Členové rodiny cystatinu jsou důležitými inhibitory cysteinových proteáz katepsinového typu a podílejí se na řadě patologií. Modifikace mají odlišný vzor pro ricistatin a iristatin, jak ukazuje krystalová struktura komplexu cystatin-proteáza, a jsou hlavním znakem, který odlišuje dvě fylogenetické skupiny cystatinů ze slin klíšťat. Všechny proteiny rodiny cystatinu inhibují cysteinové katepsiny společným mechanismem, který využívá tři vzájemně se ovlivňující segmenty.
Mají unikátní inhibiční profil, zaměřující se téměř výhradně na cysteinové katepsiny s endopeptidázovou aktivitou, které vykazovaly silné imunomodulační a protizánětlivé účinky 33,37–39. Srovnání inhibiční specifity FhCyLS-2 s jinými členy rodiny ukázalo, že FhCyLS-2 inhibuje cysteinové katepsiny s endopeptidázovou i exopeptidázovou aktivitou. Lze tedy předpokládat, že secernovaný FhCyLS-2 je schopen vychýlit proteolytickou rovnováhu stejným způsobem jako dříve popsané pravé cystatiny helmintů a klíšťat s odlišnými imunomodulačními účinky 50,51.
Strukturní vysvětlení inhibiční specifity cystatinů z klíštěte I. ricinus
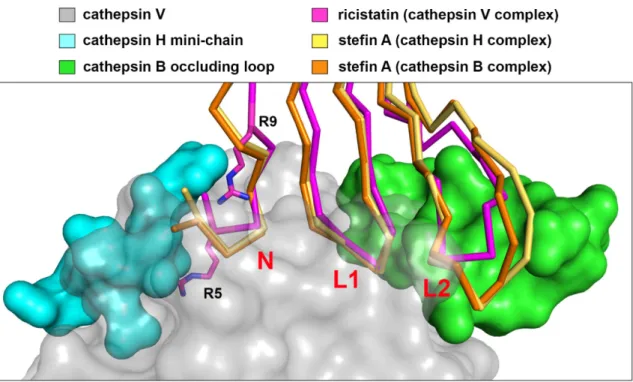
Ze struktury komplexu stefinu A a kathepsinu B je zřejmé, že interakční smyčka L2 na stefinu A je schopna vytěsnit „okluzní smyčku“ a kontaktovat podmísta S2' a S3' 59 (obr. 2). Tyto strukturální změny ve smyčce L2 ricistatinu s největší pravděpodobností způsobují neproduktivní interakci s koncovou smyčkou, což vede ke ztrátě afinity ke katepsinu B. Krystalová struktura irisstatinu ukazuje, že jeho inhibiční specifita má jiný strukturní základ než specifičnost ricistatinu.
Strukturální prvky, které omezují vazbu substrátu na aktivní místo katepsinu H (miniřetězec, azurová) a katepsinu B ("uzavírací smyčka", zelená) z komplexů se stefinem A, jsou zobrazeny v superpozici na šedém povrchu endopeptidázy katepsinu V.
Závěry
Obě skupiny slinných cystatinů tedy evolučně konvergují k úzké inhibiční specificitě, což zajišťuje fyziologickou roli v interakci klíště-hostitel.
Použitá literatura
Collagenolytic activities of the major secreted cathepsin L-peptidases involved in the virulence of the worm pathogen, Fasciola hepatica. Salivary (SD-type) cystatins: Over a billion years in the making - but to what end. The refined 2.4 A X-ray crystal structure of recombinant human stefin B in complex with the cysteine proteinase papain: a new type of proteinase inhibitor interaction.
Hlcyst-1 and Hlcyst-2 are potential inhibitors of HlCPL-A in the midgut of the ixodic tick Haemaphysalis longicornis. A salivary cystatin, HlSC-1, from the ixodid tick Haemaphysalis longicornis plays a role in blood supply processes. Bm-CPI-2, a cystatin from Brugia malayi nematode parasites, differs from Caenorhabditis elegans cystatins in a specific site that mediates the inhibition of the antigen-processing enzyme AEP.
The crystal structures of two salivary cystatins from the tick Ixodes scapularis and the effect of these inhibitors on the onset of Borrelia burgdorferi infection in a mouse model. Crystal structure and functional characterization of an immunomodulatory salivary cystatin from the soft tick Ornithodoros moubata. Crystal structure of stephine A in complex with cathepsin H: N-terminal residues of inhibitors can adapt to the active sites of endo- and exopeptidases.
The refined 2.15 Å X-ray crystal structure of human liver cathepsin B: the structural basis for its specificity. Crystal structure of porcine cathepsin H determined at 2.1 angstrom resolution: location of the minichain C-terminal carboxyl group defines the aminopeptidase function of cathepsin H.
Introduction
They are able to regulate digestion both in the intestinal tissue 25 and in the intestinal lumen 26 of the parasites. Moreover, they are involved in the process of embryogenesis 27 and in the modulation of their 28 and host immune systems 29,30. The most intensively studied tick cystatins are the true cystatins sialostatin L and L2 identified in the saliva of an I-bound tick.
They possess a unique inhibitory profile, targeting almost exclusively cysteine cathepsins with endopeptidase activity, and have shown potent immunomodulatory and anti-inflammatory effects 33,37-39. Neither the structural origin of the unusual inhibitory profile of sialostatins nor the molecular mechanism of their action is satisfactorily explained. Knockdown expression experiments demonstrated a crucial role for sialostatins in tick feeding, with 40% of ticks unable to successfully complete feeding in the absence of sialostatins 40 .
This makes tick salivary cystatins interesting antigens for the development of anti-tick vaccines that can prevent tick feeding on the host and the transmission of pathogens in the long term. Another study has shown that FhStf-2 can modulate the human immune system and exhibit anti-inflammatory effects44.
Aims of the study
Methods
- Material and laboratory equipment
- Protein expression and purification
- Biochemical methods
- Protein crystallization
- Collection and processing of diffraction data
- Solving and analysis of crystal structures
Crystallographic data collection or testing of crystal quality and cryoprotective conditions was performed at 100 K either in the Laboratory for Structural Biology at IOCB or at the MX 14.1-2 stations of the BESSY II synchrotron operated by the Helmholtz-Zentrum Berlin (HZB), Germany. Diffraction data were processed in XDS and analyzed with the Xtriage program of the PHENIX suite. The phase problem was solved either by molecular substitution in Molrep from the CCP4 suite or Phaser from the PHENIX suite or by the SIRAS method using derived crystals followed by model building with Buccaneer from CCP4.
The models were manually edited in Coot and refined in Refmac from the CCP4 suite. The quality of the model was evaluated with the Molprobity server, and the structure of the complex was analyzed with the PISA and PLIP servers.
Results and discussion
According to the sequence, FhCyLS-2 belongs to the stefin subfamily, but it also contains a secretory signal peptide and disulfides, both typical features of the true cystatin subfamily (Fig. 1A). FhCyLS-2 was found to be present in the excretory secretory products (ESP) of adult flukes and is apparently released by secretion from intestinal cells. The FhCyLS-2 molecule possesses a conserved reactive center of the stefin subfamily and thus is an efficient inhibitor of the tested cysteine cathepsins, both host and F.
Comparison of the inhibitory specificity of FhCyLS-2 with that of other family members indicated that FhCyLS-2 inhibits cysteine cathepsins with endopeptidase and exopeptidase activities. Thus, FhCyLS-2 may have a dual role in regulating exogenous and endogenous proteolytic systems in parasite-host interactions. Of note, a similar combined function has been proposed for the tick cystatin OmC2, which has immunomodulatory effects in the host environment while regulating proteolysis in the tick gut 35 .
From the combination of sequence and structural features and phylogenetic analysis, it is clear that FhCyLS-2 belongs to the stefin subfamily. These are evolutionarily distinct from the classical stephines and form a separate group on the phylogenetic branch of stephines. Analysis of the repertoire of cystatin family genes, including CyL stefins, in different groups of flukes showed significant differences.
It can be speculated that the different distribution of cystatins in the aforementioned ranks reflects different strategies associated with parasite survival in the host 53,54. Superposition of the crystal structure of FhCyLS-2 (purple, PDB: .6I1M) with human model stefin A (orange, 3KSE) and B (yellow, 1STF) on panel (A) and.
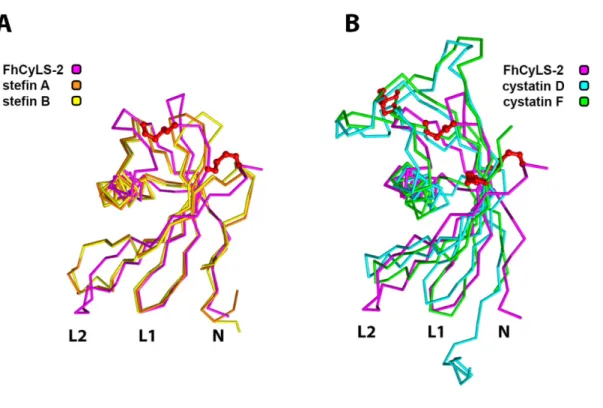
Structural basis of the inhibitory specificity of I. ricinus cystatins
For ricistatin, a crystal structure in complex with human cathepsin V, a model endopeptidase, has been successfully solved, allowing for the first time a direct explanation of the structural basis of the narrow inhibitory specificity of a salivary sign cystatin. Together, these three segments form a wedge of the reactive site that inserts into the active site of the protease. It is clear from the structure of the complex of stefin A and cathepsin B that the L2 interacting loop on stefin A is able to displace the terminating loop and make contact with the S2' and S3' subsites 59 (Fig. 2).
In addition, an additional Arg9i residue stabilizes the conformation of the entire N-terminus of ricistatin via intramolecular hydrogen bridges. These differences are significantly related to the three main binding segments of the reactive center, i.e. the N-terminus and the two surface loops L1 and L2. The flexibility of the Gly-Gly segment is essential for interaction with a wide range of target proteases, including exopeptidases.
This structural change at the top of the L1 loop will result in additional restrictions for interaction with proteases. This revealed the existence of three different groups of true cystatins in ticks of the genus Ixodes. Other important representatives are the two previously described sialostatins from I. 2) The iristatin group is evolutionarily different from the ricistatin group.
The crystal structure of iristatin shows that the inhibitory specificity has a different structural basis than that of the ricistatin group. The functional properties have probably been acquired by convergent evolution related to the role in tick saliva where they accompany members of the ricistatin group.
Conclusions
These are due to the narrow inhibitory specificity of iristatin, which is even more limited than that of ricistatin. The crystal structure of iristatin shows that the inhibitory specificity has a structurally different basis compared to the ricistatin group, with modifications of the N and L1 binding segments. Thus, both groups of salivary cystatins have converged evolutionarily towards a narrow inhibitory specificity that determines a physiological role in tick-host interactions.
It has a broad inhibitory specificity that allows interaction with tick cysteine cathepsins with endo- and exopeptidase activities. This is explained by the architecture of its reactive center, which lacks the structural constraints found in salivary cystatins. The thesis provides important new information about the evolution and structure-function relationship of the cystatin family of protease inhibitors.
Due to their critical biological roles, the studied parasite cystatins represent potential antigens for the development of antiparasitic vaccines.
The refined 2.4 A X-ray crystal structure of recombinant human stefin B in complex with cysteine proteinase-papain: a new type of proteinase inhibitor interaction. Structural basis for unique mechanisms of folding and hemoglobin binding of a malaria protease, 2006). Rhipicephalus microplus and Ixodes ovatus cystatins in tick blood digestion and evasion of the host immune response. Tick saliva Sialostatin L suppresses the initiation of immune responses by targeting IRF4-dependent transcription in murine mast cells.
Functional characterization of single-domain cystatin-like cysteine proteinase inhibitors expressed by the trematode Fasciola hepatica. The role of cysteine peptidases in hematopoietic stem cell differentiation and modulation of immune system function. Protease inhibitors in tick saliva: role of serpins and cystatins in tick-host-pathogen interaction.
Stefin A only displaces the occluding loop of cathepsin B by as much as is required to bind to the active site cleft.
Životopis / Curriculum vitae
Seznam publikací / Selected publications