PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS DA SAÚDE
AVALIAÇÃO DE EFEITOS DE UM EXTRATO DE “TRÊS BAILARINAS” EM MODELOS EXPERIMENTAIS EM DIFERENTES NÍVEIS DE ORGANIZAÇÃO BIOLÓGICA.
AVALIAÇÃO DE EFEITOS DE UM EXTRATO DE “TRÊS BAILARINAS” EM MODELOS EXPERIMENTAIS EM DIFERENTES NÍVEIS DE ORGANIZAÇÃO BIOLÓGICA.
Dissertação apresentada à Universidade Federal do Rio Grande do Norte - UFRN, para a obtenção do título de Mestre em Ciências da Saúde pelo Programa de Pós- graduação em Ciências da Saúde.
Orientador: PROF. DR. MARIO BERNARDO-FILHO Colaborador: PROF. DR. SEBASTIÃO DAVID SANTOS-FILHO
Natal, RN 2011
PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS DA SAÚDE
Coordenadora do Programa de Pós Graduação em Ciências da Saúde Profa. Dra. Técia Maria de Oliveira Maranhão
Natal, RN 2011
CATALOGAÇÃO NA FONTE
UERJ/REDE SIRIUS/BIBLIOTECA CB-A
P659 Pinto, Nelson de Souza.
Avaliação de efeitos de um extrato de três bailarinas em modelos experimentais em diferentes níveis de organização biológica / Nelson de Souza Pinto. - 2011.
57 f.
Orientador : Mario Bernardo-Filho.
Colaborador: Sebastião David Santos-Filho.
Dissertação (Mestrado) – Universidade Federal do Rio Grande do Norte. Centro de Ciências da Saúde. Programa de
Pós-graduação em Ciências da Saúde.
1. Drogas vegetais - Dissertação. 2. Eritrócitos - Dissertação. 3. Tecnécio–99m - Dissertação. 4. Escherichia coli - Dissertação. I. Bernardo-Filho, Mario. II. Santos Filho, Sebastião David dos. III. Universidade Federal do Rio Grande do Norte. Centro de Ciências da Saúde. IV. Título.
AVALIAÇÃO DE EFEITOS DE UM EXTRATO DE “TRÊS BAILARINAS” EM MODELOS EXPERIMENTAIS EM DIFERENTES NÍVEIS DE ORGANIZAÇÃO
BIOLÓGICA.
PRESIDENTE DA BANCA: Prof. Dr. Mario Bernardo-Filho (UERJ)
BANCA EXAMINADORA
Profa. Dra. Técia Maria de Oliveira Maranhão Profa. Dra. Aurimery Gomes Chermont
SUPLENTES
Prof. Dr Aldo da Cunha Medeiros
Prof. Dr. Sebastião David dos Santos Filho
Dedico este trabalho:
A minha esposa, Alcy Batista da Costa, e aos meus filhos, Alan Costa de Souza e Hugo Costa de Souza. Eles suportaram a minha ausência e vivenciaram comigo todos os momentos de estresse e as dificuldades ocasionadas pela sobrecarga de trabalho e das atividades pessoais acumuladas ao longo do período de desenvolvimento do projeto de mestrado. Precisei muito da compreensão deles. Os três são meus alicerces.
Aos meus seis irmãos, a todos os sobrinhos e sobrinhos netos. Juntos formamos uma família maravilhosa.
In memoriam, de meu pai Nelson da Silva Pinto, minha mãe Odette de Souza
Pinto e minha irmã Neize Liliane de Souza Pinto, tenho certeza que estão orgulhosos desse grande feito.
A meu orientador, Prof. Dr. Mario Bernardo Filho, por ter me acolhido.
A Deus por permitir que seu humilde servo tivesse alcançado mais essa vitória.
realização de um grande sonho, que até então parecia impossível de ser realizado. Agradeço ao Pai todo poderoso, pela minha vida, pela minha família e pelos meus amigos.
Agradeço ao meu orientador Prof. Dr. Mario Bernardo-Filho, por me ensinar o quão técnico, meticuloso, criterioso, dedicado e perseverante deve ser um pesquisador.
Ao Programa de Pós-graduação em Ciências da Saúde da Universidade Federal do Rio Grande do Norte por me proporcionar conhecimentos na área da saúde e da educação, fato que me torna um ser humano e profissional melhor. Com essas novas ferramentas adquiri um olhar mais apurado nos diferentes grupos de atuação do meu dia-a-dia: ser humano, alunos e meus pacientes.
Aos funcionários da Secretaria do Programa de Pós-graduação em Ciências da Saúde, em especial a Alana, que sempre foi atenciosa, me orientando com relação aos prazos e as normas a serem cumpridas.
Aos amigos do Laboratório de Radiofarmácia Experimental do Departamento de Biofísica e Biometria do Instituto Roberto Alcântara Gomes da Universidade do Estado do Rio de Janeiro, Márcia, Fernanda, Claudia e Daniele, molas mestras do laboratório. Aos alunos de iniciação científica: Eric e Marcela por fazerem parte dessa grande equipe.
Ao Tecnólogo em Radiologia, especialista em radiologia veterinária, Daniel Teixeira de Menezes, com a minha ausência na clínica veterinária, conduziu de forma brilhante todas as atividades internas e externas, dessa forma permitiu que eu
de radiofarmácia e desenvolvesse o projeto de mestrado. Minha eterna gratidão. Ao grande amigo Prof. Dr. Sebastião David dos Santos Filho, sem ele esse projeto não teria sido realizado. Sua amizade, paciência, dedicação e vontade de ajudar ao próximo fazem dele um ser humano impar, exemplo a ser seguido.
Sumário...viii
Lista de abreviações siglas e símbolos...x
Resumo……….………...………...xi
1. Introdução...1
2. Revisão de literatura...3
3. Artigos anexados...7
3.1. Artigo publicado...8
3.2. Artigo submetido ...21
4. Comentários, críticas e conclusões...42
5.Anexos (comitê de ética, carta de aceite)...46
6. Referências...48
7. Abstract...57
BC Blood Cell (célula sanguínea) ber base excision repair
reb reparo de excisão de bases C célula, celular
°C graus centigrados
cm centímetro
CAPES Coordenação de Aperfeiçoamento de Pessoal de Nível Superior CNPq Conselho Nacional de Desenvolvimento Científico e Tecnológico UFC unidades formadora de colônias
DNA Acido desoxirribonucléico
E. coli Escherichia coli
FAPERJ Fundação de Amparo a Pesquisa do Rio de Janeiro FI-C fração insolúvel da célula
FS-C fração solúvel da célula FI-P fração insolúvel do plasma FS-P fração solúvel do plasma
g grama
h hora
IBRAG Instituto de Biologia Roberto Alcantara Gomes kBq quilobecquerel
LB Luria Broth
LRE Laboratório de Radiofarmácia Experimental
mg miligrama ml mililitro
min minutos
mo molibdênio
mSv miliSievert NaCl cloreto de sódio
nm nanômetro
P plasma
PET tomografia por emissão de positron
% ATI porcentagem de radioatividade incorporada
PubMed base de dados da “U.S. National Library of Medicine” RBC células vermelhas do sangue
RL radical livre
rpm rotações por minuto SnCl2 cloreto estanoso Sn2+ íon estanoso
SPECT tomografia computadorizadapor emissão de fóton único Na99mTcO4 pertecnetato de sódio
99m
Tc tecnécio-99m TB Três bailarinas TCA ácido tricloroacético
culturas bacterianas, interferir na marcação de estruturas sanguíneas com tecnécio-99m (99mTc) e alterar a morfologia das hemácias. De acordo com as instruções do fabricante, a formula denominada de Três Bailarinas (TB) é sugerida para ser usada, como bebida, por pessoas que desejam ajustar o peso sem dieta. Os ingredientes dessa fórmula são a Cassia angustifolia e a Malva verticellate. Informações cientificas sobre TB não foram encontradas no indexador PubMed, e esse fato tem estimulado nossas investigações sobre seus efeitos biológicos. O objetivo deste estudo foi avaliar, em diferentes modelos experimentais, o efeito de um extrato aquoso de Três Bailarinas: (i) na sobrevivência de culturas de E. coli AB1157, ii) efeito do SnCl2 em culturas bacterianas, iii) na marcação das hemácias e proteínas plasmáticas e celulares com 99mTc e iv) na morfologia de hemácias de sangue de ratos Wistar. Os resultados encontrados demonstram que o extrato de TB não alterou a sobrevivência de cultura de E. coli AB1157 e aboliu o efeito letal do SnCl2 na sobrevivência dessa cultura bacteriana. Na marcação de estruturas sangüíneas com 99mTc o extrato de TB reduziu a percentagem de atividade (%ATI) referente ao 99m
Tc no compartimento celular e nas proteínas plasmáticas, mas não alterou a %ATI nas proteínas celulares. O extrato de TB não foi capaz de alterar a morfologia das hemácias. Os modelos experimentais realizados mostram a importância dos mesmos na avaliação de efeitos biológicos de agentes químicos, e contribui para um melhor entendimento das propriedades do extrato de Três Bailarinas. Esse trabalho abrange varias áreas do conhecimento, tais como: radiobiologia, botânica, fitoterapia e hematologia.
1 INTRODUÇÃO
O consumo e o uso de plantas medicinais vêm crescendo em todo mundo ao longo dos anos (1, 2, 3). Essas plantas são empregadas no tratamento de diversas alterações metabólicas (4).
Em relação ao produto denominado “Três Bailarinas” (TB), composto por
Cassia angustifolia, da família Fabaceae e Malva verticellata, da família Malvaceae,
segundo as instruções do fabricante (Truong Giang Corp., South El Monte, CA 91733, USA), é sugerido que extrato desse produto ajuda a perder peso sem necessidade de dieta .
Apesar da importância e uso das plantas medicinais, determinados efeitos biológicos, que poderiam acarretar reações adversas e mesmo tóxicas, são relatados o que estimula o uso de modelos experimentais que permitam uma melhor compreensão dos mecanismos de ação desses produtos naturais (5, 6, 7).
Modelos experimentais empregando culturas bacterianas são utilizados para avaliar a citotoxicidade e genotoxicidade de extratos vegetais (8, 9).
Estudos sobre o efeito de plantas medicinais na marcação de constituintes sanguíneos com tecnécio-99m (99mTc) (10, 11, 12, 13 ) e na morfologia de hemácias também têm sido realizados (14).
Na medicina nuclear, o cloreto estanoso (SnCl2) é freqüentemente usado como um agente redutor na marcação de estruturas moleculares e celulares com 99m
Tc para obtenção de radiofármacos. Apesar de seu uso, diversosautores relatam que o cloreto estanoso apresenta efeitos citotóxicos e genotóxicos (8,15, 16).
O efeito do SnCl2 também dependeria da presença de mecanismo de restauração (18). Determinados extratos vegetais diminuem ou abolem essa ação do cloreto estanoso (20, 21). Esse agente redutor também é capaz de promover a quebra de DNA plasmidial (22, 23, 24, 25, 26).
Os mecanismos de ação de TB ainda não estão bem esclarecidos, e até essa data não foi possível encontrar na literatura publicações de possíveis efeitos em culturas bacterianas e na radiomarcação de constituintes sangüíneos empregado-se o indexador PubMed (www.pubmed.com). Esse fato tem estimulado nossas investigações sobre os efeitos biológicos de um extrato de TB em modelos experimentais em diferentes níveis de organização biológica.
A hipótese dessa investigação é avaliar se o extrato de TB apresenta propriedades redoxi. O presente trabalho tem como objetivo avaliar, em modelos experimentais, o efeito de um extrato aquoso de Três Bailarinas (i) na sobrevivência de culturas de E.coli AB1157 (selvagem), (ii) efeito do SnCl2 em culturas bacterianas, (iii) na marcação dos constituintes sanguíneos com 99mTc, e (iv) na morfologia de hemácias isoladas de ratos Wistar.
2 REVISÃO DE LITERATURA
O consumo de produtos naturais vem aumentando ao longo dos tempos. É desejável que plantas medicinais possam curar muitas doenças e que não tenham toxicidade para o organismo (7, 27, 28, 30). A avaliação de forma mais cientifica dos efeitos biológicos dos produtos de origem vegetal foi progressivamente realizada através de diversas investigações ao longo dos anos (27, 28, 29).
Apesar da importância e uso de plantas medicinais, determinados efeitos biológicos que podem acarretar reações adversas e mesmo tóxicas têm sido descritos, o que estimula o desenvolvimento e implementação de estudos adicionais (7, 30). Modelos experimentais de fácil utilização e baixo custo que permitem a avaliação do efeito de extratos de produtos naturais ou drogas sintéticas têm sido propostos e importantes resultados vêm sendo obtidos.
A determinação da sobrevivência de culturas bacterianas na presença de extratos de plantas medicinais tem sido útil para a avaliação da possível citotoxicidade de produtos naturais (8, 17, 26).
Com relação aos estudos com culturas bacterianas, pesquisadores relatam potencial citotóxico e genotóxico do SnCl2 em culturas bacterianas de diferentes cepas de Escherichia coli. Cepas bacterianas com mutação em único gene, cujo produto, esteja envolvido com sistema de reparo do DNA são mais resistentes ao tratamento com cloreto estanoso do que cepas com mais de uma mutação (8, 9, 19, 54, 55, 60).
Uma das características do íon estanoso é o seu possível transporte através da membrana (41), isso poderia justificar seus efeitos tóxicos no interior da célula. Aceptores de radicais livres (como a tiouréia, benzoato de sódio e dipiridil) ou fitoterápicos (Cymbopogon citratus, Baccharis genistelloides, Maytenus ilicifolia e
Peumus boldus) aumentam a sobrevivência de culturas bacterianas, proficientes e
deficientes nos mecanismos de reparo de lesões induzidas no DNA, ao tratamento com SnCl2. O efeito protetor destes fitoterápicos ocorreria devido à presença de substâncias presentes nos extratos que atuariam como: (i) quelantes do íon estanoso, protegendo as células bacterianas contra a oxidação e diminuindo a geração de radicais livres, (ii) aceptores de radicais livres gerados pela oxidação do SnCl2, e/ou (iii) substâncias que oxidariam o íon estanoso, reduzindo os efeitos do SnCl2 (18).
A marcação de estruturas celulares ou moleculares de interesse biológico com tecnécio-99m (99mTc), empregadas como radiofármacos (radiobiocomplexos) (28) envolve a utilização do agente redutor cloreto estanoso (56). Constituintes sanguíneos, como hemácias, leucócitos, plaquetas e proteínas plasmáticas podem ser marcados com 99mTc (44, 62). Entre as aplicações das hemácias marcadas com 99m
(45, 59), medida do fluxo sangüíneo em artérias periféricas (46), localização de hemorragias gastrintestinais (47, 48) e avaliação da função excretora (49).
Um método de marcação de constituintes sangüíneos com 99mTc é utilizado também como modelo experimental (27, 28, 29). Esse modelo foi proposto e utilizado com sucesso para avaliação de efeitos biológicos de extratos de plantas medicinais (10,11, 12, 13, 27,31, 32, 33, 34, 35, 36,). Um dos métodos de marcação comumente empregado é a marcação in vitro. O sangue é isolado com anticoagulante, incubado com o extrato de planta medicinal, posteriormente com o íon estanoso e, após com íon pertecnetato.
Estudos sugerem que na marcação de hemácias com 99mTc, o ânion pertecnetato difundi-se para o espaço intracelular através da proteína da banda-3 por troca com os íons cloreto e/ou bicarbonato (20, 40). A proteína da banda-3 é uma proteína integral que constitui a principal molécula relacionada com o transporte de anions das hemácias. Estes fatos indicam que o processo de marcação ocorre em nível intracelular. O agente redutor, SnCl2, também parece ser transportado para o interior da hemácia por um sistema de transporte específico, o canal de cálcio (41, 42).
O mecanismo que envolve a ligação 99mTc-hemácias não é completamente entendido. Ainda assim, alguns autores sugerem que: (i) o íon estanho difunde-se para o interior das células, ligando-se ao componente celular, (ii) o íon pertecnetato se difunde livremente dentro e fora da célula, (iii) o íon pertecnetato, dentro da célula na presença do Sn+2 é reduzido e se liga principalmente à cadeia-beta da hemoglobina (43, 44).
encontrada ligada às proteínas plasmáticas (50, 51, 52). A marcação das proteínas plasmáticas e celulares no procedimento de marcação de hemácias com 99mTc pode ser avaliada através da precipitação das proteínas plasmáticas e celulares com ácido tricloroacético (53).
Estudos da alteração qualitativa da forma das hemácias de sangue de ratos
Wistar realizados através de esfregaços de sangue sobre uma lâmina para
microscopia de luz, também têm relevância na avaliação do efeito de extratos de plantas medicinais (57). As hemácias são células que sofreram o processo de extrusão de seu núcleo durante a diferenciação celular, o que facilita sua visualização na microscopia óptica (análise qualitativa), visto que não apresenta sistemas intracelulares de membranas (58).
3 ARTIGOS ANEXADOS
3.1 ARTIGO PUBLICADO
a - Effects of an aqueous extract of Three Ballerina on the survival of
Escherichia coli AB1157 cultures and in the action of stannous chloride. Aceito
para publicação em 2011, no periódico “The Journal of Medicinal Plants Research”, fator de impacto 0,879. Qualis internacional B.
3.2 MANUSCRITO SUBMETIDO
3.1 ARTIGO PUBLICADO
Separata
Journal of Medicinal Plants Research v. 5, suplemento 14, pp. 3256-3259, 2011.
Effects of an aqueous extract of Three Ballerina on the survival of
Escherichia coli AB1157 cultures and in the action of stannous chloride.
Full Length Research Paper
Effects of an aqueous extract of Three Ballerina on the
survival of Escherichia coli AB1157 cultures and in the
action of stannous chloride
Pinto N. S.1, Carmo F. S.2, Diniz C. L.3, Almeida D. S.3, Pereira M. O.1, Santos-Filho S. D.3*, Medeiros A. C.1 and Bernardo-Filho M.3,4
1
Programa de Pós-Graduação em Ciências da Saúde da Universidade Federal do Rio Grande do Norte, Natal, RN, Brasil.
2
Programa de Pós-Graduação de Ciências Médicas da Universidade do Estado do Rio de Janeiro, Rio de Janeiro, RJ, Brasil.
3
Laboratório de Radiofarmácia Experimental, Departamento de Biofísica e Biometria, Instituto Roberto Alcantara Gomes, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, RJ, Brasil.
4
Controladoria de Pesquisa, Instituto Nacional do Câncer, Rio de Janeiro, RJ, Brasil.
Accepted 1 April, 2011
The global interest in natural substances has increased.Three Ballerina (TB) is a natural formula that has medicinal properties. Scientific information about this formula is not found in the PubMed and this fact has stimulated investigations about its biological effects. An experimental model using a reducing agent, stannous chloride (SnCl2), was carried out. Although, SnCl2 is used in nuclear medicine to obtain technetium-99 m radiopharmaceuticals, it is capable to generate free radicals. The aim of this work was to evaluate the biological effects of an aqueous extract of TBon the survival of Escherichia coli (E. coli) AB1157 (wild type) cultures and to verify if this extract protect a E. coli culture against the action of SnCl2. E. coli AB1157 cultures (exponential growth phase) were incubated with TB extract (23.4 mg/ml) or 0.9% NaCl solution as a control. E. coli cultures were also incubated with SnCl2 (25 µg/ml) + TB extract (23.4 mg/ml) or SnCl2 (25 µg/ml) alone. Aliquots from these treatments were spread onto Petri dishes containing solidified LB medium. The colony-forming units (CFU) were counted after overnight and the survival fractions calculated. Data reveal that the TB did not alter the survival of the E. coli culture, however, it has protected the cells against the lethal effect of the SnCl2. In conclusion, the TB extract did not present cytotoxic effects, but it appears to have redox action.
Key words: Escherichia coli, three ballerina, stannous chloride, cytotoxic effect.
INTRODUCTION
The global interest in natural substances has increased over the world (Rotblatt and Ziment, 2002; Li et al., 2008; Monbaliu et al., 2010) and, there is a growing interest in the studies about various properties of medicinal and dietary plants (Bahramikia and Yazdanparast, 2008). The prevalence of obesity is growing worldwide and in the United States, it is estimated that this prevalence has reached almost 30% (Flegal et al., 2010). Due to the lack
*Corresponding author. E-mail: santos-filho@uerj.br Tel: +55-21-28688332.
“this special formula Dieters’ drink is all natural tea, soothing and relaxing especially delightful for those desiring to adjust weight, although this statement has not been evaluated by the Food and Drug Administration” (Soyuncu et al., 2008).
Different experimental models have been used to try to get scientific information about the natural products (Bahramikia and Yazdanparast, 2008; Li et al., 2008; Lima et al., 2002). Investigations to evaluate the citotoxicity of medicinal plants (Raphael et al., 2009; Almeida et al., 2007) have been performed. The effects of extracts of Baccharis genistellodes (Scheila et al., 2002) in the action of a reducing agent, the stannous chloride (SnCl2), have been assessed using Escherichia coil (E. coli) cultures.
SnCl2 is used in nuclear medicine to obtain
technetium-99m (99mTc) radiopharmaceuticals (Saha, 2010; Guedes et al., 2006). However, some deleterious effects of this substance have been described. In humans, it has been reported that it is highly irritant to the mucous membrane and skin, although it presents low systemic toxicity. In animals, it can produce stimulation or depression of the central nervous system. As for bacterial assays, SnCl2
appears to be capable of inducing and/or producing injuries in deoxyribonucleic acid (DNA), being considered as a potential genotoxic agent (Soares et al., 2004; Guedes et al., 2006). These effects may be, at least in part, attributed to free radicals (FR), generated during SnCl2 treatment (Bernardo-Filho et al., 1994; Dantas et
al., 1999; Mattos et al., 1999).
As the scientific publications about “3 Ballerina” was not found in the PubMed databank, (TB), we have investigated the effects of an aqueous extract of TBon the survival of E. coli AB1157 (wild type) cultures and if this extract protect a E. coli culture against the action of SnCl2.
MATERIALS AND METHODS
Strategy in the PubMed
A search using the key words“Three Ballerina “or “3 Ballerina” was performed in the PubMed (www.ncbi.nlm.nih.gov/sites/entrez) on March 15th 2011.
Preparation of TB extract
As the commercial extract of Three Ballerina (dried powder), (Truong Giang Corp. South El Monte, CA 91733) has only small solubility, a solution with 2.34 g of Three Ballerina extract was prepared with 100 ml of a hot (ebullition) 0.9% NaCl solution (saline). The preparation was centrifuged (clinical centrifuge, 2000 rpm, 15 min) and the supernatant was isolated. Then, the obtained solution was considered 23.4 mg/ml. Saline was used in all the dilutions. All experiments were carried out during the period of validity of this product. The quality control of the preparation of this extract was controlled by the optical density of 0.93 ± 0.01 obtained at 490 nm that was used as a marker and the quality control of each preparation of TB in the all experiments.
Preparation of the E. coli culture to be used
The E. coli AB1157, a wild-type strain, proficient to repair damage in the DNA, was used in this work and its characteristics are reported in Howard-Flanders et al. (1964). From stock (in glycerol 50% v/v) a sample (50 ȝl) of the culture was grown on liquid LB medium (5 ml, Luria and Burrous, 1957) at 37°C overnight on a shaking water bath (reciprocal water bath shaker, model R76, New Brunswick, USA) up to the stationary growth phase. A sample (200
ȝl) was taken from this culture and further incubated (20 ml, liquid LB medium) under the same conditions, for 2 h to obtain an exponential growth phase (exponential growth culture with approximately 108 cells/ml). The cells were collected by centrifugation, washed with 10 ml of saline and suspended again in the same solution until they reached 108 cells/ml.
Effect of the TB extract on the survival of E. coli culture
Samples (0.8 ml) of the washed cultures (108 cells/ml) were incubated on the shaking water bath with (1) 0.2 ml of saline, or (2) 0.1 ml of saline and 0.1 ml of the 3TB extract 23.4 mg/ml up to 60 min, at 37°C. During the assay, at 0 and 60 min, aliquots (0.1 ml) were taken and diluted with saline and spread onto Petri dishes containing solidified LB medium (1.5% agar). Colony-forming units (CFU) formed after overnight incubation at 37°C were counted and the survival fraction was calculated as described previously (Dantas et al., 1996). The survival fraction was calculated dividing the number of viable cells obtained per ml in each time of the treatment (N) by the number of viable cells obtained per ml in zero time (No).
Effect of the TB extract on the action of the stannous chloride in E. coli culture
Samples (0.8 ml) of the washed cultures (108 cells/ml) were incubated on the shaking water bath with (1) 0.1 ml of SnCl2 (25
µg/ml)and 0.1 ml of saline, or (2) 0.1 ml of the TB extract 23.4 mg/ml and 0.1 ml of saline, or (3) 0.2 ml of saline as control, on initial time and after 60 min, at 37°C. During the assay, at 0 and 60 min, aliquots (0.1 ml) were taken and diluted with saline and spread onto Petri dishes containing solidified LB medium (1.5% agar). CFU formed after overnight incubation at 37°C were counted and the survival fraction was calculated as described previously (Dantas et al., 1996). The survival fraction was calculated dividing the number of viable cells obtained per ml in each time of the treatment (N) by the number of viable cells obtained per ml in zero time (No).
RESULTS
The search that has been done in the PubMed has revealed that no items were found with the key words “Three Ballerina” or “3 Ballerina”.
Results shown in Figure 1 reveal that the TB was not capable to interfere on survival of the E. coli AB1157 culture up to 60 min with the treatment using a concentration of 23.4 mg/ml.
Figure 2 shows that the strong lethal effect of the stannous chloride has already described for different authors (Bernardo-Filho et al., 1994; Almeida et al., 2007). In addition, it is shown that the TB extract has a protective effect against the treatment with the SnCl2,
Time (min)
Survival fractions of E. coli AB1157 treated with 3 Ballerina extract
Figure 1. Absence of an effect of the TB extract on E. coli AB1157. Cells suspended in 0.9% NaCl were treated with TB extract. Survival fractions of E. coli AB1157 strain treated for different time of incubation in the presence or absence of the extract. The survival fractions were determined. Ƈ, 0.9% NaCl; ů, extract. N, number of viable cells obtained per ml in each time of the treatment; No, number of viable cells obtained per ml in zero time.
Survival fractions of E. coli AB1157 treated with 3 Ballerina extract and SnCl2
Time (min)
SnCl2
SnCl2 + Extract Control
Figure 2. Effectof the extract from TB on the inactivation induced by SnCl2 on E. coli AB1157. Cells
suspended in 0.9% NaCl were treated with SnCl. Survival fractions of E. coli AB1157 strain treated with SnCl2 for different time of incubation in the presence or absence of the extract and with the
extract alone. The survival fractions were determined. Ƈ, 0.9% NaCl; Ŷ, SnCl2; Ÿ, SnCl2 +extract. N,
number of viable cells obtained per ml in each time of the treatment; No, number of viable cells obtained per ml in zero time.
DISCUSSION
Due to the use of the medicinal plants, it is increasing around the world, and given the limited scientific information about the effect of many of them, it is highly relevant to try to assess biological properties of extract of
natural products. Concerning to the Three Ballerina, there are no publications in the PubMed. This fact stimulates scientific investigations about this formula.
in the conditions used in the experiments. This fact has already been demonstrated to another extracts, as cauliflower (Lima et al., 2002).
Although the stannous chloride has been used in the nuclear medicine (Saha, 2010), important deleterious effects associated to this reducing agent has been reported (Melo et al., 2001; Guedes et al., 2006; Souza et al., 2009). Another important finding of our work was demonstrated that the extract of Three Ballerina abolished the lethal effect of the stannous chloride (Figure 2). As the action of this reducing agent is related with the generation of free radicals, we can suggest that the TB extract has redox properties. The protective effect in E. coli AB1157 induced by the extract of TB against the inactivation produced by the treatment with SnCl2 was
also observed with Peumu boldus (Reiniger et al., 1999) and with Cymbbopogon citratus, Maytenus ilicifolia and
Baccharis genistelloides (Melo et al., 2001).
In conclusion, the findings in this work show that the chemical products present in extracts of TB, in the concentration used, are not toxic to the E. coli AB1157 culture. Moreover, as the TB extract has probably redox properties, it can prevent the generation of free radicals or act as a scavenger. Additional studies should be performed to try to elucidate the action mechanisms involved in the effects of TB extract obtained in this work.
ACKNOWLEDGEMENTS
The authors are grateful to the following CNPq, CAPES and UERJ for supporting this work.
REFERENCES
Almeida MC, Soares SF, Abreu PR, Jesus LM, Brito LC, Bernardo FM (2007). Protective effect of an aqueous extract of Harpagophytum procumbens up on Escherichia coli strains submitted to the lethal action of stannous chloride. Cell. Mol. Biol., (Noisy-le-grand) 53 Suppl: OL923-7.
Bahramikia S, Yazdanparast R (2008). Antioxidant and free radical scavenging activities of different fractions of Anethum graveolens
leaves using in vitro models. Pharmacol. Online, pp. 219-233. Bernardo FM, Cunha MC, Valsa JO, Caldeira DAA, Silva FCP, Fonseca
AS (1994). Evaluation of potential genotoxic of stannous chloride: Inactivation, filamentation and lysogenic induction of Escherichia coli. Food Chem. Toxicol., 32: 477-479.
Dantas FJS, Moraes MO, Carvalho EF, Valsa JO, Bernardo FM, Caldeira DAA (1996). Lethality induced by stannous chloride on
Escherichia coli AB1157: Participation of reactive oxygen species. Food Chem. Toxicol., 34: 959-962.
Dantas FJS, Moraes MO, Mattos JCP, Bezerra RJAC, Carvalho EF, Bernardo FM (1999). Stannous chloride mediates single strand breaks in plasmid DNA through reactive oxygen species formation. Toxicol. Lett., 110: 129-136.
Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, Chantre P, Vandermander J (1999). Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr., 70: 1040-1045.
Flegal KM, Carrol MD, Ogden CL, Curtin LR (2010). Prevalence and trends in obesity among US adults, 1999-2008. Ann. Pharmacother., 44: 1141-1151.
Guedes AP, Cardoso VN, De MJCP, Dantas FJS, Matos VC, Silva JCF, Bezerra RJAC, Caldeira DAA (2006). Cytotoxic and genotoxic effects induced by stannous chloride associated to nuclear medicine kits. Nucl. Med. Biol., 33: 915-921.
Howard-Flanders P, Simon E, Theriot T (1964). A locus that control filament formation and sensivity to radiation in Escherichia coli K-12. Genetics, 53: 237-246.
Li J, Li Q, Feng T, Li K (2008). Aqueous extract of Solanum nigrum inhibit growth of cervical carcinoma (U14) via modulating immune response of tumor bearing mice and inducing apoptosis of tumor cells. Fitoterapia, 79: 548-556.
Mattos DMM, Gomes LM, Freitas RS, Rodrigues PC, Paula EF, Bernardo FM (1999). Model to evaluate the biological effect of natural products: Vincristine action on the biodistribution of radiopharmaceuticals in Balb/c female mice. J. Appl. Toxicol., 19: 251-254.
Melo SF, Soares SF, da Costa RF, da Silva CR, de Oliveira MB, Bezerra RJ, Caldeira DAA, Bernardo FM (2001). Effect of the
Cymbopogon citratus, Maytenus ilicifolia and Baccharis genistelloides
extracts against the stannous chloride oxidative damage in
Escherichia coli. Mutat. Res., 496: 33-38.
Monbaliu S, Wu A, Zhang D, Peteghem CV, Saeger SD (2010). Multimycotoxin UPLC-MS/MS for tea, herbal infusion and the derived drinkable products. J. Agric. Food Chem., 58: 12664-12671.
Reiniger IW, Ribeiro DSC, Felzenszwalb I, De MJC, De OJF, Da SDFJ, Bezerra RJ, Caldeira DAA, Bernardo FM (1999). Boldine action against the stannous chloride effect. J. Ethnopharmacol., 68: 345-348.
Rotblatt PM, Ziment I (2002). Evidence-Based Herbal Medicine. Hanley and Belfus Philadelphia.
Saha GB (2010). Fundamentals of nuclear pharmacy. Sixth edition. Springer: New York.
Soares SF, Brito LC, Souza DE, Almeida MC, Bernardo LC, Bernardo FM (2004). Citotoxic effects of stannous salts and the action of
Maytenus ilicifolia, Baccharis genistelloides and Cymbopogon citratus
aqueous extracts. Braz. J. Biom. Eng., 20: 73-79.
Souza RS, Almeida MC, Manoel CV, Santos FSD, Fonseca AS, Bernardo FM (2009). Biological effects of an aqueous extract of Salix alba on the survival of Escherichia coli AB 1157 cultures submitted to the action of stannous chloride. Biol. Res., 42: 199-203
Soyuncu S, Cete Y, Nokay AE (2008). Portal vein thrombosis related to
Cassia angustifolia. Clin. Toxicol., (Phila), 46: 774-777.
3.2 MANUSCRITO SUBMETIDO
Russian Journal of Plant Physiology, fator de impacto 0,5. Qualis Internacional B
EVALUATION OF THE IN VITRO EFFECT OF A THREE BAILLERINA EXTRACT
ON THE LABELING OF BLOOD CONSTITUENTS OF RATS WITH
TECHNETIUM-99M AND ON THE MORPHOLOGY OF THE RED BLOOD CELL
Pinto NS1, Oliveira MP1, Carmo FS2, Diniz CL2, Missailidis S3, Santos-Filho SD2,
Medeiros AC1, Bernardo-Filho M2,4
1-Programa de Pós-Graduação em Ciências da Saúde, Universidade Federal do Rio
Grande do Norte, Natal, RN, Brasil; 2-Departamento de Biofísica e Biometria, Instituto
Roberto Alcantara Gomes, Universidade do Estado do Rio de Janeiro, Rio de Janeiro,
RJ, Brasil; 3- The Open University, UK; 4-Coordenadoria de Pesquisa, Instituto
Nacional do Câncer, Rio de Janeiro, RJ, Brasil.
Corresponding author:
Sebastião David Santos-Filho
Universidade do Estado do Rio de Janeiro
Instituto de Biologia Roberto Alcantara Gomes
Departamento de Biofísica e Biometria
Laboratório de Radiofarmácia Experimental
Av. 28 de setembro, 87, fundos, 4º andar
20551-030, Vila Isabel, Rio de Janeiro, RJ, Brasil
Phone/Fax: 55-21-2568-8332; Mobile: 55-21-8715-1371
Abstract
The aim of this work is to verify the effect of a TB extract on the labeling of blood
constituents with 99mTc and on the morphology of the red blood cells (RBC). Some
physical characteristics, as visible absorbance spectrum, electric conductivity, and
refractive index of this extract were also determined. Plasma (P) and blood cells (BC)
were labeling with 99mTc, and also isolated and precipitated with trichloroacetic acid
and soluble (SF) and insoluble fractions (IF) were separated. The %ATI in these
samples was calculated. The morphology of the treated RBC showed no shape’s
qualitative alterations. The TB extract was characterized with an absorbance peak at
490nm (0.93±0.01), electric conductivity of 1.35±0.04mSv/cm and refractive index of
2.21±0.15%BRIX. TB extract also decreased significantly (p<0.05) the radioactivity
distribution in cellular compartment of 96.97±1.30% to 88.48±7.13%, and in IF-P from
74.29±4.12 to 14.26±5.73%. In conclusion, some physical chemical parameters could
be suitable to characterize an extract of TB. Moreover, substances present in the TB
extract should probably have an effect on transport of the ions through the RBC
membrane and/or should have redoxi properties justifying the effect on the fixation of
the radioactivity on the plasma proteins.
Keywords: blood constituents, technetium-99m, red blood cell, Three Baillerina,
Introduction
Technetium-99m (99mTc) has been the most utilized radionuclide in the single
photon emission computed tomography (SPECT) (Harbert et al, 1996; Prech et al, 2006;
Rasilla et al, 2009; Schinkel et al, 2010) and it has also been used in basic research
(Burke et al, 2005; Pettersson et al, 2005; O'Connor et al, 2009; Gropler et al, 2010).
Chemical compounds or cellular structures (red blood cells-RBC, platelets and white
blood cells) have been labeled with this radionuclide and used as radiopharmaceutical
(radiobiocomplex) (Bernardo-Filho et al, 2005, Rasilla et al, 2009; Schinkel et al, 2010).
99mTc-labeled RBC scan is the nuclear study best suited for identifying
slow-bleeding sources as gastrointestinal slow-bleeding (Karacalioglu et al, 2003;
Manning-Dimmitt et al, 2005; Kiratli et al, 2009), as well as (i) in the determination of the left
ventricular function by measuring the ejection fractions (Leitha et al, 2001; Gropler et
al, 2010) and (ii) in the evaluation of wall motion abnormalities (Sampson, 1999; Prech
et al, 2006; Schinkel et al, 2010), in cardiovascular nuclear medicine. The RBC has
been labeled with 99mTc for in vitro, in vivo or in vivo/in vitro techniques. The labeled
process with 99mTc, as sodium pertechnetate, depends on a reducing agent and
stannous ion (Sn2+) is usually used for this purpose (Harbert et al, 1996; Oliveira et al,
2003; Moreno et al, 2005; Prech et al, 2006). When whole blood is used for the labeling
of RBC with 99mTc, radioactivity is mainly found on RBC, however it is also bound on
plasma proteins (Saha, 2004, Bernardo-Filho et al, 1990). Authors have reported that
some herbal medicines are capable to alter the labeling of blood constituents with
technetium99m (O’Neill et al, 1998; Oliveira et al, 2000; Lima et al, 2002; Moreno et
al, 2002; Oliveira et al, 2002; Diré et al, 2004; Santos-Filho et al, 2004; Santos-Filho et
Qualitative morphological analysis has been used as a method to evaluate if the
effects of drugs on this radiolabeling process could be related to changes of shape of
RBC (Benarroz et al, 2007).
The use of medicinal plants has grown in world (Rotblatt and Ziment, 2002;
Gullett et al, 2010), and the development and implementation of experimental models
(Júnior et al., 2005; Nakhai et al, 2007) are important to permit a better comprehension
of the action mechanisms of these natural products.
Three Baillerina (TB) is a formulae and experimental data suggest a great
pharmacologic potentiality of the substances that there are on it. The activities described
are laxative and against complement activity (Soyuncu et al., 2008; Tomoda et al.,
1992).
A useful physical chemical property to aid to characterize and to estimate the
purity or to determine the concentration of a substance or solution is the refractive index
(Castilho et al, 2006). Electric conductivity and the absorbance spectrum profile are
other physical parameters that could be measured and also used to characterize a
preparation of unknown composition, such as an extract of a medicinal plant (Néhémie
et al, 2007).
Publications about physical properties of TB extract were not found yet in
PubMed (www.pubmed.com). Moreover, since that the extract of TB can be used also
humans and several effects of this natural product are not well understood, the aim of
this work was to characterize some physical-chemical properties and to evaluate the
effect of TB aqueous extract on the labeling of blood constituents with 99mTc using an
in vitro experimental model, as well as to verify the consequences of this extract on the
morphology of the RBC.
Animals
The experiments were performed with rats maintained in a controlled
environment. The animals had free access to water and food and the ambient
temperature was kept at 25±2 °C. The experiments were carried out without sacrificing
the animals. Heparinized whole blood was withdrawn by cardiac puncture from adult
male Wistar rats (9 animals, 3 months of age, 250±15 g of weight). All the experimental
procedures have followed the Ethical Guidelines of the Instituto de Biologia Roberto
Alcantara Gomes, Universidade do Estado do Rio de Janeiro (Protocol
CEA/024/2009).
Preparation of TB extract
As the commercial extract of Three Baillerina (dried powder, Astron Comercial
LTDA, São Paulo, Brasil, Lot 1563) has only small solubility, a solution with 2.34 g
of Three Baillerina extract was prepared with 100 ml of a hot (ebullition) 0.9% NaCl
(saline). The preparation was centrifuged (clinical centrifuge, 2000 rpm, 15 min) and
the supernatant was isolated. Then, the obtained solution was considered 23.4 mg/ml
(100%). Saline was used in all the dilutions. All experiments were carried out during the
period of validity of this product.
Spectrophotometry of TB extract
Absorbance spectrum (Spectrophotometer, 800M, Analyser Comércio e
Indústria Ltda., São Paulo, Brazil) was determined with the TB extract (23.4 mg/ml)
prepared as described above in the range of 400–700 nm. Saline solution was used as
considered as the marker of the reproducibility of the conditions used to prepare all the
extracts utilized in the assays.
Electric conductivity of TB extract
Electric conductivity (mS/cm) of the TB extract was performed with a
conductivimeter (Marte Balanças e Aparelhos de Precisão Ltda., São Paulo). Saline
solution was used as the control.
Refractive index of TB extract
The refractive index (%BRIX) of TB extract was measured with a refractometer
(Ningbo Utech International Co. Ltd., Ningbo, People’s Republic of China) at room
temperature. Saline solution was also used as the control.
In vitro radiolabeling of blood constituents
Samples of 0.5 ml of whole blood were incubated with 100 µl of different
concentrations of diluted (0.9% NaCl) TB extract (6.25, 12.5, 25, 50 and 100%) for 1
hour at room temperature. A sample of heparinized whole blood was incubated with
saline solution (NaCl 0.9%) as control. All the tubes used in this experiment were
previously closed with a rubber cap and a syringe was used to reduce the air atmosphere
(vacuum) inside the vials. Then, 0.5 ml of a freshly prepared stannous chloride solution
(1.2 µg/ml), as SnCl2 (Sigma, USA) was added and the incubation continued for another
1 hour. After this period of time, 99mTc (0.1 ml with 370kBq), as sodium pertechnetate,
recently milked from a 99Mo/99mTc generator (Instituto de Pesquisas Energéticas e
Nucleares, Comissão Nacional de Energia Nuclear, Brasil), was added and the
(P) and BC cells were separated. Samples (20 µl) of P and BC were also precipitated
with 1 ml of trichloroacetic acid 5% and soluble (SF) and insoluble fractions (IF) were
separated. The radioactivity in P, BC, IF-P, SF-P, IF-BC and SF-BC were determined in
a well counter (Automatic Gamma Counter, Packard Instrument Co, USA). After that,
the % of radioactivity (%ATI) was calculated as described elsewhere (Oliveira et al,
2002; Oliveira et al, 2003).
Morphological evaluation of RBC
Preparations for morphological (microscopic) analyses were carried out with
blood samples treated in vitro with TB extract at different concentrations for 60 minutes
at room temperature or with saline solution as the control group. Blood smears were
prepared, dried, fixed, and staining by the May-Grünwald-Giemsa method (Barcia,
2007). After that, images of the RBCs were acquired (CANON, model Power Shot
SX200 IS) from blood smears to analyze the qualitative morphology by optical
microscopy (NIKON, model E 200, x 1000). Three independent researches with
expertise in analysis of blood smears have done a qualitative analysis of the RBC.
Analysis of the results
The data were analyzed using the GraphPad InStat (version 3.01 for Windows
95/NT, GraphPad Software, San Diego Ca, USA). Data from the analyses were tested
for any differences between treatments using one-way analysis of variance (ANOVA).
The means and standard errors of the means (mean ± SE) are also reported. Test with
Results
Physical chemical determinations, as, spectrum of absorbance, electric
conductivity and refractive index of the extract of TB were performed. Figure 1 shows
the spectrum of absorbance of the TB extract at higher concentration used (23.4mg/ml)
in the range of 400–700 nm. The data show an absorption peak of the extract
(0.93±0.01) at 490 nm. This value was considered as the marker of the reproducibility
of the conditions used to prepare the extracts used in the assays. The value of electric
conductivity (1.35±0.04mS/cm) of the extract at higher concentration was used as a
second marker of the reproducibility of the conditions used to prepare the extract. The
value of the refractive index (2.21±0.15%BRIX) of the extract at higher concentration
was used as a third marker of the reproducibility of the conditions used to prepare the
extract.
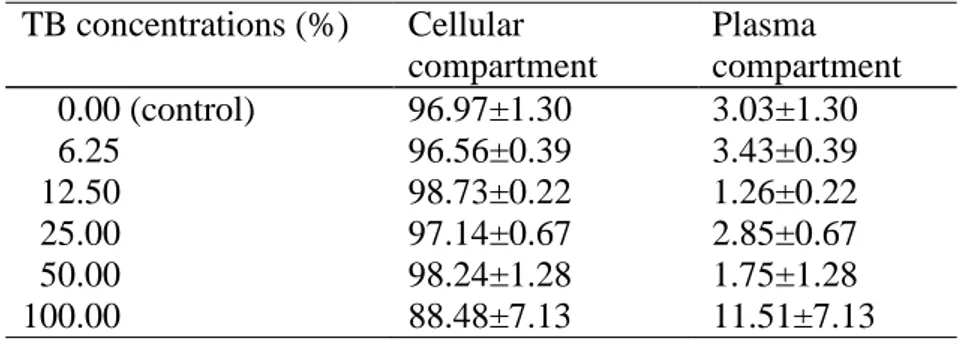
Table I shows the distribution of the radioactivity in the cellular and plasma
compartments isolated from whole blood treated with different concentrations of the TB
extract. The results indicate that there is a significant decrease (p<0.05) in the
distribution of the 99mTc in the cellular compartment from 96.97±1.30% to
88.48±7.13% in presence of TB extract.
Table II shows the distribution of the radioactivity on the soluble and insoluble
fractions of the cellular compartment isolated from whole blood treated with different
concentrations of the TB extract. There is no alteration in the 99mTc fixation by the
cellular proteins (IF-BC) in presence of TB extract.
Table III shows the distribution of the radioactivity in the soluble and insoluble
fractions of the plasma compartment isolated from whole blood treated with different
radioactivity fixation in the plasma proteins (IF-P) in presence of TB extract from
74.29±4.12 to 14.26±5.73%.
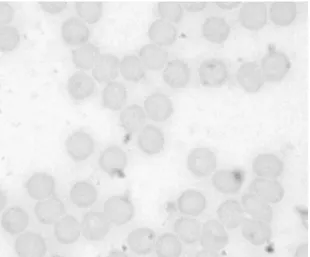
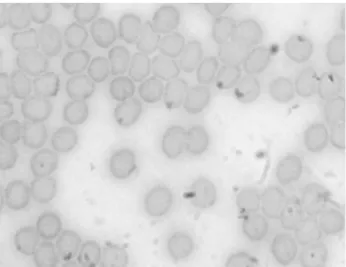
Figures 2 and 3 show photomicrographs of smears from blood treated with
saline solution (control) and with TB extract at the higher concentration used
(23.4mg/ml), respectively. The qualitative morphological analysis suggests that the
treatment with TB extract does not induce important changes in the shape of RBC
observed under optical microscopy.2
Discussion
To our knowledge, there is no description about physical chemical properties of
TB extracts. This fact has also stimulated this investigation, and a commercial TB was
used. This study shows that TB extract could be characterized by an absorbance
spectrum at 490 nm (Figure 1), an electric conductivity of 1.35±0.04mSv/ml and a
refraction index of 2.21±0.15%BRIX. These physical parameters could be used in other
studies to characterize the preparation conditions of an aqueous TB extract.
Nuclear medicine procedures as SPECT and PET have proven increasingly
effective imaging modalities in the study of several clinical disorders (Kumakura et al,
2004; Lodge et al, 2005; Palestro et al, 2005; Kiratli et al, 2009; Gropler et al, 2010).
Besides the disease, some authors have reported that these procedures could be altered
by medications that the patient is undergoing (Hesslewood and Leung, 1994). Natural
products could alter the biodistribution of radiobiocomplexes in a specific target or the
fixation of the radionuclide 99mTc to blood constituents (Sampson, 1999; Caprilles et
al, 2002; Moreno et al, 2002; Oliveira et al, 2002; Oliveira et al, 2003; Diré et al, 2004;
Fonseca et al, 2005). Other products are not capable to interfere with this labeling
as an in vitro assay to verify some important properties, as chelating/redox activities or
interactions on cellular membrane, of products used daily by humans (Benarroz et al,
2007; Fonseca et al, 2007).
Although, there are data concerning to the interaction of diagnostic agents,
including radiobiocomplexes, with therapeutic drugs (synthetic and natural),
investigations about these interactions are worthwhile. Knowledge about drug–
radiopharmaceutical interactions could contribute to reduce the risk of misdiagnosis
and/or repetition of the examinations (Saha, 2004) that could contribute to increase the
radiation dose to patients and staff in nuclear medicine. The development and use of in
vitro assays may provide important information about possible drug–
radiopharmaceutical interactions (Fonseca et al, 2007).
The analysis of the data presented in Tables 1 and 3 indicates that the TB extract
could alter the distribution of 99mTc between P and cellular compartments or the
fixation of this radionuclide on plasma proteins. In general, the labeling of blood
constituents could decrease because of action of drugs by (a) alteration of the plasma
membrane structure or modifying the transport systems of stannous and pertechnetate
ions into cells, (b) direct oxidation or generation of free radicals that could oxidize the
stannous ion, (c) direct inhibition (chelating action) of the stannous and pertechnetate
ions, or (d) binding at same sites on the blood constituents.
Interactions involved with the ion transport systems could alter the transport of
stannous and pertechnetate ions, decreasing the labeling of RBC with 99mTc and
explain, in part, the data obtained (Table 1). In fact, other researchers have
demonstrated that drugs that interact with calcium channels (Gutfilen et al, 1992) and
band-3 protein (Callahan and Rabito, 1990) to alter the labeling of RBC with 99mTc.
were not found when blood samples were incubated with TB extract (Figures 2 and 3),
suggesting that other mechanisms could be involved in the decrease of the labeling
efficiency of RBC with 99mTc (Table 1).
The redox properties of phenolic compounds present in medicinal plants have
been related to various mechanisms: free radical scavenging activity, transition metal
chelating, and singlet oxygen-quenching capacity (Shan et al, 2005). These substances
could act as chelators on stannous ions, decreasing the distribution of radioactivity in
blood cell compartment (Table 1) and the fixation of 99mTc on Plasma proteins (Table
3). In fact, experimental data have suggested antioxidant and pro-oxidant actions of
caffeine and its metabolites (Azam et al, 2003). Other data have suggested that caffeine
could alter the labeling of blood constituent at higher concentrations (Frydman et al,
2004). These findings and our morphological data from RBC could indicate that TB
extract could alter the labeling of blood constituents with 99mTc due to chelating/redox
properties of chemical compounds at the highest concentration of TB.
Although scientific information about the pharmacokinetics and
pharmacodynamics of the products present in TB is scarce, in general, the actions of
drugs has been shown to be depend on the plasma protein binding (Musteata et al,
2006). Despite previous data indicating that caffeine at low concentrations does not alter
the radiolabeling of plasma and cellular proteins (Frydman et al, 2004), other chemical
compounds in TB extract could decrease the fixation of 99mTc on these proteins (Table
3).
An unexpected finding is the no alteration of the labeling of the blood cell
proteins in presence of extract of TB (Table 2). Probably, the quantity of the TB that is
inside the RBC is not capable to interfere also on the fixation of the 99mTc on the blood
Conclusion
In conclusion, our data show some physical chemical parameters that could be
suitable to characterize an extract of TB. Moreover, substances present in the TB extract
should probably have an effect on transport of the ions through the RBC membrane
and/or should have redoxi properties and the stannous ion would decrease and could
justify the effect on the fixation of the radioactivity on the plasma proteins. Moreover,
although our experiments were carried out with animals, it is suggested precaution in
the interpretation of the examinations that use labeled blood constituents in patients who
are using TB extract.
References
-Azam S, Hadi N, Khan NU, Hadi SM: Antioxidant and prooxidant properties of
caffeine, theobromine and xanthine. Med Sci Monit 2003;9:BR325–BR330.
-Barcia JJ: The Giemsa stain: its history and applications. Int J Surg Pathol
2007;15:292–296.
-Benarroz MO, Fonseca AS, Rocha GS, Frydman JN, Rocha VC, Pereira MO,
Bernardo-Filho M: Cinnamomum zeylanicum extract on the radiolabelling of blood
constituents and the morphometry of red blood cells: in vitro assay. Appl Radiat Isot
2007;66:139–146.
-Bernardo-Filho M, Nogueira J, Sturm J, Boasquevisque E: Plasma proteins labelling
with 99mTechnetium. Braz. Arch. Biol. Technol. 1990;33, 811–817.
-Bernardo-Filho M, Santos-Filho SD, Moura EG, Maiworm AI, Orlando MMC, Penas
ME, Cardoso VN, Bernardo LC, Brito LC: Drug interaction with radiopharmaceuticals:
-Burke IT, Boothman C, Lloyd JR, Mortimer RJ, Livens FR, Morris K: Effects of
progressive anoxia on the solubility of technetium in sediments. Environ. Sci. Technol.
2005;39:4109–4116.
-Callahan RJ, Rabito CA: Radiolabeling of erythrocytes with technetium-99m: role of
band-3 protein in the transport of pertechnetate across the cell membrane. J Nucl Med
1990; 31:2004–2010.
-Caprilles PVSZ, Dias APM, Costa TEMM, Oliveira MBN, Faria MVC, Moura EG,
Abreu BAL, Bernardo-Filho M: Effect of eggplant (Solanum melongena) extract on the
in vitro labeling of blood elements with technetium-99m and on the biodistribution of
sodium pertechnetate in rats. Cell. Mol. Biol. 2002;48:771–776.
-Diré G, Gomes ML, Lima EAC, Jales RL, Faria MC, Bernardo-Filho M: Assessment
of a fruit extract (Sechium edule) on the labeling of blood elements with
technetium-99m. Afr. J. Biotechnol. 2004;3:484–488.
-Fernandes JFO, Brito LC, Frydman JNG, Santos-Filho S, Bernardo-Filho M: An
aqueous extract of Pfaffia sp. does not alter the labeling of blood constituents with
technetium-99m and the morphology of the red blood cells. Braz J Pharmacogn
2005;15:126–132.
-Fonseca AS, Frydman JNG, Santos R, Bernardo-Filho M: Influence of antipyretic
drugs on the labeling of blood elements with technetium-99m. Acta Biol Hung
2005;56:275–282.
-Fonseca AS, Frydman JN, Rocha VC, Bernardo-Filho M: Acetylsalicylic acid
decreases the labeling of blood constituents with technetium-99m. Acta Biol Hung
2007;58:187–198.
-Frydman JNG, Oliveira MBN, Santos AEO, Fonseca AS, Santos R, Bernardo-Filho M:
Pak J Biol Sci 2004;4:521–524.
-Gropler RJ, Beanlands RS, Dilsizian V, Lewandowski ED, Villanueva FS, Ziadi MC.
Imaging myocardial metabolic remodeling. J Nucl Med. 2010; 51:88S-101S.
-Gullett NP, Ruhul Amin AR, Bayraktar S, Pezzuto JM, Shin DM, Khuri FR, Aggarwal
BB, Surh YJ, Kucuk O. Cancer prevention with natural compounds. Semin Oncol.
2010;37:258-81.
-Gutfilen B, Ribeiro BLAR, Mattos MF, Ribeiro CR, Bernardo-Filho M: Labeling of
thymine with technetium-99m: suggestion of a chemical model. Arq Biol Tecnol
1996;39:69–74.
-Harbert JC, Eckelman WC, Neumann RD (1996): Nuclear Medicine Diagnosis and
Therapy. Thieme Medical Publishers, New York.
-Hesslewood S, Leung E: Drug interactions with radiopharmaceuticals. Eur. J. Nucl.
Med. 1994;21:348–356.
-Júnior VFV, Pinto A C. Plantas Medicinais: Cura Segura? Quim Nova 2005;28:519-28
-Karacalioglu O, Ilgan S, Arslan N, Ozguven M: Uterine doughnut in early proliferating
phase: potential pitfall in gastrointestinal bleeding studies. Ann. Nucl. Med.
2003;17:685–687.
-Kiratli PO, Aksoy T, Bozkurt MF, Orhan D. Detection of ectopic gastric mucosa using
99mTc pertechnetate: review of the literature. Ann Nucl Med. 2009; 23:97-105.
-Kumakura Y, Danielsen EH, Reilhac A, Gjedde A, Cumming P: Levodopa effect on
[18F]fluorodopa influx to brain: normal volunteers and patients with Parkinson’s
disease. Acta Neurol. Scand. 2004;110:188–195.
-Leitha T, Gwechenberger M, Pruckmayer M, Staudenherz A, Bailer H, Kronik G: Does
motion analysis in postexercise gated sestamibi SPECT reflect rest left ventricular
-Lima EAC, Diré G, Mattos DMM, Freiras RS, Gomes ML, Oliveira MBN, Faria
MVC, Jales RL, Bernardo-Filho M: Effect of an extract of cauliflower (leaf) on the
labeling of blood elements with technetium-99m and on the survival of Escherichia coli
AB1157 submitted to the treatment with stannous chloride. Food Chem. Toxicol.
2002;40:919–923.
-Lodge MA, Braess H, Mahmoud F, Suh J, Englar N, Geyser-Stoops S, Jenkins J,
Bacharach SL, Dilsizian V: Developments in nuclear cardiology: transition from single
photon emission computed tomography to positron emission tomography-computed
tomography. J. Invasive Cardiol. 2005;17:491–496.
-Manning-Dimmitt LL, Dimmitt SG, Wilson GR: Diagnosis of gastrointestinal bleeding
in adults. Am. Fam. Physician. 2005;71:1339–1346.
-Néhémie DT, Fotso, Sanonne, Dénis ON. Morpho-physical variation of fruits and
impact on almond production of djansang (Ricinodendron heudelotii Baill.) in west and
centre of Cameroon. Pak J Biol Sci. 2007;10:2838-43.
-Misra N, Sharma M, Raj K, Dangi A, Srivastava S, Misra-Bhattacharya S: Chemical
constituents and antifilarial activity of Lantana Camara against human lymphatic
filariid Brugia malayi and rodent filariid Acanthocheilonema viteae maintained in
rodent hosts. Parasitol. Res. 2007;100:439–448.
-Moreno SRF, Diré, G, Freitas RS, Farah MB, Lima-Filho GL, Rocha EK, Jales RLC,
Bernardo-Filho M: Effect of Ginkgo biloba on the labeling of blood elements with
technetium-99m: in vitro study. Rev. Bras. Farmacognosia 2002;12:62-63.
-Moreno SRF, Carvalho JJ, Nascimento AL, Pereira M, Rocha EK, Diré G, Arnobio A,
Caldas LQA, Bernardo-Filho M: Bioavailability of the sodium pertechnetate and
morphometry of organs isolated from Wistar rats: study of possible pharmacokinetic
issue):73–78.
-Musteata FM, Pawliszyn J, Qian MG, Wu JT, Miwa GT: Determination of drug plasma
protein binding by solid phase microextraction. J Pharm Sci 2006;95:1712–1722.
-Nakhai LA, Mohammadirad A, Yasa N, Minaie B, Nikfar S, Ghazanfari G, Zamani
MJ, Dehghan G, Jamshidi H, Boushehri VS, Khorasani R, Abdollahi M. Benefits of
Zataria multiflora Boiss in Experimental Model of Mouse Inflammatory Bowel Disease.
Evid Based Complement Alternat Med. 2007;4:43-50.
-O'Connor M, Rhodes D, Hruska C. Molecular breast imaging. Expert Rev Anticancer
Ther. 2009; 9:1073-80.
-O’Neill MJ, Lewis JA, Noble HM, Holland S, Mansat C, Farthing JE, Foster G, Noble
D, Lane SJ, Sidebottom PJ, Lynn SM, Hayes MV, Dix CJ: Isolation of
translactone-containing triterpenes with thrombin inhibitory activities from the leaves of Lantana
camara. J. Nat. Prod. 1998;61:1328–1331.
-Oliveira JF, Braga AC, de Oliveira MB, Avila AS, Caldeira-de-Araujo A, Cardoso VN,
Bezerra RJ, Bernardo-Filho M: Assessment of the effect of Maytenus ilicifolia
(espinheira santa) extract on the labeling of red blood cells and plasma proteins with
technetium-99m. J. Ethnopharmacol. 2000;72:179–184.
-Oliveira JF, Avila AS, Braga ACS, Oliveira MBN, Boasquevisque EM, Jales RL,
Cardoso VN, Bernardo-Filho M: Effect of extract of medicinal plants on the labeling of
blood elements with technetium-99m and on the morphology of red blood cells: I – a
study with Paullinia cupana. Fitoterapia 2002;73:305–312.
-Oliveira JF, Santos-Filho SD, Catanho MTJA, Srivastava SC, Lima-Filho GL,
Bernardo Filho M: Effect of extract of medicinal plants on the labeling of blood
elements with technetium-99m and on the morphology of red blood cells (RBC):
2003;18:52–56.
-Oliveira JFF, Brito LC, Frydman JNG, Santos-Filho SD, Bernardo-Filho M: An
aqueous extract of Pfaffia sp. does not alter the labeling of blood constituents with
technetium-99m and the morphology of the red blood cells. Rev. Bras. Farmacognosia
2005;15:126–132.
-Palestro CJ, Tomas MB, Tronco GG: Radionuclide imaging of the parathyroid glands.
Semin. Nucl. Med. 2005;35:266–276.
-Pettersson F, Vogt AM, Jonsson C, Mok BW, Shamaei-Tousi A, Bergstrom S, Chen Q,
Wahlgren M: Whole-body imaging of sequestration of Plasmodium falciparum in the
rat. Infect. Immun. 2005;73:7736–7746.
-Prech M, Grajek S, Marszalek A, Lesiak M, Jemielity M, Araszkiewicz A,
Mularek-Kubzdela T, Cieslinski A: Chronic infarct-related artery occlusion is associated with a
reduction in capillary density: Effects on infarct healing. Eur. J. Heart Fail.
2006;8:373–380.
-Rasilla JM, Arboniés JC, Gutiérrez LL. Bone SPECT-CT with 99mTc-labelled
bisphosphonates. Rev Esp Med Nucl. 2009; 28:307-8.
-Rotblatt M and Ziment I. 2002. Evidence-Based Herbal Medicine. Hanley & Belfus,
Philadelphia.
-Saha GB: Fundamentals of Nuclear Pharmacy. Springer-Verlag, New York, 2004, pp.
46–128.
-Sampson CB (1999): Textbook of Radiopharmacy Theory and Practice. Gordon and
Breach, Amsterdam
-Santos-Filho SD, Diré GL, Lima E, Oliveira MN, Bernardo-Filho M: Effect of Mentha
crispa (mint) extract on the labeling of blood elements with technetium-99m: A possible
-Santos-Filho SD, Bernardo-Filho M: Effect of Hypericum perforatum extract on in
vitro labeling of blood elements with technetium-99m and on biodisponsibility of
sodium pertechnetate in Wistar rats. Acta Cir. Bras. 2005;20:121–125.
-Schinkel AF, Valkema R, Geleijnse ML, Sijbrands EJ, Poldermans D. Single-photon
emission computed tomography for assessment of myocardial viability.
EuroIntervention. 2010; 6:G115-22.
-Shan B, Cai YZ, Sun M, Corke H: Antioxidant capacity of 26 spices extracts and
characterization of their phenolic constituents. J Agric Food Chem 2005;53:7749–7759.
-Soyuncu S, Cete Y, Nokay AE. Portal vein thrombosis related to Cassia angustifolia.
Clin Toxicol (Phila). 2008;46:774-7
-Tomoda M, Asahara H, Gonda R, Takada K. Constituents of the seed of Malva
verticillata. VIII. Smith degradation of MVS-VI, the major acidic polysaccharide, and
Tables
Table I: Effect of TB extract on the distribution of the radioactivity in the cellular and plasmatic compartments
TB concentrations (%) Cellular
compartment
Plasma compartment
0.00 (control) 96.97±1.30 3.03±1.30
6.25 96.56±0.39 3.43±0.39
12.50 98.73±0.22 1.26±0.22
25.00 97.14±0.67 2.85±0.67
50.00 98.24±1.28 1.75±1.28
100.00 88.48±7.13 11.51±7.13
Samples of heparinized blood were incubated with different concentrations of TB extract. A sample of heparinized whole blood was incubated with saline solution (NaCl 0.9%) as control. Then, stannous chloride (1.2µg/ml) and 99mTc, as sodium pertechnetate were added. The % ATI in plasma and cellular compartments were determined in a well counter and the percent of radioactivity was calculated.
Table II: Effect of TB extract in the labeling of soluble (SF) and insoluble (IF) fractions of the BC with 99mTc
TB concentrations (%) IF-BC SF-BC
0.00 (control) 93.45±7.64 6.54±7.64
6.25 96.49±1.69 3.50±1.69
12.50 98.26±0.72 1.73±0.72
25.00 95.45±4.67 4.54±4.67
50.00 97.66±0.99 2.33±0.99
100.00 96.43±1.95 3.56±1.95
Samples of heparinized blood were incubated with different concentrations of TB extract. A sample of heparinized whole blood was incubated with saline solution (NaCl, 0.9%) as control. Then, stannous chloride (1.2µg/ml) and 99mTc, as sodium pertechnetate were added. ATI% in IF-BC and SF-BC were determined in a well counter and the % of radioactivity was calculated.
Table III: Effect of TB extract in the labeling of soluble (SF) and insoluble (IF) fractions of the plasma (P) with 99mTc
TB concentrations (%) IF-P SF-P
0.00 (control) 74.29±4.12 25.70±4.12
6.25 72.11±0.84 27.88±0.84
12.50 71.38±0.23 28.61±0.23
25.00 8.64±7.59 91.35±7.59
50.00 9.58±8.95 90.41±8.95
100.00 14.26±5.73 85.73±5.73
Samples of heparinized blood were incubated with different concentrations of TB extract. Sample of heparinized whole blood was incubated with saline solution (NaCl, 0.9%) as control. Then, stannous chloride (1.2µg/ml) and 99mTc, as sodium pertechnetate were added. The %ATI in IF-P and SF-P were determined in a well counter and the percent of radioactivity was calculated.