Abbreviations
Introduction
Heart as an endocrine organ
Cardiac natriuretic peptides
Synthesis and Structure
Corin, the pro-natriuretic peptide convertase
Regulation of natriuretic peptide level
This is supported by the observation that while 60% of circulating BNP is of ventricular origin, mature BNP tissue concentration in the ventricles is only 1% of that in the atria [20, 21]. a, Regulators of natriuretic peptide synthesis and secretion. Shortly after the discovery of ANP, mechanical stretching of the atria emerged as a physiological regulator of ANP release [42].
Natriuretic peptide receptors and effects of natriuretic peptides
Pathophysiology
As previously mentioned, there is no known disease caused by overexpression or undersecretion of natriuretic peptides. However, natriuretic peptides have an important and extensively studied role in the maintenance of cardiovascular homeostasis (for a recent review see [106].
Pathophysiology of natriuretic peptides in cardiovascular diseases
As a result, patients with overt heart failure suffer from natriuretic peptide deficiency, despite the measured high plasma natriuretic peptide concentration [113]. In addition, recent animal studies have shown that upregulation of phosphodiesterase in heart failure impairs cGMP signaling of natriuretic peptide receptors [115], resulting in decreased effects of natriuretic peptides even with normal/high hormone concentrations.
Pathophysiology of natriuretic peptides beyond cardiovascular diseases
Clinical use
Free from the clinically insignificant fluctuations, BNP has been proven to be a more reliable and sensitive marker for various cardiovascular diseases [134]. They found that elevated BNP levels were independently predictive of risk of death, heart failure, atrial fibrillation, stroke and transient ischemic attack.
Screening
Diagnostic use
In the differential diagnosis of acute dyspnea, an early study found that BNP more accurately predicted the final diagnosis of heart failure than left ventricular ejection fraction [139]. Furthermore, a recent study [140] found that NT-proBNP was even 17% more specific for the diagnosis of heart failure in dyspneic patients than BNP.
Prognostic use
Measurement of BNP levels may be particularly useful in the diagnosis of acute coronary syndromes, particularly in unstable angina and non-ST elevation myocardial infarction (NSTEMI). In mitral regurgitation, aortic regurgitation, and stenosis, BNP levels correlate with disease severity [141-143].
Monitoring therapy
In atrial fibrillation, both pre- and post-cardioversion natriuretic peptide levels predict recurrent atrial fibrillation after cardioversion [106]. Knowledge of the likelihood of postcard ioversion-reversion to atrial fibrillation may help select patients who would benefit more from rate control and anticoagulant therapy.
Therapeutic use
Aims
- In vitro studies: physiologic mechanism of ANP secretion from cardiomyocytes
Regulation of ANP secretion from cardiomyocytes
Characterization of corin, the pro-ANP convertase
In vivo studies: natriuretic peptide secretion in animal model of diabetes combined with
Natriuretic peptides and endothelin are counterregulatory hormones involved in cardiovascular complications of diabetes mellitus. The aim of our experiments was to characterize the changes of ANP and endothelin secretion in response to acute hemodynamic load in experimental diabetes.
Clinical practice: natriuretic peptide secretion in bradycardia
Although there are numerous reports of these changes in the basal state, little is known about the responses to increased acute hemodynamic stress in diabetes.
Material and methods
- Cloning, bacterial expression, and purification of bacterial proteins
- Antibodies
Proteins were analyzed by SDS-PAGE and Western blotting using mouse anti-GST tag (Santa Cruz Biotech., Inc., Santa Cruz, CA) or anti-His-tag antibodies (QIAGEN Inc., Valencia, CA).
Polyclonal antibody generation
The specificity of the polyclonal sera was tested against the original immunizing antigens and tissue extracts of mouse heart and other tissues by Western blotting.
Monoclonal antibody generation
Protein extraction
Protein extraction from tissue
Protein extraction from cultured cells
- Plasma peptide extraction and radioimmunoassay
- Concanavalin A-Sepharose binding assay and cell surface labeling
- Western-blotting
- Immunoprecipitation
- Cardiomyocytes and HL-1 cell culture
- Immunofluorescence staining
- Cell permeabilization, secretion assay and ANP measurement
- Flow cytometry analysis
- Diabetes induction and arterio-venous shunt preparation in dogs
- Statistics
- Animals
- Results
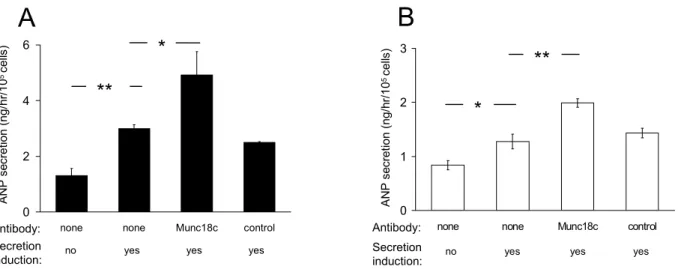
- Natriuretic peptide secretion from cardiomyocytes is regulated by Munc18c
Cells were plated at high density (10 5 /well) on fibronectin/gelatin-coated 24-well plates and incubated overnight in a 4:1 mixture of Dulbecco's Modified Eagle Medium (DMEM). The following day, non-adherent cells were removed and the medium was replaced with a modified complete serum-free medium (CSFM [64]) containing: DMEM/F12 (Invitrogen), 25 mM HEPES (Sigma-Aldrich, St. Louis, MO , USA), 1.7 μM insulin (Sigma-Aldrich), 1 μM transferrin (Sigma-Aldrich), 10 nM sodium selenite (Sigma-Aldrich), 1 nM tri-iodo-thyronine (Sigma-Aldrich), 0.2% BSA (Sigma-Aldrich), 190 nM dexamethasone (Sigma-Aldrich), 1 mg/l arachidonic acid (Sigma-Aldrich), 0.5 mg/ml docosahexaenoic acid (Sigma-Aldrich), and 100 units/ml penicillin/streptomycin (invitrogenycin) )). HL-1 cells were cultured according to the published protocol [169] in Claycomb medium (JRH Biosciences, Lenexa, KA) supplemented with 10% fetal bovine serum (JRH Bioscience), 100 μg/ml penicillin/streptomycin, 0, 1 mM norepinephrine (Sigmphrin) ) and 2 mM L-glutamine (Life Technologies).
Non-permeabilized cells were washed with ice-cold PBS containing 3% fetal bovine serum, collected, centrifuged at 2000 g for 2 minutes, resuspended at a density of 4x106 cells/ml and stained with pooled anticorin (12G8/16E6/14H1, 10 μg/ml ) or nonspecific control pooled (IgM/IgG/IgM, 10 μg/ml) MAbs in culture medium for 30 min. After several washings and incubation for 15 minutes with PBS containing 1% fetal goat serum, the cells were stained for 35 minutes with Alexa Fluor 488-labeled goat anti-mouse IgG/IgM (0.5 μg/ml).
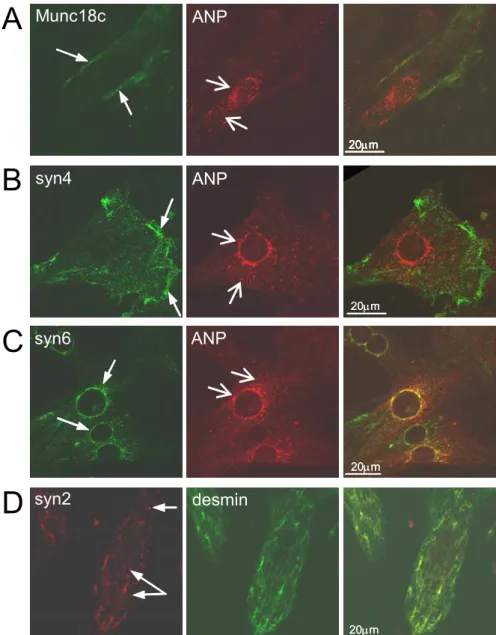
Syntaxin 4 and Munc18c colocalize and interact at the plasma membrane of
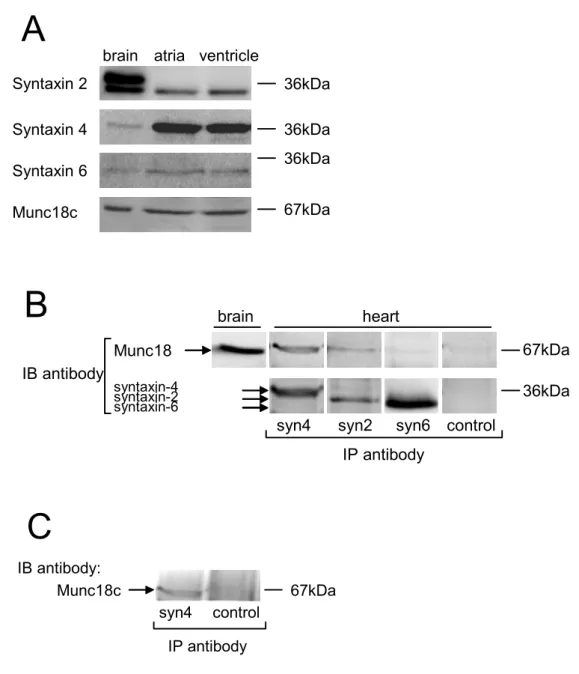
Left panel: subcellular localization of Munc18c, syntaxin 4, syntaxin 6 and syntaxin 2 proteins in cardiomyocyte culture cells. Right panel: merged images of left and center panels show Munc18c, syntaxin 4 and syntaxin 6, and syntaxin 2 localization in cardiomyocytes identified by pro-ANP or desmin staining. Immunoblotting studies showed that syntaxin 6 was present in both atrial and ventricular tissues at an expected relative molecular mass of ∼34 kDa ( Fig. 2A ).
Co-immunoprecipitation studies revealed only a few Munc18–syntaxin 2 interactions when immunoblotted by a pan-anti-Munc18 antibody (Fig. 2B). Immunoprecipitation was performed with an anti-syntaxin-4 antibody or rabbit IgG (control) as described in panel B.
Ca 2+ –induced ANP secretion in permeabilized, primary cardiomyocytes
Pro-natriuretic peptide-convertase corin is localized on the cardiomyocyte surface
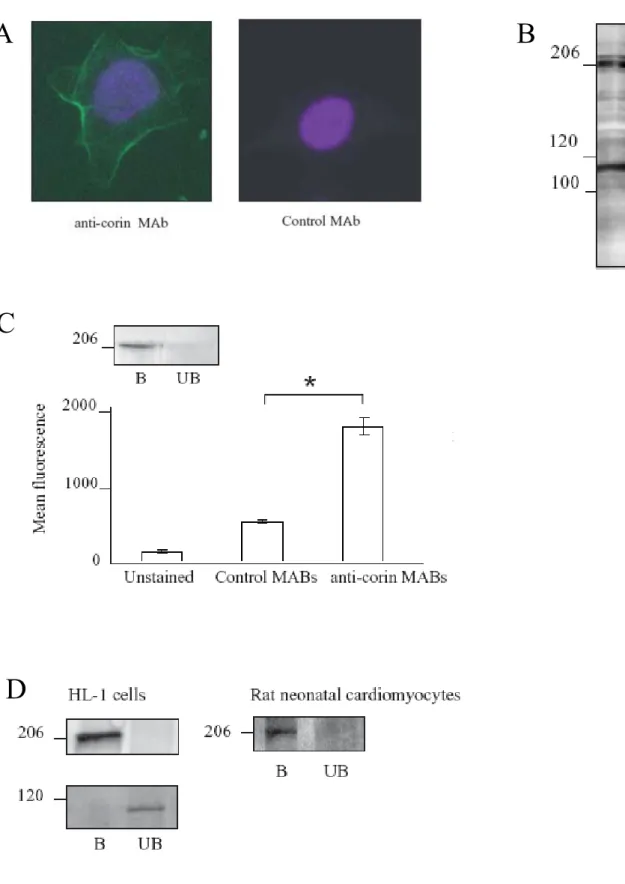
Anti–corin polyclonal and monoclonal antibody production and characterization
The disulfide bond (S-S) connecting the stem and protease domains after zymogen activation is shown. B) Specificity of polyclonal anti-corin antibodies for recombinant corin fragments. Western blots (reducing conditions) against recombinant corin protease domain or nonspecific recombinant protein (negative control) (2) were probed with anti-corin MAbs as indicated. Cultured non-permeabilized neonatal atrial cardiomyocytes were analyzed by immunofluorescence staining with anti-corin antibodies (Figure 6A).
To investigate whether native corin is expressed in cardiomyocytes that also express pro-ANP, permeabilized neonatal atrial cardiomyocytes were double stained with mouse anti-corin protease domain MAb (19C10) and rabbit polyclonal anti-pro-ANP antibodies and analyzed by confocal microscopy. Immunofluorescence staining of non-permeabilized (A) or permeabilized (B) neonatal rotatrial cardiomyocytes. A) Cells were stained with anti-corin antibody as indicated and detected with Alexa Fluor 594-labeled secondary antibody.
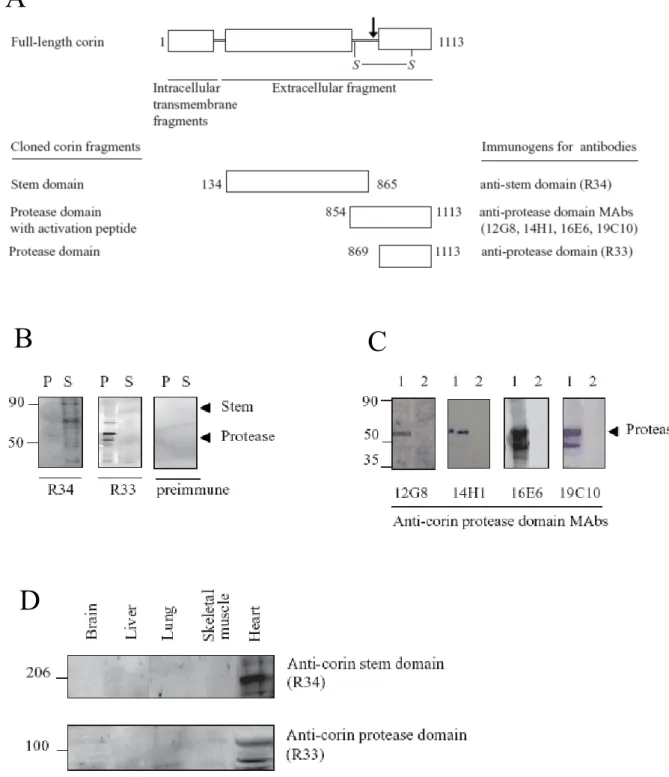
Molecular size and structure of native corin in mouse heart tissue
Antibody against the root stem domain (R34) detects corin fragments with a relative mass of 180 kDA and 110 kDa (Figure 7B). These fragments were not recognized by anti-corin protease domain antibodies and therefore may correspond to structures containing mainly the parent domain. Corin protease domain antibody (R33) detects corin fragments with a relative mass of 45-50 kDa and 90 kDa (Figure 7B).
These fragments were not recognized by the antibody against the stem domain (R34) and may correspond to structures containing mainly the protease domain. Western blot of membrane fractions of mouse heart extract under reducing conditions with anti-corin stem domain antibody (R34) and anti-corin protease domain.

Corin expression in cardiomyocyte-like HL-1 cells
Acute hemodynamic load increases natriuretic peptide level both in healthy and diabetic
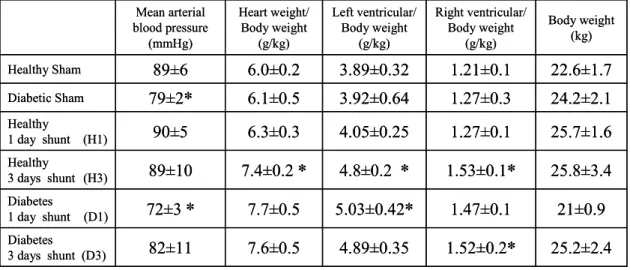
Changes in cardiovascular parameters due to acute hemodynamic load in diabetic and
Mean arterial blood pressure, heart weight/body weight, left and right ventricle weight/body weight ratios, and body weight data.
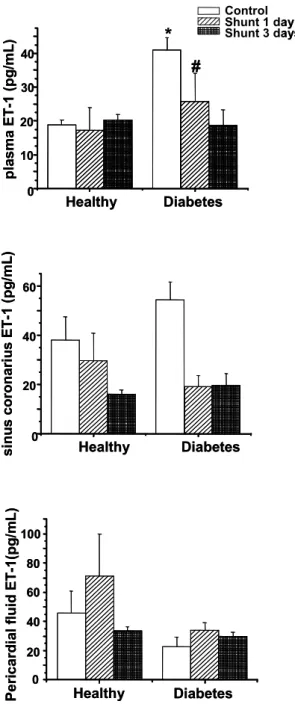
Endothelin levels in healthy and diabetic dogs with normal circulation and after acute
Endothelin-1 (ET-1) levels in healthy and diabetic dogs with normal circulation and 1 and 3 days after shunting. metabolically healthy control of 3. A) ET-1 The level in the peripheral plasma.
Acute hemodynamic load increases NT-proANP levels in different compartments of
Discussion
- Mechanism of ANP secretion
Munc18s and SNAREs
Lower affinity interactions between syntaxin 2 and Munc18c have been detected using in vitro systems, but few if any interactions between these molecules have been detected in cells [182]. The diffuse granular distribution of syntaxin 2 throughout the cells raises the possibility that it may colocalize with ANP-containing granules, but we could not confirm this due to limitations with the antibody reagents for these molecules. However, syntaxin 6 is known to be a ubiquitous member of the trans Golgi network and endosomes in most cells [ 194 – 196 ].
The ability of syntaxin 6 to form SNARE complexes is regulated by Vps45, another member of the Sec1/Munc18 gene family [195]. In addition, we found that priming the cells with ATP was necessary for Ca2+ -triggered secretion (data not shown).
Corin
ANP and endothelin in response to hemodynamic strain in diabetes mellitus
Acute hemodynamic stress further lowered the blood pressure in diabetic dogs, while there was no change in blood pressure in the control group. In the coronary sinus and in the pericardial fluid, endothelin levels were higher than in the systemic plasma, which is consistent with the previous reports [213], suggesting that the heart actively contributes to the maintenance of endothelin level. Left ventricular dP/dt in the healthy animals was not different from the diabetic shunted animals.
However, the increase in NT-proANP levels due to shunting was slightly higher in the diabetic group than in the metabolically healthy controls (Fig. 10). Taken together, the observed increased ANP level in the diabetic animals in our experiments could be explained by the increased need for natriuretic peptides due to acute cardiac stress, and the parallel decreased concentration of natriuretic peptide receptors due to the hypoinsulinemia .
Bradycardia and increased natriuretic peptide level
Furthermore, an earlier study reported an impaired vasodilator response to atrial natriuretic peptide in diabetic patients [ 219 ]. They reported that the loss of atrioventricular synchrony could cause increased plasma BNP level, similar to our observation, where the lack of adequate atrial contraction resulted in increased BNP level. The latter shows that the loss of atrioventricular synchrony – or the lack of sufficient atrial contraction, as in our case – can also cause increased plasma BNP level.
These observations are further supported by the finding that in hypertonic patients, heart rate lowering beta-blocker therapy increases plasma atrial natriuretic peptide (ANP) and BNP concentrations [223]. Increased stroke volume results in increased wall tension, which consequently triggers natriuretic peptide secretion.
Conclusion
- Munc18c regulates ANP secretion
- Corin is co-expressed with pro-ANP and localized on the cardiomyocyte surface in both
- Cardiac strain reduces ET-1 and increases ANP level in diabetes mellitus
- Bradycardia can induce increased plasma BNP level
Furthermore, we showed for the first time that native chorin is expressed in cardiomyocytes that also express its substrate, pro-ANP (Fig. 6B), providing further evidence that chorin may contribute to the regulation of the natriuretic peptide system in vivo by pro- ANP processing. Pericardial fluid contained higher ET-1 levels in both metabolically healthy and diabetic animals, and its level did not change as a result of shunting either in diabetes or in the healthy controls. metabolically healthy controls, although NT-proANP levels tend to be higher in the diabetic animals. In the case of an elderly woman, we observed extremely elevated natriuretic peptide level due to bradycardia caused by sinus arrest with a compound escape rhythm provoked by beta-blocker therapy.
Discontinuation of the medication resulted in sinus rhythm and a rapid decrease in the natriuretic peptide level. According to these results, we conclude that elevated natriuretic peptide levels may indicate subclinical cardiomyopathy induced by bradycardia.
Summary
Evaluation of atrial natriuretic peptide and brain natriuretic peptide in atrial granules of rats with experimental congestive heart failure. Cellular mechanisms for the synthesis and secretion of atrial natriuretic peptide and brain natriuretic peptide in cultured rat atrial cells. Atrial natriuretic peptide (ANP) inhibits its secretion via ANP(A) receptors: altered effect in experimental hypertension.
Similar bradycardic baroreflex actions of atrial natriuretic peptide and natriuretic peptide types B and C in conscious rats. Amino-terminal pro-brain natriuretic peptide to diagnose congestive heart failure in patients with impaired renal function. Use of brain natriuretic peptide concentration in plasma to aid in the diagnosis of heart failure.
List of publications
- Related
- Unrelated
Acknowledgement