Voevodsky Institute of Chemical Kinetics and Combustion, Siberian Branch of the Russian Academy of Sciences (ICKC SB RAS). Vorozhtsov Novosibirsk Institute of Organic Chemistry, Siberian Branch of the Russian Academy of Sciences, Lavrent'ev Avenue Novosibirsk, Russia.
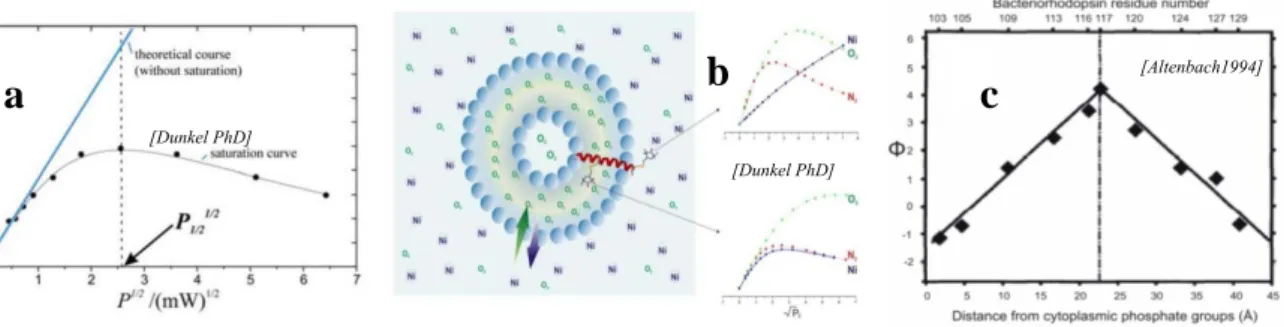
Membrane proteins study by nitroxide labeling combined with Continuous Wave EPR
Bifurcation transitions in chemical systems under low magnetic fields
Advances in nitroxides chemistry and new opportunities in biophysical research and structural biology
Quadrupolar nuclei. NMR crystallography approach
CPMG sequence with arbitrary refocusing angle
Peculiarities of NMR Relaxation in ionic liquids
Novel possibilities and difficulties in interpretation
Multiconfigurational reference methods for calculation of magnetic coupling in highly correlated materials
NMR of biomolecules: Current scope and future prospects
Introduction to Dynamic Nuclear Polarization
История развития метода магнитно-резонансной томографии в СО АН СССР / СО РАН
ESR for studies of organic radical anions
NMR spectroscopy in structure elucidation of natural products and spectral profiling of plant extracts
Vanadyl as a stable spin probe for in situ studies of fluid molecular dynamics and phase heterogeneities at high
Multifunctional rapid scan EPR imaging
NMR methods for studying ionic and molecular transport in synthetic and biological membranes
EPR spectroscopy for the study of catalyst active sites
Electron and proton transfer and water penetration
The application of the ESR in situ often involves recording the spectra at elevated temperatures and pressures. The FMR spectra contain information about the structure, morphology and size distribution of the nanoparticles2.
Electron transfer in chiral linked systems
Investigation of CIDNP kinetics by means of flash-photolysis and NMR
Parahydrogen-induced polarization for mechanistic investigations of heterogeneous hydrogenation and
The influence of spin-diffusion effect in conformational analysis of small molecules by 2D NOESY
Stereoselectivity of charge transfer processes in naproxen-based chiral dyads
1 H NMR spectroscopy in vitro of rat tissues and biofluids after impact with low and high alcohol doses
New approaches for room-temperature structural EPR studies of nucleic acids
Structure of niobium surface sites grafted on alumina with low and high niobium content from the first-principle
Phase transitions in graphite oxide/acetonitrile systems studied by EPR spectroscopy
Production of hyperpolarized contrast agents using heterogeneous hydrogenation with parahydrogen
NMR study of glycirrhizin-membrane interaction
Application of High Resolution NMR spectroscopy to the metabolomic profiling of biological tissues
Study of the relaxation properties of nitroxides in the trehalose matrix at room temperature
Investigation of magnetite nanoparticles in iron-containing arabinogalactan in the process of thermal degradation
EPR spin trapping study of redox reactions of deferiprone chelating complexes
New sterically shielded pyrrolidine nitroxide spin labels for in-cell structural measurements
Biomimetic iron-catalyzed asymmetric epoxidation
Dramatic effect of carboxylic acid structure on the electronic nature of the active oxidizing species
The study of the structure of 1,2,3,5-tetrafluorobenzene radical anion by Optically Detected ESR technique and
Multimodal molecular probes based on homocysteinylated human serum albumin for 19 F-MRI and fluorescence imaging
Advanced synthesis of sterically shielded pyrrolidine nitroxides via 1,3-dipolar cycloaddition of azomethine ylides
CIDNP study
Magnetic behavior features of heterobimetallic Cu-Pb complex
Enantioselective Solid NMR: The homochiral metal-organic framework [Zn 2 (S-lac)(bdc)(dmf)]
FMR study of catalytic corrosion processes during the reaction of halogenated compounds
The effect of the support phase transformations on the state of the spin-probed electron donor sites of Pd-Rh/Alumina.
The effect of the support phase transformations on the state of the spin-probed electron-donor sites of Pd-Rh/Alumina
Exciplex generation under optical and X-ray excitation
Spin trapping investigation of short-lived intermediates of furfural photonucleation
EPR of phosphorus-related defects in diamond
Supramolecular systems of dipeptides and cucurbit[7]uril
CW EPR parameters reflect binding modes in cytochrome P450 enzymes
Magnetic field effects on the emission of X-ray generated exciplex in alkane solutions
Recombination kinetics of charge carriers in conductive polymer P3HT and fullerene PCBM solutions studied
Time-domain shape of electron spin echo signal
Spin dynamics at early steps of charge separation
The development of MRI method for gas-phase visualization
NMR relaxation and diffusion study of self-association of glycyrrhizic acid
Investigation of electron-acceptor sites on the surface of metal oxides using EPR
Structure and HFC parameters of organic radical ions
DFT calculations
Therefore, the PES of the low-symmetry species have a pseudo-rotational shape, which is a consequence of avoiding the intersection. The mirror symmetry of the out-of-plane distortions results in a doubling of the number of stationary structures. The PES of this RA is a pseudorotational surface, but the height of the pseudorotational barrier is relatively large.
Adiabatic PESs of the alkylcyclohexane RCs, c-C6H11R+• (R = Me, Eth, iso-Pr, tert-Bu) are also pseudorotation surfaces. The energy difference between the stationary structures of minimum and maximum energy is the measure of the RC structural flexibility. Stationary temperature dependence of the reacting system on external radiation intensity in the presence and in the absence of magnetic field.
It can also be used to change the spin state in iron complexes in Fe-ZSM-5 zeolites [6]. In many cases, this approach could be used to predict the activity of the studied catalysts.

Magnetic resonance spectroscopy of nitrogen-vacancy centers in diamond
The first evidence of the nature of the spin triplet of the NV ground state was obtained through the study of optical hole burning and magnetic circular dichroism. One of the attractive features of the NV center is the phenomenon of optically induced spin polarization of the triplet ground state. The most striking line is observed at 1024 G, which comes from a Level Anti-Crossing (LAC) of the triplet levels in the NV─ center (LAC line).
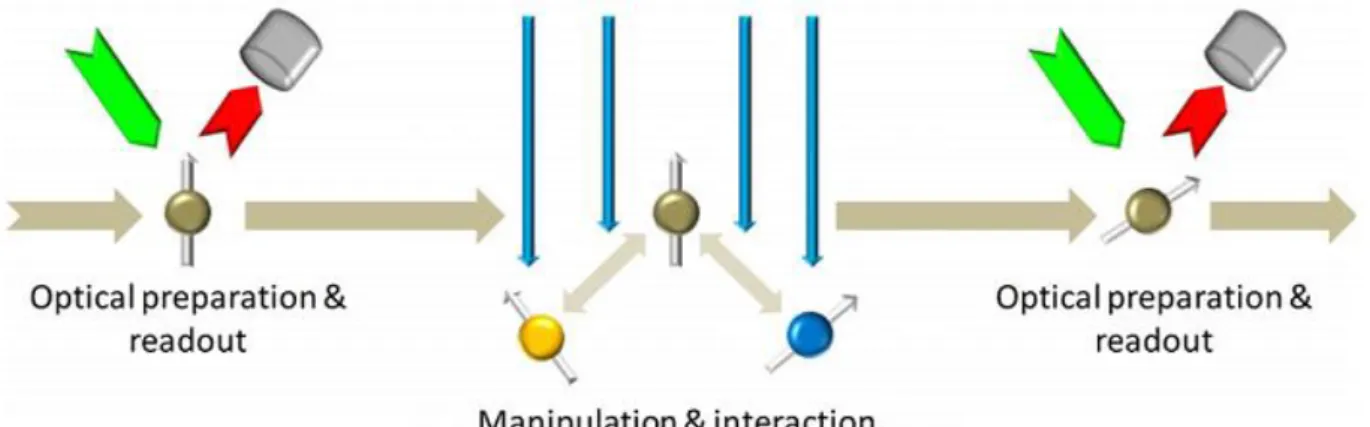
LC spectrum of the NV− centers in diamond single crystal measured with magnetic field modulation with lock-in amplifier. Each of the room temperature implementations of the NV− center as a spin qubit uses the same preparation-manipulate/interact-readout mode (see Fig. 7). Schematic representation of the preparation-manipulate/interact-readout mode of the NV− spin qubit.
The luminescence of the NV− center is monitored while it is manipulated with pulsed microwave radiation. The blue line shows the sign of the NV− center electron spin and its variation in time.
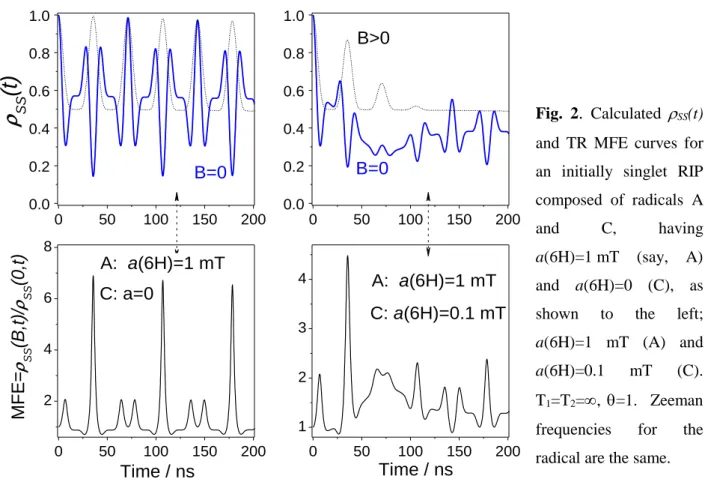
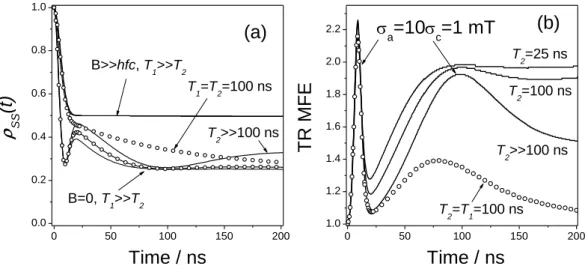
Studying spin-correlated radical ion pairs in organic media using the time resolved magnetic field effects
The wavy line is to emphasize that the fluorescence can only appear due to the recombination of RIP in its singlet state. In a relatively strong magnetic field, these interactions, as well as the Zeeman interactions of the radicals that make up the RIP, result in the mixing of the singlet with, approximately, only one triplet state, T0. Therefore, if the population of the singlet spin state were monitored via fluorescence from the S state, then, in the idealized situation neglecting spin lattice relaxation, the recombination fluorescence intensity after singlet–triplet mixing would increase by a factor of 2 in response to turning on the magnetic field.
The fraction of the initially singlet-correlated RIPs is assumed to be constant, and the other, uncorrelated RIPs are assumed to be in the singlet state with probability 0.25. As demonstrated below, the spin state evolution of spin-correlated RIP at B=0 shows quite diverse behavior, but, unlike the case of a strong magnetic field, the corresponding ρss(t) dependence can only be observed in a few specific cases are calculated analytically. At long enough T2, the maxima of these peaks are directly related to the EPR spectrum width of the RIP partners.
When both recombining radical ions exhibit isotropic HFC with equivalent protons, the theory predicts a more complicated quantum beat pattern (Fig. 2) both at B=0 and B>>aHFC. Additional advantages of the TR MFE method are its ability to directly determine relaxation times in a wide range of magnetic fields in addition to HFC and g values, as well as the ability to follow the transformations of a radical ion on the nanosecond time scale.
Electron spin relaxation in solids: Materials, proteins and coupled spins
In this lecture I will give a brief description of modern solid-state NMR of quadrupolar nuclei and in some examples I will demonstrate NMR crystallography approaches for oxide-based systems. In the Hahn-echo sequence, the pulse durations are related as P2 = 2 P1, which means multiples of the pulse /2. The application of the MAS technique (magic angle spinning of the sample) is mainly aimed at narrowing the first-order interactions according to the perturbation theory (dipolar interaction, magnetic shielding anisotropy, first-order quadrupole interaction).
Spinning at various speeds allows the determination of the isotropic shift (a line whose position does not change at a variable speed of sample spinning). Nowadays, in addition to the structural ensemble, wwPDB requires the deposition of the assigned chemical shifts as well as the geometrical constraints used in the structure determination and refinement. DFT calculations then provide insight into the NMR parameters for the substituted atom itself and typical changes in the NMR parameters of the surrounding atoms.
Calculations showed that the chemical shift in 19F was sensitive to the nature of the species at the two closest anion sites, at 2.7 and 3.2 Å, respectively, leading, as shown in Fig. The results of comparing the calculated and the experimental echo amplitudes are depicted in Fig.
Pecu Novel
R Relaxa d difficu
In particular, the present talk is devoted to the basics of multiconfigurational wave functional methods. Then, the wave function space for the model Hamiltonian can be bounded by products of local ground states. Then, one can only change the spin projections of the magnetic electrons and deal with pure spin functions.
The quality of the model can be tested by means of the effective Hamiltonian theory, the approach was formalized by Bloch in 1958. After the construction of the effective Hamiltonian can be compared with the model Hamiltonian to determine the significance of the model. Thus, the singlet selection criterion is the dominant neutral character of the singlet.
It reflects the ability of magnetic electrons to move from one place to another in a singlet state. The role of the 1h-1p class is to effectively lower the energy (stabilization) of the ionic determinants (dynamic charge polarization) and correct the direct exchange integral (spin polarization).
Vanadyl as a stable spin probe for in situ studies of molecular dynamics and phase heterogeneities at high temperatures and
Simulation of the VO 2+ ESR spectra at different rotational rates
The equation for ∆Hpp(mI) is used in most works devoted to the study of the dynamics of molecules containing vanadyl. However, this approach is not applicable to studying the dynamics of large vanadyl-containing molecules, because in the latter case τ is usually greater than 0.2 ns within the temperature range of interest. Calculation of the ESR spectrum at 50 ps ~< τ ~< 500 ns is a rather complex computational problem.
Despite the fact that this problem was solved in principle in the 1970s, the exact numerical calculation of the ESR lineshape for these intermediate cases became widely available only recently after the release of the EasySpin v.2.5 MATLAB package in 2007. Today, this package allows us to calculate of VO2+ ESR spectra for any molecular reorientation time τ. Experimental spectra for the VOTPP/toluene solution were obtained in the temperature range from 150 K to 370 K.
It was shown that the registered spectra can be accurately described by the simulated ones using τ equal to the τ derived from the SED formula for the corresponding temperature and size of the VOTPP molecule (D = 1.2 nm). Thus, we have a tool not only for calculating the spectrum of a known molecule containing vanadyl, but also for determining the size of unknowns by analyzing their spectra in a solvent of known viscosity.
Line shape VO 2+ ESR spectra in the case of spin exchange
All this makes VO2+ a relatively universal spin probe for studying the dynamics of different molecules under a variety of conditions. Here we would like to present some results how the ideology described above can be successfully applied to study heterogeneous systems in situ at elevated temperatures and pressures. It has also been demonstrated that ESR of slowly rotating vanadyls is a unique and effective tool to quantitatively monitor the size distribution of vanadyl-containing species (asphaltenes) in complex multicomponent systems (for example, heavy oils and their components) at elevated temperatures in situ.
ESR experimental methods at high pressures and temperatures
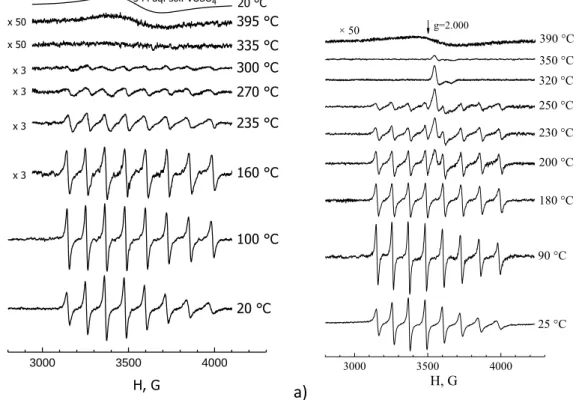
The figure shows the temperature of the middle part of the capillary when the spectrum was recorded. A typical temperature dependence of the ESR spectrum of oil and its heavy component dissolved in benzene is shown in the figure. Wide scans produce more harmonics of the modulation frequency and the signal is directly detected.
The ESR spectrum of the sample shows a single narrow line with high intensity and the characteristic shape, which corresponds to the formation of superparamagnetic nanoparticles in the sample. 4 shows the successively measured in situ ESR spectra during annealing of the sample directly in the spectrometer resonator at a temperature of 793 K. The possible applications of the ESR technique for studying magnetic nanoparticles are not limited to the processes of nanoparticle formation.
5 shows the result of an ESR in situ experiment on the process of sulphidation of the -Fe2O3 nanoparticles at elevated temperatures. In combination with other magnetic methods, the ESR technique can be a very precise tool to study the magnetic structure of the complex systems. 6 represents the observed phenomena — the formation of a paramagnetic ordered subsystem, which was found to correspond to the surface of -Fe2O3.
5 in situ ESR spectra of the sulfidation process of -Fe2O3 nanoparticles at elevated temperatures.
![Fig. 8. Measuring the magnetic field of a nuclear spin oscillating at its resonance frequency [8]](https://thumb-eu.123doks.com/thumbv2/pdfplayernet/427049.46493/84.892.97.797.151.499/fig-measuring-magnetic-field-nuclear-oscillating-resonance-frequency.webp)