affect the synthesis of volatile odor and flavor compounds depending on the presence of oxygen (Mauricio et al., 1997).
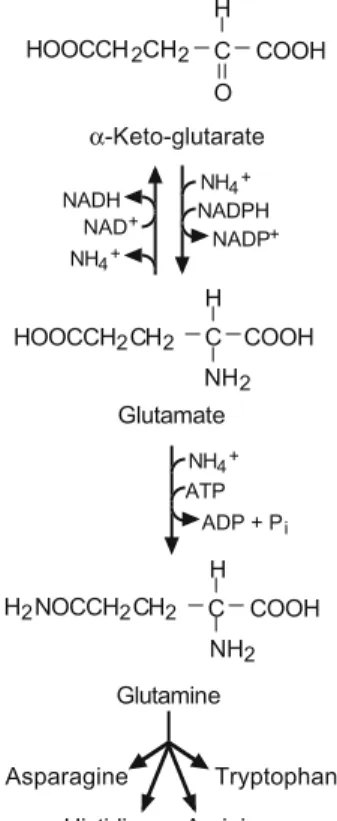
METABOLISM .1 Glucose
- Sulfur
- Odor/Flavor Compounds
- Glycosidases
- Mannoproteins
Adapted from Boulton et al. 1999) with the kind permission of Springer Science and Business Media. These compounds are distributed in various locations in the grape berry (Wilson et al., 1986).
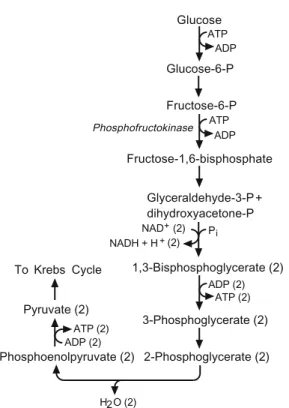
CHAPTER 2
L ACTIC A CID B ACTERIA
- INTRODUCTION
- TAXONOMY
- NUTRITIONAL REQUIREMENTS
- METABOLISM .1 Glucose
- Arginine
- Malate
- Mannitol and Erythritol
- Diacetyl and Other Odor/Flavor Compounds
- CHAPTER 3
Xiyyeeffannoo l-arginine dabaluu fayyadamuun Pilone et al. oeni dhuguma ammooniyaa oomishaa ture. Poolman fi kkf irraa kan fooyya’e. (1991) hayyama gaarummaa Joornaalii Baakteeriyooloogii irraa argateen.

A CETIC A CID B ACTERIA
- INTRODUCTION
- TAXONOMY
- NUTRITIONAL REQUIREMENTS
- METABOLISM .1 Carbohydrates
- Ethanol
- CHAPTER 4
This differs from the last edition of Bergey's Manual where Acetobacter and Gluconobacter were the only genera recognized (De Ley and Swings, 1984; De Ley et al., 1984). However, these bacteria can directly oxidize glucose to form gluconates and, to a lesser extent, ketogluconates (Weenk et al., 1984;. While glycolysis is either absent or very weak (De Ley et al., 1984) , some species are capable of oxidizing polols to the corresponding ketoses.
Acetic acid bacteria can produce large amounts of acetaldehyde, approaching 250 mg/L (Du Toit and Pretorius, 2002).

M OLDS AND O THER M ICROORGANISMS
- INTRODUCTION
- ECOLOGY HABITATS
- TAXONOMY
- NUTRITIONAL REQUIREMENTS
- METABOLISM .1 Glucose
- Mycotoxins
- Odor/Flavor Compounds
- OTHER MICROORGANISMS .1 Bacillus
Streptomyces has been implicated in the formation of "moldy" compounds in corks as well as in wineries (Silva Pereira et al., 2000). Moreover, Silva Pereira et al. 2000) noted that cork stains may originate from other microorganisms. Darriet et al. 2001) detected geosmin present in a "mossy" Cabernet Sauvignon wine, specifically the - isomer (−), which has a much lower sensory threshold than geosmin (+).
Since geosmin has been found in freshly crushed grapes (Darriet et al., 2000), microorganisms that develop on grapes can therefore potentially produce "musty" wines.

SECTION II
V INIFICATION AND
W INERY P ROCESSING
CHAPTER 5
M ANAGING M ICROBIAL G ROWTH
- INTRODUCTION
- PRESERVATIVES AND STERILANTS
- Sulfur Dioxide
- Dimethyl Dicarbonate
- Lysozyme
- Sorbic Acid
- Other Preservatives and Sterilants
- FILTRATION
- Perpendicular-Flow Filtration (“Nominal” or “Depth”) Depth fi ltration systems use either paper pads, diatomaceous earth (“DE”
- Perpendicular-Flow Filtration (“Absolute”
- Cross-Flow/Tangential Filtration
- CHAPTER 6
Plant phenolics are known to react with enzymes and thereby reduce activity (Rohn et al., 2002). Based on this observation, De Rosa et al. 1983) recommended that sorbates should not be used in the production of sparkling wines. The most important function of fumaric acid is its ability to inhibit malolactic fermentation (Ough and Kunkee, 1974; Pilone et al., 1974).
However, fumaric acid is broken down during alcoholic fermentation by Saccharomyces forming l-malic acid (Pilone et al., 1973).

M ICROBIAL E COLOGY D URING V INIFICATION
- INTRODUCTION
- NON-SACCHAROMYCES AND SACCHAROMYCES YEASTS .1 Grapes and Musts
- Alcoholic Fermentation
- Post-fermentation
- DEKKERA/BRETTANOMYCES
- Grapes and Musts
- Alcoholic and Post-fermentation
- LACTIC ACID BACTERIA .1 Grapes and Musts
- Alcoholic and Malolactic Fermentations
- Post-fermentation
- ACETIC ACID BACTERIA .1 Grapes and Musts
- Alcoholic and Post-fermentation
- MICROBIAL INTERACTIONS
- Other Interactions
- CHAPTER 7
Adapted from Huang et al. 1996) and with kind permission from the American Journal of Enology and Viticulture. Adapted from Huang et al. 1996) with kind permission from the American Journal of Enology and Viticulture. Due to the increase in pH, MLF may be favorable for the growth of other lactic acid bacteria such as Pediococcus (Davis et al., 1986a).
Adapted from Edwards et al. 1994) with kind permission of the American Journal of Enology and Viticulture.

H ARVEST AND
P RE - FERMENTATION P ROCESSING
- INTRODUCTION
- HARVEST AND TRANSPORT
- FRUIT QUALITY ASSESSMENT
- Soluble Solids
- pH and Titratable Acidity
- Microbial Spoilage
- MUST PROCESSING
- Enzymes
- Suspended Solids
- Pre-fermentation Maceration (Cold-Soak)
- Thermovinifi cation
- Inert Gassing
- PROCESSING MICROBIALLY DETERIORATED FRUIT
- Enzymatic Browning
- Fermentation Diffi culties
- Clarifi cation Concerns
- Management Strategies
- JUICE STORAGE (MUTÉ)
- CHAPTER 8
Open canopies favor not only air circulation but also spray penetration (English et al., 1990). Changes in the fatty acid composition of musts have also been reported (Varela et al., 1999). At least one report noted differences in volatile acidity depending on the clarification procedure (Aragon et al., 1998).
Addition of glucanases either to the juice during clarification or after fermentation results in hydrolysis of polymers (Dubourdieu et al., 1981).

F ERMENTATION AND
P OST - FERMENTATION P ROCESSING
INTRODUCTION
MUST SUPPLEMENTATION
The nitrogenous components of grapes and must that are metabolically available to yeast are present as ammonium salts (NH4+) and primary amino acids, known collectively as “yeast assimilable nitrogen” (YAN). The concentration of assimilable yeast nitrogen present in must is vineyard specific, varying with climate and soil type, grape variety, roots, fertilization and irrigation practices, harvest maturity (degree of ripeness), as well as the degree of microbiological deterioration. which may have occurred before harvest (Ough and Bell, 1980; Huang and Ough Spayd et al., 1994). In the United States, the maximum level of (NH4)2HPO4 legally allowed to correct nutrient deficiencies is 960 mg/L (8 lb/1000 gal), a concentration that provides 203 mg N/L of assimilable nitrogen.
Because some ingredients in these formulations may not currently be approved for use in the United States, winemakers must petition the US.

ALCOHOLIC FERMENTATION
- Historical Perspective of Starter Cultures
- Preparation of Starter Cultures
- Strain Selection
- Temperature
- Immobilized Yeast
Each strain has different characteristics, including different fermentation rates and H2S production (Ough and Groat, 1978; Jiranek et al., 1995c). Relatively few yeasts (<103/mL) escape the encapsulation matrix (Yokotsuka et al., 1993), but they nevertheless carry out active alcoholic fermentation. Non-Saccharomyces yeasts are known to produce a variety of odor and aroma molecules that alter wine quality (Holloway and Subden, 1991; Gil et al., 1996; Lema et al., 1996).
In one study, Jolly et al. pulcherrima with Saccharomyces cerevisiae in Chenin blanc must.
FERMENTATION PROBLEMS .1 Sluggish/Stuck Fermentations
- Hydrogen Sulfi de
For example, Wang et al. 2003) reported that fermentations of a synthetic grape juice medium containing only 60 mg N/. As noted by Sea et al. 1998), yeast strains that consistently produce low sulfur content are not commercially available, even with hundreds of strains screened. This observation has not been previously reported and casts doubt on the belief that adding nitrogen to grape must will always reduce H2S problems (Tamayo et al., 1999).
Although elemental sulfur used in the vineyard can also be a source of H2S (Acree et al., 1972; Eschenbruch, 1974), very high concentrations may be required on treated grapes (Thomas et al., 1993).

MALOLACTIC FERMENTATION
- Preparation of Starter Cultures
- Strain Selection
- Timing of Inoculation
These cultures sometimes require rehydration and adaptation in diluted grape juice or wine before inoculation (Lafon-Lafourcade et al., 1983a; Krieger et al., 1990; Pompilio, 1993; Henick-Kling, 1995). Adapted from Semon et al. 2001) with the kind permission of the Australian Journal of Grape and Wine Research. To avoid the potential problems associated with early inoculation, some advocate inoculation of bacterial starter cultures after completion of the alcoholic fermentation (Ribéreau-Gayon, 1985; Krieger et al. Henick-Kling, 1993; Nygaard et al., 1998).
Malolactic bacteria can also benefit from nutrients released by yeast autolysis after alcoholic fermentation (Fornachon, 1968; Beelman et al., 1982; Henick-Kling, 1993).

POST-FERMENTATION PROCESSING
- Aging and Storage
- Adjustment of Volatile Acidity
However, because the atomic weight of N2 (28 g/mol) is close to O2 (32 g/mol), efficient displacement of the oxygen is questionable. Volatile acidity adjustment in salvageable wines has historically been achieved through blending with other wines containing a much lower level of acetic acid. Direct attempts to chemically neutralize the acetic acid are not possible due to preferential reduction of the more strongly fixed ones (tartaric and malic acids).
In this case, only the permeate containing acetic acid, ethanol and water passes through the anion exchange column, which removes the acetic acid (Figure 8.4).
BOTTLING
Regardless of whether the wine must be sterile filtered or stabilized with chemical preservatives, microbiological examination must be performed after bottling to ensure the effectiveness of the treatment and operation. Bottles must be collected off-line at (minimum) 1 hour intervals during the bottling process. Since populations of microorganisms in the packaged product should be low or undetectable, wines should be screened using membrane filtration (Section 14.5.3).
If viable microorganisms that cause wine spoilage are found, the integrity of the filter membranes should be checked.
CHAPTER 9
W INERY C LEANING
AND S ANITIZING
- INTRODUCTION
- SAFETY ISSUES
- WATER QUALITY
- PRELIMINARY CLEANING
- DETERGENTS, CLEANERS, AND SURFACTANTS
- Alkali
- Acids
- Surfactants
- RINSES
- SANITIZERS
- Iodine
- Quaternary Ammonium Compounds
- Acidulated Sulfur Dioxide
- Peroxides
- Chlorine and Chlorinated Formulations
- Hot Water and Steam
- Ozone
- SANITATION MONITORING
- SCHEDULES AND DOCUMENTATION
- CHAPTER 10
In addition, water should be analyzed two to four times a year, depending on the source (well or city). Once most of the dirt and film has been removed, the surface should be cleaned. However, gels provide additional contact time with the surface and can be used in low-pressure systems (Wirtanen and Salo, 2003).
In both cases, the temperature should be monitored at the point farthest from the steam source (eg, end of line, fill nozzles, etc.).

Q UALITY P OINTS P ROGRAM
- INTRODUCTION
- DEVELOPING QP PROGRAMS
- PROCESSING AND FLOWCHART
- QUALITY FACTOR ANALYSIS
- Examples of Quality Factors
- Preventive Measures
- Critical Quality Points
- CRITICAL LIMITS
- MONITORING PROCEDURES
- CORRECTIVE ACTIONS
- RECORD-KEEPING AND DOCUMENTATION
- VERIFICATION
- CHAPTER 11
In this case, it is important that the winemaking staff agree, before crushing, on the preventive measures to be taken if the amount of nitrogen in the grape must is below a specified concentration. Enumeration of microorganisms (Chapter 14) should be incorporated into sanitation programs, GMPs or as part of verification. When a critical limit has been violated, it is important to determine what corrective actions should be taken (Table 10.1).
For example, this step indicates that a program has been implemented and is effective for a given operation.

W INE S POILAGE
INTRODUCTION
SPOILAGE MICROORGANISMS .1 Acetobacter
- Film Yeasts
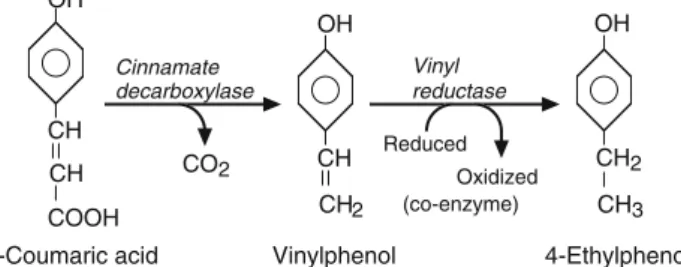
These odors are due to the synthesis of a variety of volatile compounds including 4-ethyl guaiacol and 4-ethyl phenol (Fugelsang et al., 1993; . Licker et al., 1999). Perhaps a simpler method was that described by Chassagne et al. 2005) where yeast sludge was used to remove volatile phenolics. Adapted from Chatonnet et al. 1992) and reprinted with the express permission of John Wiley & Sons Ltd.
For example, Brettanomyces is known to produce isovaleric acid, an odorous compound described as “rancid” (Wang, 1985; Licker et al., 1999).
WINE FAULTS .1 Volatile Acidity
- Ethyl Carbamate
- Mousiness
- Post-MLF Bacterial Growth
- Geranium Odor/Tone
- Biogenic Amines
- Acrolein
- Mannitol
- Ropiness
- Tartaric Acid Utilization
However, more recent information (Uthurry et al., 2006) suggests that some lactic acid bacteria, particularly O. Adapted from Ough et al. 1988b) with kind permission of the American Journal of Enology and Viticulture. Evidence for this alternative pathway in lactic acid bacteria is lacking (Moreno-Arribas et al., 2003).
Adu ti agparang a β-d-glucans (Llaubéres et al., 1990) ngem dagiti dadduma pay a monosaccharides ket mabalin nga adda (Mungesa e Nadra ken Strasser de Saad, 1995).
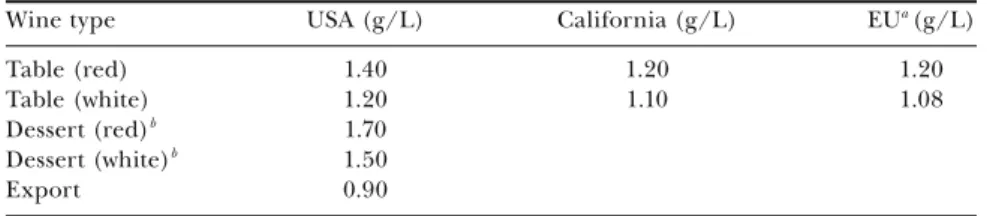
SECTION III
L ABORATORY P ROCEDURES
AND P ROTOCOLS
CHAPTER 12
B ASIC M ICROSCOPY
INTRODUCTION
MICROSCOPES
- Magnifi cation
- Resolution
- Contrast
- Fluorescence Microscopy
The first and most obvious purpose of microscopy is to magnify the image of the microorganism so that details can be easily seen by the human eye. The total magnification is the product of the contributions of the objective lens and the eyepiece (ocular). One factor that affects resolution is the refractive index of the medium between the objective lens and the coverslip on the microscope slide.
Since increasing the refractive index of the medium will result in better resolution, immersion oil is placed between the objective and the coverslip.