The microsporidium Nosema lymantriae is a pathogen of the European gypsy moth, Lymantria dispar, a polyphagous insect herbivore that causes defoliation of temperate hardwood forests during mass outbreaks. Infectious spores are released into the environment from faeces and decaying carcasses and provide the source for new infections as they contaminate the host's feeding sites on leaves. This setup was performed three times in a row with larvae in early, middle and late stages of disease progression.
Niederschläge zu Zeiten, in denen sich die Wirte im Spätstadium der Erkrankung befinden, können die Infektionsprävalenz in der Bevölkerung erheblich erhöhen. Das Mikrosporidium Nosema lymantriae ist ein Krankheitserreger des Schwammspinners Lymantria dispar, eines polyphagen Insektenschädlings, der in gemäßigten Laubwäldern zu massivem Befall führen kann. Dieses Verfahren wurde dreimal hintereinander mit Raupen im frühen, mittleren und späten Stadium der Infektion durchgeführt.
Introduction
Lymantria dispar
- Morphology and biology
- Distribution
- Host plants
- Gradations
- Damage
- Gypsy moth and climatic conditions/weather
- Parasitoids, Predators
- Pathogens
In North America in particular, outbreaks occur irregularly and are difficult to predict (Liebhold et al., 2000). Warm and dry weather conditions (Wellenstein & Schwenke, 1978), especially in spring (April-June) (Hoch et al., 2006; Kalbacher, 2008), favor the development and survival of gypsy moth larvae and therefore promote gradations . Grades are limited by density-dependent mortality factors (Liebhold et al., 2000), such as food shortage or increased susceptibility to disease, especially viruses (Wellenstein & Schwenke, 1978).
In addition, endemic herbivore species are sometimes displaced in competition for food resources or sun exposure due to defoliation, leading to unfavorable microhabitat changes (Hajek et al., 2003). As a result of different temperature and weather conditions, regional differences in German moth population dynamics can be observed (e.g. in Hoch et al., 2001). Nucleopolyhedrosis virus (LdNPV) in particular has been an important factor in stopping massive outbreaks in both Europe and North America (Weiser, 1998; Hoch et al., 2001; Hajek et al., 2003).
Microsporidia
- Taxonomy
- Life cycle
- Transmission pathways
- Host specificity
- Microsporidia in Lymantria dispar
- Nosema lymantriae
- Environmental factors affecting microsporidian transmission
At the beginning of their intracytoplasmic development, microsporidia do not cause visible damage to the host cell (Vávra & Lukeš, 2013). Within the digestive system, a combination of stimuli triggers spore germination (Weiser, 1961; Vávra & . Lukeš, 2013). Second, the disease appears slowly, due to the long latent period of the pathogen (Solter & Becnel.
Furthermore, they report that no microsporidia have been detected in North American populations of the gypsy moth. They are easily recognized by the visible vacuole on one side of the spore (Weiser, 1998). The only ontogenetic stage of microsporidia that occurs outside the host is the environmental spore.
Aims and rationale
20 Nevertheless, the pathogen can have an impact on the dynamics of the host population as a naturally occurring enemy (Hoch & Goertz, 2009). It enables microsporidian spread and is therefore the agent of disease transmission (Undeen & Vávra, 1997; Vávra & Lukeš, 2013). Environmental spores are quite sensitive to abiotic factors, such as UV radiation, heat or low temperatures.
Due to their sensitivity to external factors and their need to enter the host gut before germination, a large number of spores is required to ensure the survival of the pathogen (Weiser, 1961; Vávra & Lukeš, 2013). The faeces of leaf-feeding larvae are hard, dry and unlikely to stick to leaf surfaces. Host larvae at later stages of disease progression cause a greater number of new infections in healthy larvae than individuals infected at an early stage of disease progression.
Material and Methods
- Insects
- Pathogen
- Experimental inoculation
- Incubation periods and stages of disease progression
- Dietary adjustment: change from wheat germ diet to foliage
- Cage system
- Contamination phase
- Simulation of rain
- Transmission phase
- Control group
- Temperature measurement
- Dissection for diagnosis
- Analysis of fecal particles
- Data analysis
To prepare a suspension containing 1000 spores per microliter, the concentration of the mixture was determined by counting spores in a Neubauer hemocytometer, followed by appropriate dilution with water. Larvae were placed separately in wells and overnight in a climate chamber. 20 dpi was chosen as the late stage variant representing the terminal stage of infection before larval death.
After completion of the first trial, it became clear that most of the larvae had reached a very advanced stage of disease by day 20. The hornbeam twigs were taken from a tree in the garden of the institute on the day of the test setup. The experiment was set up in the garden of the institute on days 12, 16 and 20 after inoculation (in the first trial) and days 12, 15 and 18 after inoculation (in trials two and three), respectively.
At the end of the infestation phase, the inoculated larvae were removed and the two-story cage system was deconstructed. 28 Ten healthy test larvae were placed on their first day of the third instar in each of the resulting cages, regardless of their previous position (top or bottom). During the periods of the experiments in the institute garden, the air temperature was recorded with a Hobo Pendant data logger.
Upon deconstruction of the two-story cages, they were collected in plastic zipper bags and stored in a freezer until examination. Dried samples were taken from the desiccator and weighed on a microbalance (Mettler Toledo MT5), particles were counted. This is due to the fact that many of the inoculated larvae died before reaching the intended 'late stage of infection'.
To determine significant differences between "rain" and "no rain" variants, the Mann-Whitney U test was applied.

Results
- Disease progression in inoculated larvae
- Early stage of disease progression: 12 days post inoculation
- Middle stage of disease progression: 15 and 16 days post inoculation
- Late stage of disease progression: 18 and 20 days post inoculation
- Disease transmission in experimental populations of healthy test larvae
- Contamination in top and bottom cages
- Leaf contamination in top cages
- Leaf contamination in bottom cage
- Feces
- Temperature profiles
- First trial
- Second trial
- Third trial
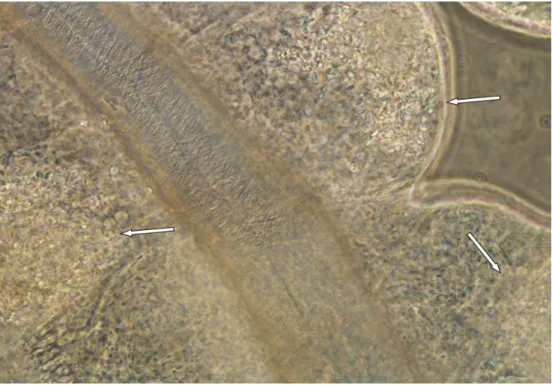
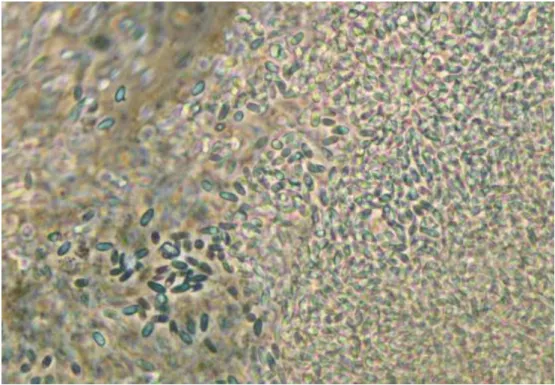
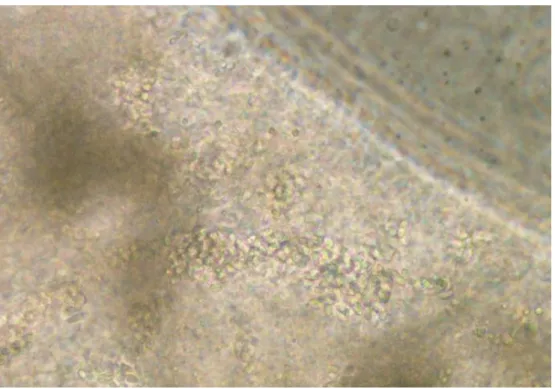
The number of environmental spores had also increased in the fat body, there were still numerous primary spores to be found (fig. 8). Although the contents of the Malpighian tubules had increased in density, the spores were easier to distinguish than before (Fig. 9). 36 3.1.3 Late stage of disease progression: 18 and 20 days after inoculation In the late stage of disease progression, all organs were densely filled with environmental spores (Fig. 10-12 ).
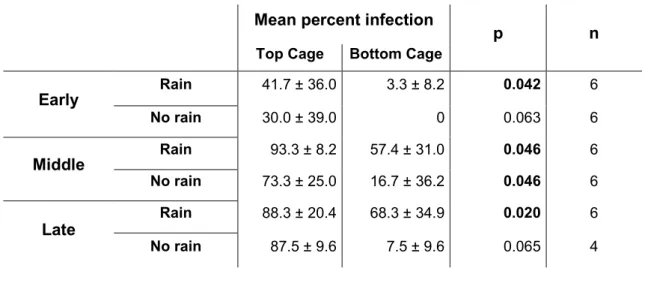
Therefore, data from rainfed and non-rainfed variants were pooled to investigate the effects of disease progression. While there was no difference between the infection rates of the middle and late stages, both were significantly higher than that of the early stage (U-test: early-mid: p = 0.001; early-late p = 0.001, Bonferroni corrected α = 0.0167) . When leaves were contaminated with feces only, no transmission was observed in test larvae during the early stage of infection (all dry cages and five of six water-sprayed cages).
In the middle and late stages of disease progression, transmission of microsporidia from larval faeces was significantly affected by simulated rain (Fig. 15). Pairwise Mann-Whitney U tests showed no significant difference for the early stages of infection, but significant differences for the middle and late stages of infection. When no rain was simulated, the percentage of infection in the test larval population did not change significantly over the course of disease progression (Kruskal-Wallis H Test: χ² = 3.163, df . = 2, p = 0.2).
16 Linear and logarithmic regression of change in transmission over time in cages with simulated rain and faecal contamination. A difference between stages was indicated for the dry weight of faecal pellets in cages without simulated rain (Kruskal Wallis H-test: χ p = 0.001). For the variant with rain, dry weights were also significantly different between stages, (H-test: χ²= 6.382, p = 0.041), but only the pairwise comparisons of early and middle stages of infection were significantly different (U-test: p = 0.009 ).
In the early phase, faecal particles collected in cages with simulated rain were significantly lighter than pellets from the dry version (U-test p = 0.009). Different capital letters indicate significant differences between early, middle and late stages of disease progression. The faecal particles of the larvae used for the middle setting of the second experiment were found to be heavier than those collected during the other two experiments.

Discussion
- Disease progression in inoculated larvae
- Disease transmission
- Transmission via contaminated larvae and feces
- Transmission via contaminated feces
- Effects of rain on disease transmission
- Problems encountered during the experiment
- Spore extraction from feces
- Ambient temperature during trial 2
- Conclusion
Simulated rain did not cause a significant change in the microsporidia transmission rate - it was equally high in the dry and wet cages. In the current experiment, transmission occurred in the upper cages of the early setup (12 dpi) but virtually not in the lower cages. Larvae in this experimental setup contaminated the foliage in the lower cages with feces, but direct contact with test larvae was prevented.
In the variant with simulated rain, a correlation was also indicated between percent infection and course of disease. According to Goertz & Hoch (2008a), the increase in fecal trace content follows a logarithmic trend over the course of disease progression. Although it may not affect the transmission effect, it is still interesting that environmental traces in the feces of infected individuals appear to have species.
Therefore, moisture is an important factor in dealing with possible contamination by faecal matter in the field. Wet surfaces lead to increased mud adhesion, resulting in an accelerating effect on transfer. Simulated rain in the present experiments even caused a significant decrease in the dry weight of the frass pellets.
In this series of tests, live hosts continuously shed infectious spores—partially embedded in fecal pellets—even after simulated rain. In contrast, no negative effects of simulated light rain on transmission were observed in the upper cages in this study. In another trial, there was almost no transfer of microsporia between the early and mid-stage settings.
The physiological change of the larvae during the second experiment is also reflected in the dry weight of the faecal pellets.
Modeling horizontal transmission of microsporidia infecting gypsy moth, Lymantria dispar (L.), larvae. Microsporidia) from Lymantria dispar (L.) (Lepidoptera: Lymantriidae). Quantification of horizontal transmission of Nosema lymantriae, a microsporidian pathogen of the gypsy moth, Lymantria dispar (Lep., Lymantriidae) in field cage studies. The gypsy moth revisited: Studies on the natural enemy complex of Lymantria dispar L. Lep., Lymantriidae) during an outbreak in a known gypsy moth area.
Gamma radiation-induced pseudoparasitism as a tool to study interactions between host insects and parasitoids in the system Lymantria dispar (Lep., Lymantriidae)-Glyptapanteles liparidis (Hym., Braconidae). Effects of Glyptapanteles liparidis (Hym.: Braconidae) parasitism, polydnavirus and venom on the development of microsporidia-infected and uninfected Lymantria dispar (Lep.: Lymantriidae) larvae. The natural enemy complex of the gypsy moth, Lymantria dispar (Lep., Lymantriidae) in different phases of its population dynamics in Eastern Austria and Slovakia – a comparative study.
Untersuchung des Überlebens und der Entwicklung der Larven zweier Herkünfte des Schwammspinners (Lymantria dispar L. Lep.: Lymantriidae) in Bezug auf die Futterpflanze. Pollan, S. 2009: Einfluss der Temperatur auf die Entwicklung des Mikrosporidiums Nosema lymantriae und das Fortschreiten der Krankheit im Wirt Lymantria dispar. Einfluss der Temperatur auf die Entwicklung des Mikrosporidiums Nosema lymantriae und das Fortschreiten der Krankheit im Wirt Lymantria dispar (L. 1758).
Zur Bionomie und Bedeutung von Glyptapanteles liparidis (Hym., Braconidae) als Regulator von Lymantria dispar (Lep., Lymantriidae) in Gebieten mit unterschiedchenchen Populationsdichten. Host specificity of microsporidia (Protista: Microspora) from European populations of Lymantria dispar (Lepidoptera: . Lymantriidae) to native North American Lepidoptera. The effect of mixed infections of three types of microspores isolated from negara, Lymantria dispar L.
The potential of Entomophaga maimaiga to control the gypsy moth Lymantria dispar (L.) (Lepidoptera: Erebidae) in Europe.
Index of figures
Index of tables