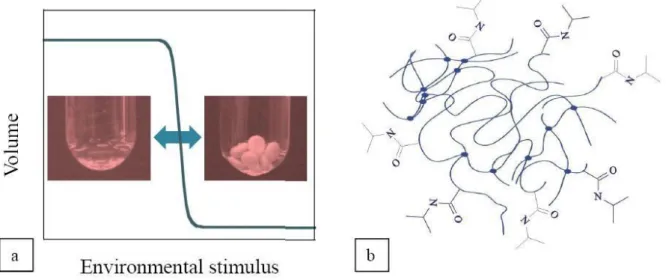
Introduction
Polymer hydrogels
- The poly(N-isopropylacrylamide) hydrogel
- Intrinsic ionic behaviour in cross-linked PNIPA hydrogel
- Interactions of PNIPA with dissolved molecules
- Drug uptake and release in hydrogels
- PNIPA – carbon nanoparticle hybrid gels
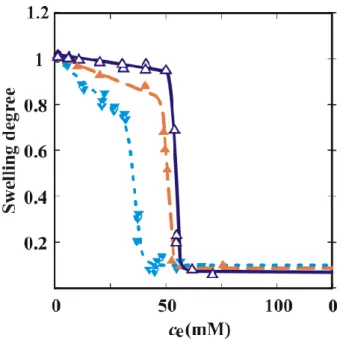
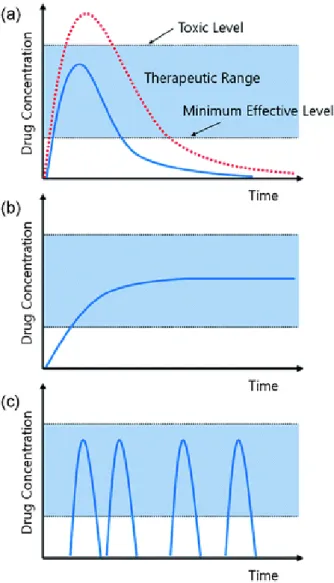
The volume phase transition properties of the PNIPA hydrogel can be significantly influenced by the dissolved species of the aqueous medium. Dependence of the drug concentration profile in body fluids on the release mechanism: (a) immediate release from conventional pharmaceutical forms (injection or tablet) or
Objectives of the PhD thesis
Materials and methods
Materials
- Aromatic guest molecules
- Carbon nanoparticles
- Synthesis of PNIPA gel
- Synthesis of PNIPA – carbon nanoparticle hybrid gels
To obtain composite gel, aqueous suspensions of carbon nanoparticles of the required concentration were mixed with the precursor solution of NIPA, BA and TEMED. In the case of CNT containing compounds ultrasound (ultrasonic bath Elmasonic X-tra 150H, 4 h C, cleaning mode, 45 kHz) was used to ensure the homogeneity of the distribution.
Methods
- Equilibrium swelling degree
- Mechanical properties
- Kinetics of temperature induced phase transition
- Differential scanning microcalorimetry (DSC)
- Calculation of aromatic uptake
- Scanning electron microscopy (SEM)
- Simultaneous thermal analysis (STA)
- X-ray powder diffraction (XRD)
- Infrared (IR) irradiation
The shrinkage caused by the thermal shock was captured photographically by monitoring the diameter D of the discs. The temperature of the irradiated gels was determined as the average temperature of 20 positions within the laser-illuminated zone.

Results and discussion
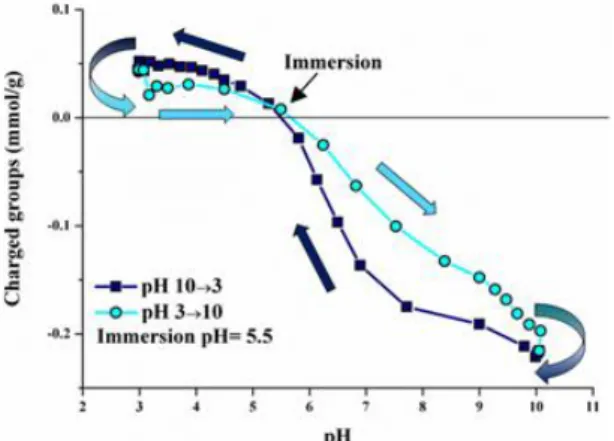
A search for evidence of intrinsic ionic behaviour in the response of the PNIPA
Comparison of the A value of the cross-linked PNIPA hydrogel (Eq. 10) with the value of the reference solution of the non-cross-linked polymer (Eq. 11) is an important indicator of the presence of electrostatic interactions in the gel. Despite the experimental uncertainties inherent in the two independent estimates of A in Eq. This asymmetry is also indicative of the preferential affinity of the PNIPA gel for anions.
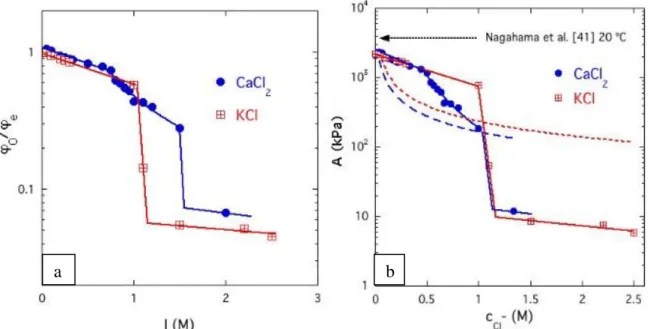
The maximum in 0/e around pH 3 is the consequence of induced polyelectrolyte behavior associated with protonation of. The effect of the ionic salts KCl and CaCl2 on the degree of swelling and the osmotic pressure of the gels is shown in Figure 16. When plotting φ0/φe as a function of the ionic strength I of the solution surrounding the gel, with these two salts the VPT occurs at different values of I (Fig. 16a).
This comparison again shows that the ion content of the gel is much less than 1%. a) Ionic strength dependence of the equilibrium swelling ratio 0/e of PNIPA gels in KCl (squares) and CaCl2 (circles) solutions at 20 °C (b) Dependence of osmot. Although ionic salts affect the osmotic pressure, the phase transition temperature does not appear to be directly related to the osmotic pressure value at the transition threshold. The response of the degree of swelling to the ionic salts KCl and CaCl2 confirms that the VPT gel is not governed by the ionic strength, but rather by the concentration of anions in the surrounding solution.
Interactions of small aromatic molecules with PNIPA hydrogel
- Phenols
- Dopamine and ibuprofen
- Interactions between small aromatic molecules and the PNIPA hydrogel seen in
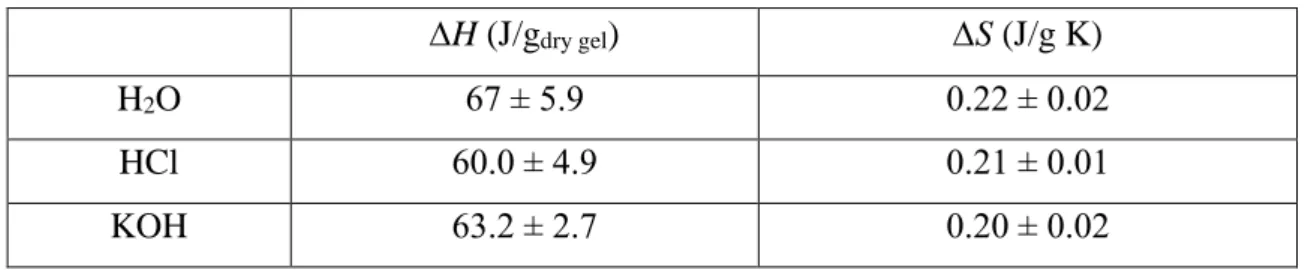
ΔH values of the phase transition of PNIPA hydrogel swollen in aqueous phenol and catechol solutions.181. ΔH values of the phase transition of PNIPA hydrogel swollen in aqueous dopamine and ibuprofen solutions. Similar to dopamine, in the CRAMPS spectra of the ibuprofen-loaded gel, intermolecular interactions between the drug and the polymer could not be clearly detected.
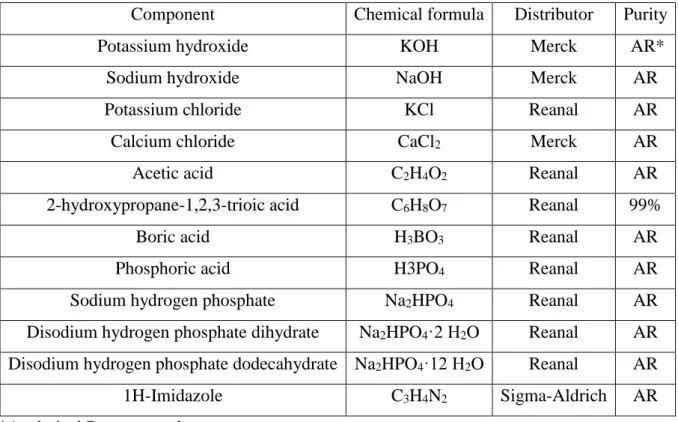
When investigating the phenol – PNIPA systems, it was found that all phenols have a great influence on the phase transition of the PNIPA gel. The range depends on both the number and the position of the hydroxyl groups. The sharpness of the melting peak and the corresponding enthalpy (9.4 kJ/mol) indicate a lack of sublimation.185 V.
Comparison of the DTG response of PNIPA gel samples loaded with different drug molecules in N2 flow. Rapid crystallization of the drug can be observed in the gel contained in ibuprofen sodium (Fig. 27e). XRD spectra of the guest molecules and the guest PNIPA gel systems during the drying process: (a) phenol (b) phenol-loaded PNIPA gel (c) dopamine hydrochloride (d) dopamine hydrochloride-loaded PNIPA gel (e) ibuprofen sodium (f ) ibuprofen sodium loaded.
PNIPA – carbon nanoparticle hybrid gels
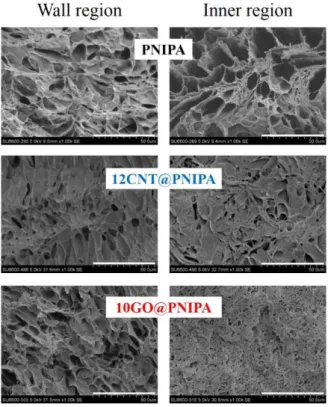
Morphology of hybrid gels
The pore size distributions of the composites containing CNTs (Fig.30b) are not much different from pure PNIPA (Fig.30a). On the other hand, the GO content clearly has a stronger influence on the pore structure (Fig. 30c and 30d).
Swelling and mechanical properties
Response of hybrid gels to various stimuli
- Temperature induced phase transition
- Infrared light sensitivity of PNIPA hybrid gels
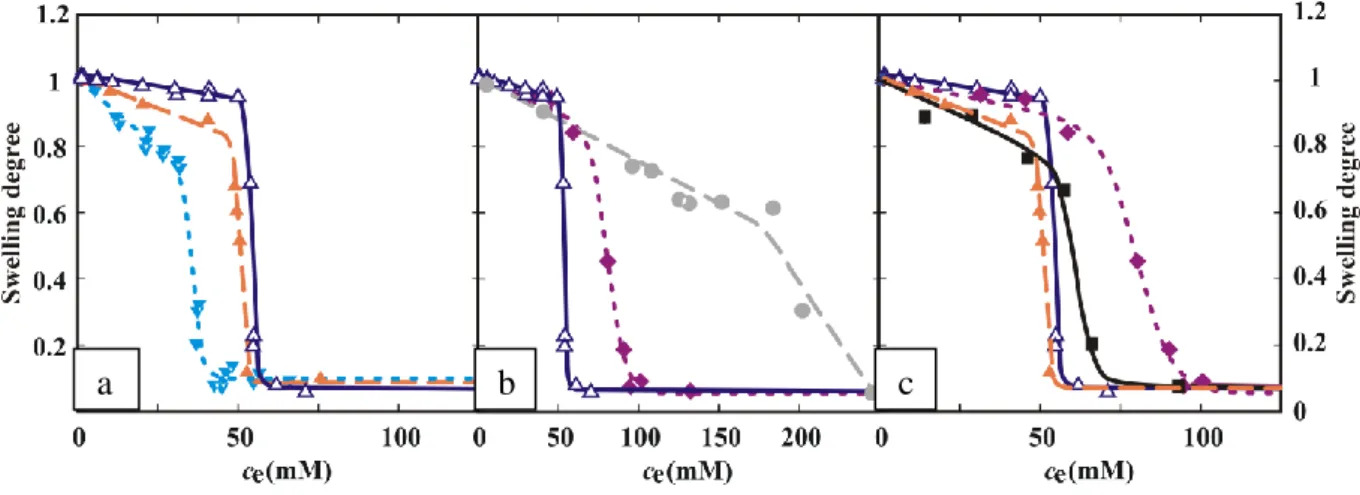
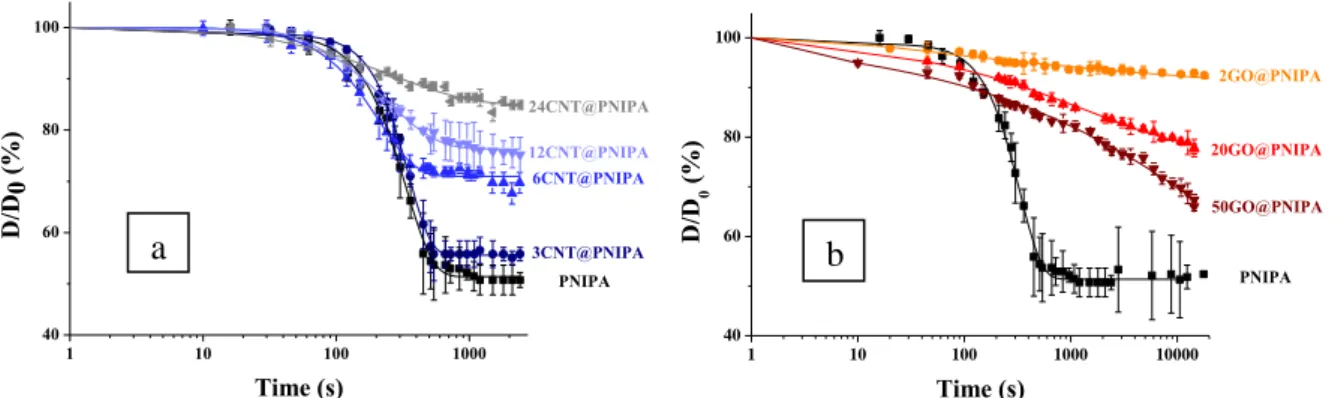
- Comparison of the effect of CNT and GO
For the GO incorporated samples, values of the fit parameters (Table 11) can only be used for qualitative comparisons, since the absence of measured swelling limit. Rapid shrinkage upon exposure to IR laser irradiation, and rapid recovery of the gels after the exposure were observed in all cases. The measured temperature fluctuations can be attributed to the low thermal conductivity of the gels, even in the presence of nanoparticles (Fig. 34c).
Mean temperature values of PNIPA composites during IR laser exposure of the lyophilized gel films.159. This behavior influences the response of the two different nanoparticles during the swelling and stress load observations. The stronger IR absorption at low GO concentration seems to reveal that these complex processes suppress the IR responsiveness of the hybrid.
Incorporation of CNT and GO leads to different effects in the behavior of the hybrids. The mechanical properties of PNIPA gel were improved by incorporating GO, while the swelling degree of the GO – PNIPA systems decreased significantly. The time constant and the swelling ratio of the temperature-induced shrinkage can therefore be adjusted by choosing the type and amount of nanoparticle loading.
Summary
This phenomenon can be attributed to the strong chemical bond between phenol and PNIPA, which was confirmed by NMR studies. In NMR and simultaneous thermal analytical (STA) experiments, no interaction between the drug and PNIPA was observed, allowing crystallization of the guest molecules in the dry polymer matrix. In the same way as phenols, ibuprofen reduces the degree of swelling of the gel, but only to a small extent and does not induce phase transition at 20°C.
This problem can be overcome by fabricating composite hydrogels by incorporating nanocarbons into the gel matrix. My goal was to obtain a comparable evaluation of the impact of carbon nanotubes (CNT) and graphene oxide (GO) nanoparticles on the hydrogel, by examining composite systems synthesized under identical conditions. It was observed that nanocarbons can only be added to the polymer in limited amounts, however both CNT and GO exert a strengthening effect at the applied low concentrations by improving the fracture stress tolerance of the hydrogel.
The modulus of elasticity and degree of swelling of the composite containing CNTs are similar to those of pure PNIPA gel. In contrast, GO increases the elastic modulus and significantly reduces the degree of polymer swelling. I also found that both nanocarbons improve the infrared sensitivity of the hydrogel and that the kinetics of temperature-induced VPT can be controlled by careful selection of type and concentration.
New scientific results
Both carbon nanotubes (CNTs) and graphene oxide (GO) increase the compressive strength of the PNIPA hydrogel even at low concentrations. The kinetics of the temperature-induced phase transition can be controlled by choosing the type and concentration of the loading nanoparticles. I have demonstrated that the infrared (IR) sensitivity of the hydrogel can be enhanced by both CNTs and GO, and that the effect depends on the dispersion of the nanoparticles.
Pulsatile drug delivery systems using hydrogels Ryo Yoshida A, Kiyotaka Sakai A, Teruo Okano B, and Yasuhisa Sakurai B. Small-angle neutron scattering study on poly(N-isopropylacrylamide) gels near their volume-phase transition temperature. Effect of tacticity of poly(N-isopropylacrylamide) on the phase separation temperature of its aqueous solutions.
Dual thermo- and pH-sensitive poly(N-isopropylacrylamide-co-acrylic acid) hydrogels with rapid response behavior. The effect of salts and pH-buffered solutions on the phase transition temperature and swelling of thermoresponsive pseudogels based on N-isopropylacrylamide. Effect of some physiological and non-physiological compounds on the phase transition temperature of thermoresponsive polymers intended for oral controlled drug delivery.
Roles of hydrophobic interaction in a bulk phase transition of alkylacrylamide gel induced by the hydrogen-bond-driving alkylphenol bond. Effect of drug physicochemical properties on swelling/swelling kinetics and pulsatile drug release from thermoresponsive poly(N-isopropylacrylamide) hydrogels. Dynamics and distribution of aromatic model drugs in the phase transition of thermoreversible poly(N-isopropylacrylamide) in solution.
Publications related to the PhD thesis
Egyidejű hőelemzés a gyógyszeradagolás kutatásában. A Magyar Tudományos Akadémia Termoanalitikai Munkabizottságának éves ülése.
Kozmetikai Szimpózium 2014, Magyar Kémiai Társaság Kozmetikai és Állami Kémiai Szakosztálya szervezésében (MKE Kozmetikai és Háztártásvegyipari Társasaga, Kozmetikai Szimpózium Budapest, Magyarország. A gyógyszerek interferáló szerepe a gyógyszerszállításban és az akrilamid gyógyszermolekulák) hidrogél kis környezeti aromás mólokkal és orvosbiológiai jelentősége.
A BME Oláh György Doktori Iskola konferenciája (XI. Oláh György Doktori Iskola Konferencia Budapest, Magyarország. Nemzetközi Kolloid- és Interfésztudósok Szövetségének konferenciája 2012 (IACIS Sendai, Japán) Poszter előadások.
Appendix
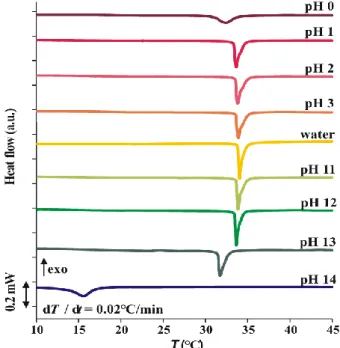
In contrast, for buffers of constant ionic strength, the degree of swelling is practically independent of the pH of the solution. In phosphate buffers, a systematic downward shift of the onset temperature and broadening of the phase transition with increasing pH is observed, but there is no correlation between Tonset and I (Fig. 14). In Britton-Robinson buffers at fixed I = 0.15 M, Tonset shifts only slightly to lower temperatures with increasing pH, with no apparent change in either the shape of the endothermic transition peak or the correlation between Tonset.
It can be concluded that the swelling properties are affected not only by the pH set by the buffer, but also by the composition of the buffer. DSC response of PNIPA swollen in (a) phosphate buffer solutions of different pH, (b) in Britton-Robinson buffers pH 4.5 of different ionic strengths and (c) in different buffers. The NMR measurements were carried out by the NMR research group of the Research Center for Natural Sciences of the Hungarian Academy of Sciences.
The on-resonance position of the RF field is outside the spectral response (–5 kHz from the center of the proton spectra). Single pulse spectra provide information about the mobile species, while the signals of the less mobile species are broadened by dipolar interactions. The CRAMPS method suppresses this broadening effect of dipole-dipole interactions that better resolve the signals of the less mobile components, but distort those of the mobile components.