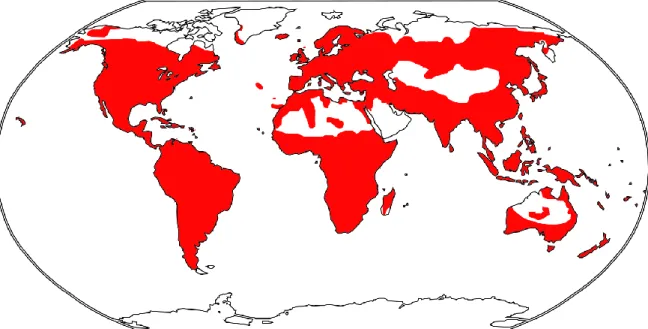
One of the demanding topics is the evolutionary origin of weeds with two main areas of interest (Pyšek & Richardson, 2006; Pyšek et al., 2008; Richardson & Pyšek, 2008). The extent of the mentioned characteristics is also associated with the effect of polyploidy (often preceded by hybridization), which is accepted as an important evolutionary driver that can change not only the ecology and physiology, but also the genetic composition and the morphology of the plants (Pandit et al., 2011, in Beest et al., 2012; see also Glennon et al., 2014). Several life history traits such as the formation of an abundant and persistent seed bank, intensive vegetative propagation by underground stolons (horizontal stems used for vegetative propagation), the ability to regenerate from separated parts of stolons, and polyploidy, may contribute significantly to the weed, widespread distribution and the formation of dense and monospecific vegetation (Ivins, 1952; Thompson & Grime, 1979; Wheeler, 1981; Roberts & Boddrell, 1984; Hara & Šrůtek, 1995; Šrůmann, 1908; Taylor, 1908; Rejlová et al. al., 2019).
On the other hand, the introduced representatives of the species partially complement the role of food plants for the rare and endangered butterflies (Vanessa gonerilla Fabricius, together with their described subspecies, and Vanessa itea Fabricius; Fig. 1) and thus help to increase their populations (Chudleigh, 1999; Barron et al., 2004; Barron, 2007). In addition, man has used this 'herb' or 'crop' plant for centuries, which may have led to its wider distribution (Di Virgilio et al., 2015). Nettle fibers were found with Ötzi the Iceman, who probably used them as a bowstring or to attach fletching and arrowheads (Junkmanns et al., 2019).
The widely useful plant (currently used e.g. in applied research – treatment of diabetes and prostate diseases, textile industry, cosmetics; Bredemann & Garber, 1959; Farzami et al., 2003; Bacci et al., 2009; Mohammadi et al., 2009; Mohammadi et al. ., 2016) has become an anthropochorous, troublesome weed species (especially in Central Europe).
GENERAL INTRODUCTION TO THE NETTLE FAMILY
The family Urticaceae Juss
Vanessa gonerilla gonerilla Fabricius – New Zealand red admiral (A) mature individual (photo by Kimberley Collins; file is licensed under CC BY‑SA 4.0); (B) favorite food plant Urtica ferox G. D) pupa (photo by Roger Frost; file is licensed under CC BY‑NC); (E) lower arm (photo by Tony Wills; file is licensed under CC BY‑SA 3.0).

BIOSYSTEMATIC INSIGHT INTO NETTLES
- The genus Urtica L
- Evolutionary mechanisms in the genus Urtica
- Gender distribution and sex ratio in the genus Urtica
- Seeds dispersal as the main mean of gene flow
Previously, a number of studies have investigated the predominance of individual types of polyploids with conflicting results (Clausen et al., 1945; Soltis et al., 2007). Different cytotypes often occur sympatrically, and according to recent knowledge, up to sixteen percent of vascular plants consist of several cytotypes (Rice et al., 2015). Within the genus Urtica, different species can be found with different chromosome numbers (Rice et al., 2015); however, there is no clear evidence that this leads to significant diversification.
Dioecy is one of the phenomena that shapes not only the genus Urtica, but also the entire family (Wu et al., 2018). Diecy likely recurred throughout history, with polyploidization possibly associated with its origin (Volz & Renner, 2008; Ashman et al., 2013). The primary vectors for long-distance dispersal are primarily hydrochory (dispersal by water), anemochory (dispersal by wind), and animal dispersal (zoochory; Cain et al., 2000).
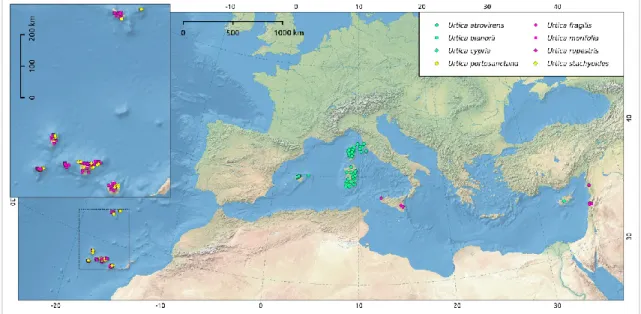
According to a recent phylogenetic study (Grosse‑Veldmann et al., 2016b), most island endemics are the result of at least two independent colonizations (with the exception of Hawaii and the Juan Fernández Islands).
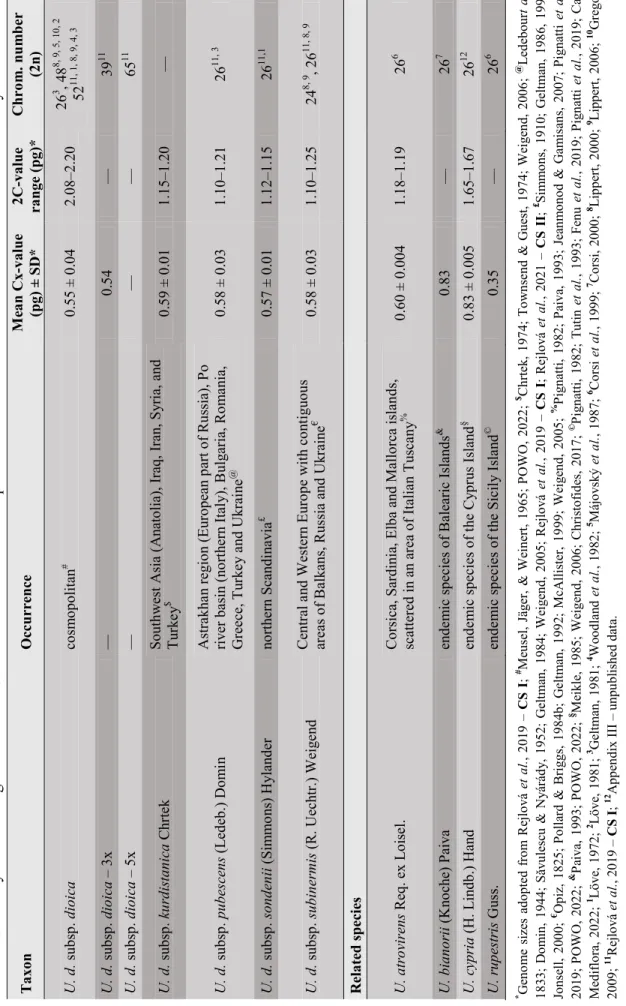
INTRASPECIFIC CLASSIFICATION OF URTICA DIOICA
Nominate subspecies
The more natural populations occur mainly in moist floodplain forests, around and near streams and in ravine forests (Ivins, 1952; Geltman, 1986; Chrtek, 1997; Šrůtek & Teckelmann, 1998; Kaplan, 2019). Detail of carpel flowers (C) with stigmas and staminate flowers (D) with anthers (white arrow) and pistillode (a rudimentary pistil; red arrow) of Urtica dioica subsp. For more detailed floral morphology within wind-pollinated representatives of urticalean rosids see Pedersoli et al., 2019.

Diploid subspecies of Urtica dioica in Europe
The plants are described with the typical character of narrow leaves, which is often described in diploids. The center of occurrence is in northern Scandinavia, where it grows in forests, along small streams and on scree (Fig. 11A). The plants are characterized by a light green color, an elongated leaf blade, almost a lack of stinging trichomes, and a reduced amount of unicellular trichomes (Fig. 12C).
The total area where it occurs includes mainly Central and Western Europe with adjacent areas of the Balkans, Russia and Ukraine, but the literature on its distribution is often contradictory (Opiz, 1825; Pollard & Briggs, 1984b; Geltman, 1992; McAllister, 1999). ; Weigend, 2005).

The Asian diploid subspecies of Urtica dioica
ENDEMIC SPECIES OF THE GENUS URTICA FROM THE MEDITERRANEAN
A stinging nettle is small in stature and easily identified by its warty (warty) leaf lamina (Fig. 15B), which is due to its noticeable stinging trichomes. It is a monoecious and perennial plant (Fig. 15A), usually found in nitrogen rich and shaded sites on rocky outcrops with frequent grazing. The individuals are characterized by scattered stinging trichomes, mainly on the veins of the underside of the leaf lamina and stem, entirely without unicellular trichomes.
The plants are monoecious, with the male flowers usually in the lower half of the plant and the female flowers above (Fig. 16). The stem is wooded at the base (Fig. 18A); the plants have different male and female individuals (dioecious; individuals with a transient sexual state have also been recorded for more details see Grosse‑Veldmann & Weigend, 2018 and CS III). The typical habitats of occurrence are rocky outcrops in mesophilic holm oak forests, often on carbonate substrates (Fig. 18B), at an altitude of 0-600 m above sea level.
EN; individuale U4213 van verbouing; versamelaar Eliška Havlíčková, foto deur Ludmila Rejlová) detail van blaarlamina (B; foto deur Zuzana Chumová).

AIMS OF THE THESIS
What are the delimitation and relationships between European and Southwest Asian diploid taxa growing in a diverse range of 'primary' habitats. H1: European lowland diploids with overlapping areas are more closely related to each other than to the mountain Asian diploid. Are the morphological and genetic pattern in congruence, that is, is the morphological differentiation reflected in the genetic pattern and vice versa.
METHODS
Targeted enrichment combined with genome mapping (Hyb-Seq approach) was used to evaluate phylogenetic relationships. This method provides hundreds of useful low-copy core loci in addition to flanking regions, high-copy repeats, and organellar genomes. A wide range of genomic data is suitable for a phylogenomic study (Weitemier et al., 2014; Yu et al., 2018) to distinguish individual lineages (species, subspecies, cytotypes) within U.
Low-copy nuclear (LCN) probes for target enrichment were designed based on a combination of transcriptome and genome skimming data. To assemble the hundreds of orthologous low-copy loci, the script Sondovač (Schmickl et al., 2016) was used. Subsequently, SNP sites have been extracted from the obtained Hyb-Seq data (Page et al., 2016; CS II).
The use of the RAD-Seq (restriction site-associated sequencing) method was also considered suitable for phylogenetic studies below the genus level, although with lower data recovery from the other genomic data (Yu et al., 2018).
KEY RESULTS
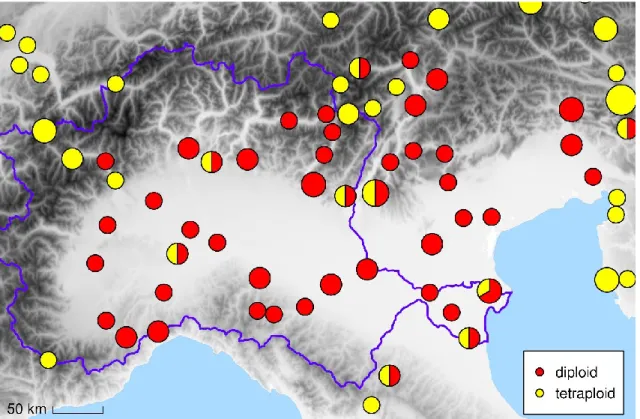
This is related to the deforestation and long-term agricultural use of the Po Field (Marchetti, 2002). Subsequent canonical discriminant analyzes (individual plants assigned to groups based on ploidy level) have shown only marginal differentiation of the two cytotypes. A combination of multiple biosystematic methods has been applied to contribute to the elucidation of the variability of U.
Nevertheless, the distinction between the taxa is complicated due to the high variability and plasticity across the group. In addition, it has contributed to delimiting the occurrence of the currently recognized diploid subspecies in U. The geographic distribution of some subspecies is more or less clearly defined, especially the occurrence of U.
In addition, in the morphological part of the study, plants between these subspecies have been collected in the region between Norway and the Finnish Lakeland.
DISCUSSION
The type specimen is from the Astrakhan region (Ledebour et al., 1833), and Geltman (1986) even treated this taxon as endemic to the Volga River Delta. In addition, when examining the original sites in the Volga River Delta, no individuals were found. As a result, it was impossible to provide an acceptable explanation for this disjunction and prevalence of diploids in the Po Plain.
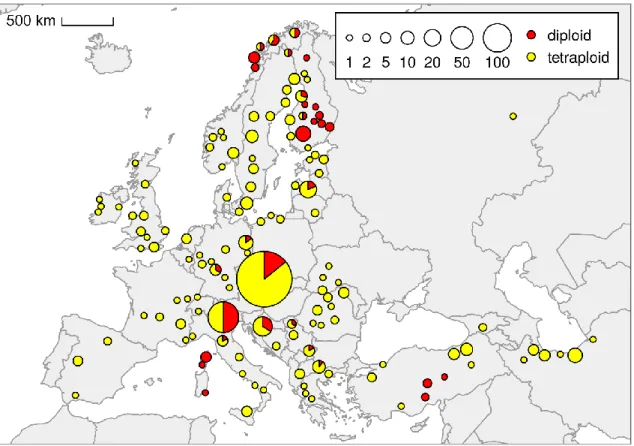
In the cytogeographic study, the predominance of the tetraploid cytotype (commonly referred to as U. d. subsp. dioica) has been confirmed, which is common in different synanthropic habitats and has a cosmopolitan distribution (Meusel et al., 1965; POWO, 2022; CS I). Chen et al., 2003) outside the study area and migrated to the study area. Although the different sets of molecular markers were used and all plants were checked by flow cytometry, no structure has been found in Urtica dioica (CS II), as in the case of previous phylogenetic studies (Henning et al., 2014; Grosse‑Veldmann et al., 2016b).
Genome size can aid in the detection of taxa and can be an indication of genetic distance.
CONCLUSIONS AND FUTURE PERSPECTIVES
Systematic and ecological-geographic characteristics of the species from the affinity of Urtica dioica (Urticaceae) in the flora of the U.S.S.R. Fifty years of invasion ecology – the legacy of Charles Elton: the legacy of Charles Elton. However, although relatively many chromosome counts have been published (e.g. [64]), the distribution pattern has so far only been studied marginally and locally.
Absolute genome size of the 4x cytotype (U. d. subsp. dioica), 2x cytotype (U. d. subsp. kurdistanica, subsp. pubescens, subsp. sondenii, subsp. subinermis) and closely related species (U. atrovirens, U. bianorii , U. d. subsp. cypria, U. kioviensis and U. simensis). Additional occurrences of the alluvial diploid cytotype (~U. d. subsp. subinermis) can be expected in Western Europe (especially in France and the United Kingdom). Simultaneous analysis – from left: 2x – diploid cytotype, 3x – triploid, 4x – tetraploid, 5x – pentaploid, Bellis perennis – the internal standard.
Species closely related to the U. dioica clade in recent phylogenies, namely: U. The upper left portion shows one population of U. The size of the circles represents the number of populations. Weeding the nettles III: Named nonsense versus named morphotypes in European Urtica dioica L. The geometry of sex: hyperdiversification of sexual systems in Urtica L. Simultaneous analysis – from left: 2x – diploid cytotype, 3x – triploid, 4x – tetraploid, 5x – pentaploid, Bellis perennis – the internal standard.
16 distance from the widest point of the leaf to the top of the leaf mm. Grosse‑Veldmann B, Nürk NM, Smissen R, Breitwieser I, Quandt D, Weigend M. Pulling the sting out of nettle systematics – a comprehensive phylogeny of the genus Urtica L. Weeding the nettles III: named nonsense versus named morphotypes in European Urtica dioica L The role of adaptation strategies in plant naturalization.
Urtica kioviensis Rogow. × Urtica dioica L
Urtica kioviensis Rogow. in Nature Reserve “Plačkův les a říčka Šatava”
Chromosome count of Urtica cypria (H. Lindb.) Hand
Examples of the morphology of the achenes of species within the Urtica genus
Author contribution to included case studies
Copyright permissions
Collection permissions
Curriculum vitae