Our results provide evidence of an impact of aging on the global sperm metabolome, with a profound alteration of several key metabolic pathways associated with male reproductive potential. Our results provide evidence of an impact of aging on the global testicular and seminal metabolome, with a profound alteration of several key metabolic pathways associated with male reproductive potential.
Introduction
Contents
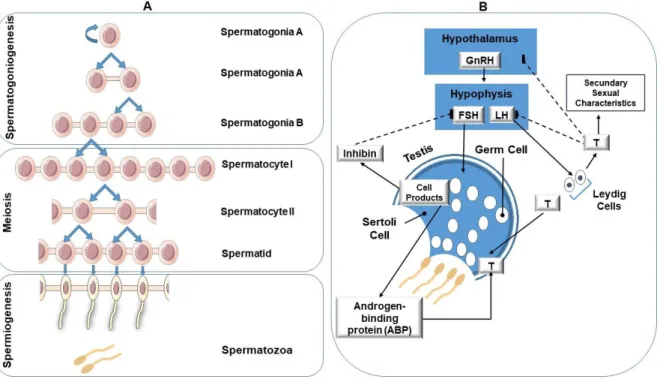
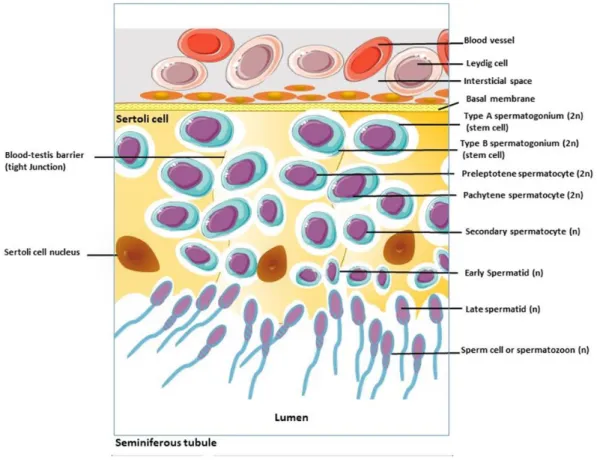
In most other mammals, the seminiferous tubules are surrounded by only 2–4 layers of myofibroblasts [16]. Fritz, I.B., et al., Regulation by FSH and dibutyryl cyclic AMP of androgen-binding protein formation in Sertoli cell-enriched cultures.
Biochemical changes in the reproductive function of the aging male
No, D., et al., Age-related structural and metabolic changes in pelvic reproductive end organs. Koppers, A.J., et al., Importance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa.
Fertility and sperm quality in the aging male
Harman, S.M., et al., Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Morley, J.E., et al., Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Wang, C., et al., Male reproductive aging: using the Norway brown rat as a human model.
Hossain, A., et al., Assessing the relationship of sperm morphology to seminal and other clinical conditions of sperm donors. Nakamura, B.N., et al., Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age-dependent manner. Belloc, S., et al., Paternal age and sperm DNA decay: discrepancy between chromomycin and aniline blue staining.
Plastira, K., et al., The effects of age on DNA fragmentation, chromatin packaging and conventional sperm parameters in spermatozoa from oligoasthenoteratozoospermic patients.
Objectives
A major limitation of most studies is the lack of a mechanistic approach as they are mostly focused on sperm parameters and DNA analysis. We aimed to study the underlying mechanisms in sperm associated with male aging, particularly metabolic and oxidative profiles. However, the studies were only focused on the evaluation of sperm parameters or DNA fragmentation in older men.
While those direct measures are the endpoint of male reproductive performance, we propose to study molecular mechanisms that may control that outcome. Herein, we hypothesize that paternal age is associated with changes in sperm parameters and that physiology is noted only when specific changes occur in sperm metabolism and oxidative status. We propose to study sperm metabolism and oxidative status and correlate them with sperm parameters and physiology and ultimately with aging and ART outcomes.
To assess the oxidative status of sperm and oxidative damage by determining the antioxidant potential of sperm and studying protein carbonylation and nitration as well as lipid peroxidation;.
Senescence and declining reproductive potential
Jarak I*, Almeida SP*, Carvalho RA, Sousa M, Barros A, Alves MG, Oliveira PF (*both authors contributed equally) (2018) Senescence and declining reproductive capacity: understanding molecular mechanisms through testicular metabolomics.
Senescence and declining reproductive potential: insight into molecular mechanisms through testicular
Abstract
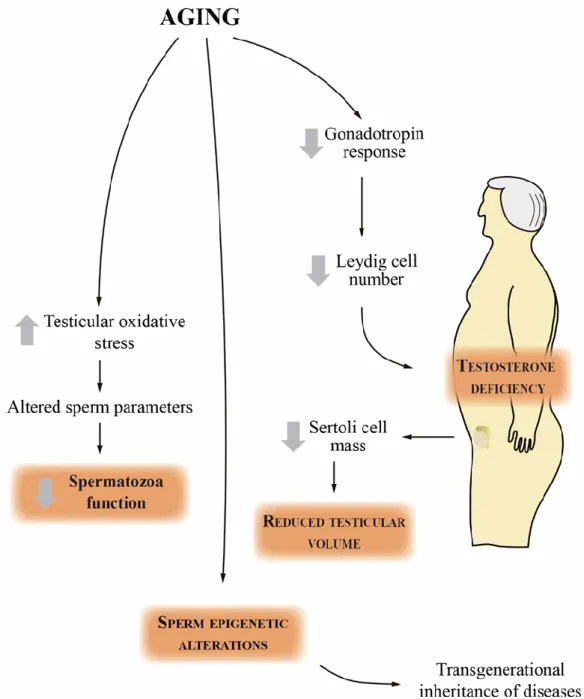
As a result, sperm concentration and total number decrease, and their motility and morphology deteriorate in the aging male. The difficulty of obtaining human testicular tissue for the study of age-related changes in the reproductive system highlights the importance of using animal models in obtaining data on the effects of aging on the testes. Typically, rats live 35-40 months, making it easy to investigate the mechanisms by which age-related changes occur in the absence of other confounding factors [7].
Therefore, we propose to reveal relevant molecular bases of testicular aging by performing a comprehensive study of the global testicular metabolome during natural aging of male Wistar rats (Rattus norvegicus). This work represents the first study using a metabolomic approach to reveal age-related changes in the total metabolome of testes in a mammalian model. Mammalian Protein Extraction Reagent and BCA Protein Assay Kit were purchased from Thermo Scientific (Whalthan, MA, USA).
After processing, 1H noesy spectra were binned using one-point bucketing (all intensity values) in the region 0.6-9.0 ppm, with signal-free, water and fumarate regions excluded from data matrix used for multivariate analysis.
Results
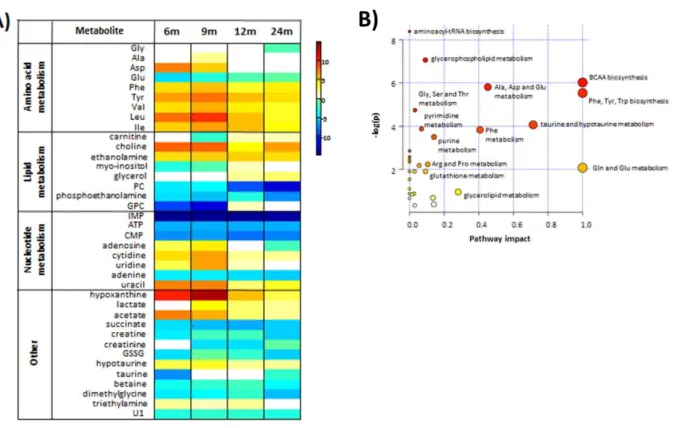
There is a marked increase in branched chain (BCAA) and aromatic amino acids (Phe, Tyr) in the testes of the 6 month group compared to the 3 month group. Scores scatter plots obtained by PCA (A) and PLS-DA (B) analysis of H NMR spectra of polar testicular extracts in 3 and 6 month old group samples. Most of the metabolites present in testes show greater variability in the 12- and 24-month-old groups than those in younger age groups.
Testicular metabolome changes associated with aging compared to testicular tissue from 3-month-old animals. Levels of lipid oxidation marker, 4-hydrononenal (4-HNE), were elevated up to 9 months of age. In the samples from the older rats, there was no significant difference between testicular 4-HNE compared to that from the testis of 3-month-old rats.
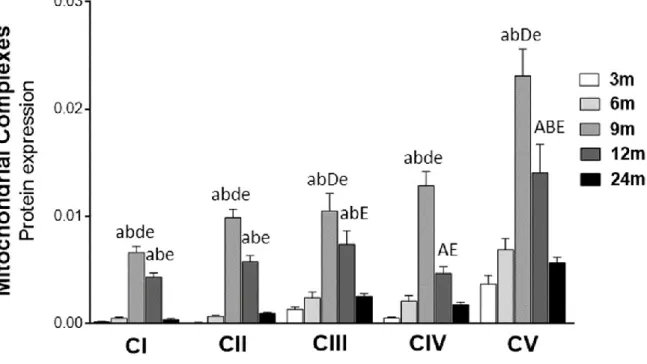
Interestingly, the peak of expression of mitochondrial complexes was observed in the testis of 9-month-old rats.
Discussion
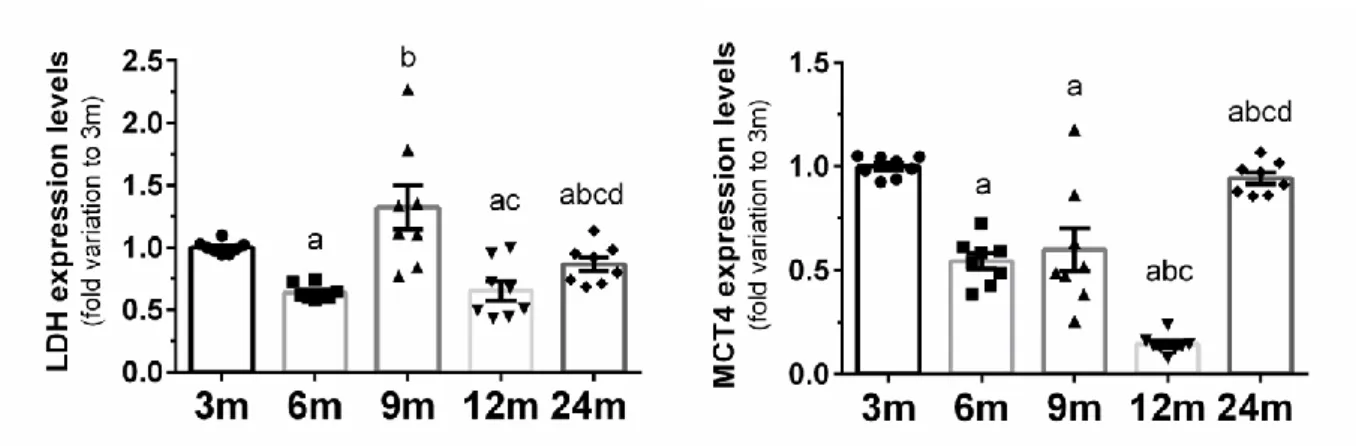
The observed decrease in the amount of MCT4 in the testes of older rats (6 to 12 months) confirms the possible accumulation of lactate in Sertoli cells and reduced bioavailability for the developing germ cells. A strong increase in MCT4, presumably of a compensatory nature, was observed in the oldest group of animals. Consistent with our findings, taurine is reported as the major free β-amino acid in the male reproductive system [37].
In the presence of high metabolic rates, as observed in spermatogenesis, mitochondrial OXPHOS can promote overproduction of ROS [41]. However, this increase was also accompanied by an increase in the expression levels of other OXPHOS complexes. However, in older animals (24-month-old mice), the expression levels of OXPHOS complexes were significantly decreased (compared to 12-month-old mice), suggesting impairment of mitochondrial function, as observed by others [44] .
Concentrations of other phospholipid precursors, myo-inositol and glycerol, were also increased in the testes of the same age groups.
Conclusions
Krishna, Testicular glucose and its transporter GLUT 8 as a marker of age-dependent variation and its role in steroidogenesis in mice, Journal of experimental zoology. Viant, Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI), Metabolomics : Official Journal of the Metabolomic Society. Russell, Germ cell genotype controls cell cycle during spermatogenesis in the rat, Biology of reproduction.
Agarwal, The role of metabolic biomarker analysis in the management of male infertility, Molecular Diagnostics Expert Review. Yoshida, Integrated NMR-based metabonomic investigation of early metabolic effects of ethylene glycol monomethyl ether (EGME) on male reproductive organs in rats, Journal of toxicological sciences. 19, a cell death regulatory gene product, is a subunit of bovine mitochondrial NADH:ubiquinone oxidoreductase (complex I), The Journal of Biological Chemistry.
Wu, Antioxidant Mechanism of Betaine without Free Radical Scavenging Ability, Journal of Agricultural and Food Chemistry.
Seminal plasma metabolites and aging: Impact on male reproductive potencial
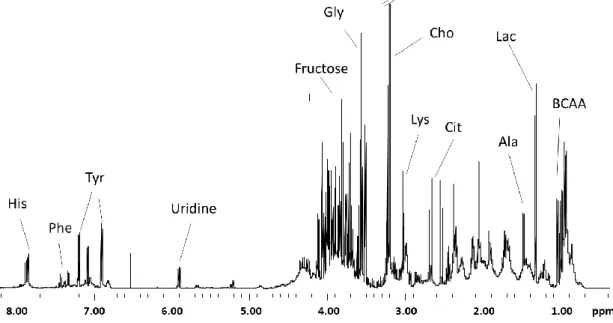
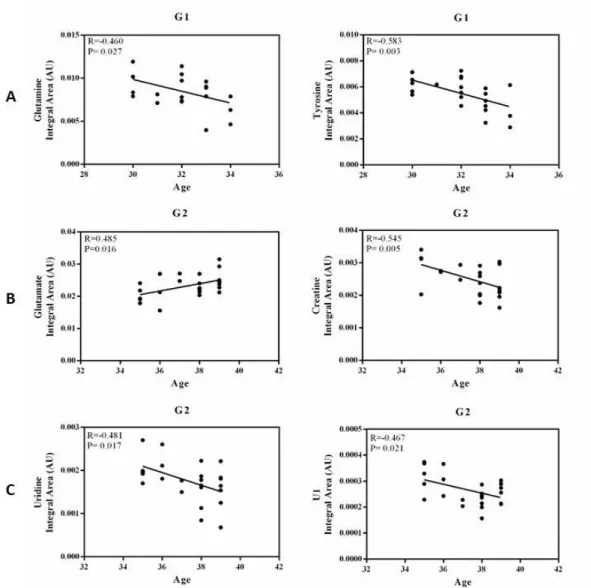
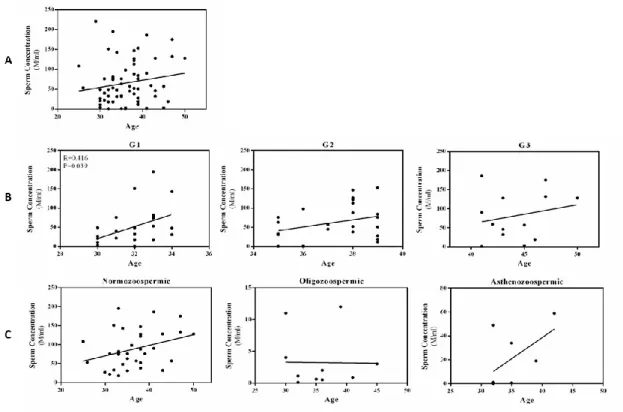
1A - Total sperm volume (ejaculate volume in mL) of samples from men of all ages (25-50 years; N=65) by individual age. 2C - Sperm count (millions/mL) of samples from men of the normozoospermic (N=35), oligozoospermic (N=11) and asthenozoospermic (N=8) groups according to individual age. A clear separation of 3 classes of samples of different ages can be observed in the graphs of the PLS-DA results (Fig. 4).
Statistical analyzes of metabolites present in samples from all individuals showed no correlation with age (Supplementary Table S1). Relative content (Integral Area - AU) of metabolites obtained by NMR in the seminal plasma of male samples from normozoospermic, oligozoospermic and asthenozoospermic groups according to individual age. Relative quantification (Integral Area - AU) of metabolites obtained by NMR in the seminal plasma of male samples of normozoospermic (N=35), oligozoospermic (N=11) and asthenozoospermic (N=8) groups.
In this context, it is imperative to elucidate the molecular basis underlying the age-related changes in the reproductive parameters.
Concluding Remarks
However, in the older animals (24-month-old rats), expression levels of OXPHOS complexes decreased significantly (compared to 12-month-old rats), suggesting a deterioration in mitochondrial function, as noted by others [29]. Hamamah, S., et al., 1H nuclear magnetic resonance studies of seminal plasma from fertile and infertile men. Gupta, A., et al., 1H NMR spectroscopic studies on human seminal plasma: a classification model for the analysis of discriminant function.
Baracca, A., et al., Reduction of Mitochondrial Complex I Is Responsible for Bioenergetic Dysfunction in K-ras Transformed Cells. Johnson, A.A., et al., The role of DNA methylation in aging, rejuvenation, and age-related diseases. Rufo, G.A., Jr., et al., Purification and characterization of a calcium transport inhibitory protein from bovine seminal plasma.
Kumar, A., et al., Fertility-associated metabolites in bull seminal plasma and blood serum: 1H nuclear magnetic resonance analysis.
Copyrights
Supplementary Material
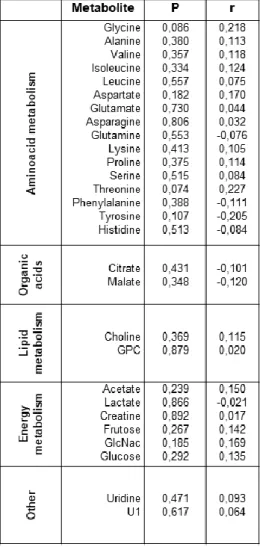
Capital letters indicate statistical significance at the FDR q<0.01 and lower case letters indicate significance at q<0.001. Capital letters indicate significance at the FDR q<0.01 and lower case letters indicate significance at q<0.001. Significance values and Pearson's correlation coefficient values of identified NMR-derived metabolites in seminal plasma.
The relative quantification of NMR-derived metabolites was evaluated by calculating Pearson's correlation coefficients (r) assuming a Gaussian distribution and a 95% confidence interval. 145 Supplementary Table S2: Significance values and Pearson's correlation coefficient values of metabolites obtained by NMR in seminal plasma samples of male samples of normozoospermic (N=35), oligozoospermic (N=11) and asthenozoospermic (N=8) groups according to individual age. 146 Supplementary Table S3: Significance values and Pearson correlation coefficient values of metabolites obtained by NMR in the seminal plasma of male samples of different ages (G1 - group 1: 30 to 35 years; . G2 - group 2: 35 to 40 years) years; G3 - group 3: over 40 years) according to the age of the individual.
Legend: U1- the metabolite we could not identify; GlcNac - N-Acetylglucosamine; GPC - glycerophosphocholine; r=Pearson's correlation coefficient; P=Significance; *P<0.05.