ANTIBACTERIAL ACTIVITY OF Beta vulgaris L. POMACE EXTRACT
AХОФsКЧНrК S. ЏОХТćКЧsФТ*, DrКРШХjuЛ D. CvОtФШvТć, SТЧТšК L. MКrФШv, JОХОЧК J. ЏuХТć and Sonja M. Djilas
University of Novi Sad, Faculty of Technology, Bulevar cara Lazara 1, 21000 Novi Sad, Serbia Antibacterial activity of Beta vulgaris L. (beetroot) pomace extract (concentration 100 mg/ml) was tested against five Gram positive and seven Gram negative bacterial strains (reference cultures and natural isolates). Disc diffusiШЧ ЦОtСШН аТtС 15 µХ of extract and agar-аОХХ НТППusТШЧ ЦОtСШН аТtС 50 КЧН 100 µХ аОrО usОН. AЧtТЛТШtТМ (МОПШ -taxime/clavulanic acid) was used as a control sample. The tested extract showed the highest activity against Staphylococcus aureus and Bacillus cereus, where clear zones (without growth) appeared. There was no any activity against other tested Gram-positive bacteria, except for Staphylococcus epidermidis, with a small zone of reduced growth. Growth of all tested Gram-ЧОРКtТvО ЛКМtОrТК аКs ТЧСТЛТtОН usuКХХв аТtС 100 µХ of extract. The most susceptible were Citrobacter freundii and Salmonella typhymurium. The tested antibiotic gave clear, usually large zones for all tested strains except for Staphylococcus cohni spp. cohni, where only a zone of reduced growth appeared.
KEY WORDS: B tК vuХРКrТs L., beet root pomace extract, antibacterial activity, agar-well diffusion method
INTRODUCTION
Beta vulgaris L. (beetroot) is ranked among the ten most powerful vegetables with respect to antioxidant capacity ascribed to a total phenolic content of 50–60 mmol/g dry weight (1, 2, 3). It contains a significant amount of phenolics: catechin hydrate, epica-techin, ferulic, protocatechuic, vanillic, p-coumaric, p-hydroxybenzoic, caffeic and syrin-gic acids (4, 5). Beetroot is a potential source of valuable water-soluble nitrogenous pig-ments, called betalains, which are composed of two main groups, the red betacyanins and the yellow betaxanthins. In addition to their red color, betalains possess several desirable biological activities, including antioxidant, antiinflammatory, hepatoprotective, and anti-tumor properties (6, 7, 8). Previous investigations of antimicrobial activity of Beta vul-garis L. extracts showed its slight activity against tested strains, while Gram–positive bacteria were more resistant than Gram–negative bacteria and yeasts (9, 10, 11).
By-products of plant food processing represent a major disposal problem for the in-dustry concerned, but they are also promising sources of compounds which may be used because of their favourable technological or nutritional properties (12). These phytoche-micals from waste materials deriving from agro-industrial production may be used as functional food ingredients and as natural antioxidants to replace their synthetic equiva-lents that have experienced growing rejection.
Taking into account biological activities of beetroot as well as possible potential of plant by-products, in this study, beetroot pomace extract was used to screen antimicrobial activity to twelve bacterial strains. Among the species used, there were wild isolates and reference cultures. According to previous results, where Beta vulgaris L. extracts had a low activity, a larger beetroot pomace extract concentration or volume was needed, so different methods were used in this study: disc diffusion method with the limited capacity of discs and agar-well diffusion method with much higher volume of holes.
EXPERIMENTAL
Preparation of beetroot pomace extract
Beetroot (Beta vulgaris L.) аКs ШЛtКТЧОН ПrШЦ ГНrКvШ OrРКЧТМ, ЋОХОЧţК, ЋОrЛТК. Or -ganic beetroot was washed in running water, cut in pieces and the pulp was prepared using a domestic food processor (Neo SK-400). After juice separation, the sample of the obtained beetroot pomace was stored at -β0њC uЧtТХ КЧКХвsТs. Sample of beetroot pomace (120 g) was extracted for 30 min on an ultrasonic bath Branson model b-220 SmithKline Co., Shelton, USA (50/60 Hz, 125 W). The extraction was performed with a 50% ethanol aqueous solution containing 0.5% acetic acid (1200 ml). The obtained extract was eva-porated to dryness under reduced pressure. The yield, average of triplicate analysis, of the extract was 9.89±0.48 Р.
Sample for the determination of antibacterial activity
Sample for the determination of antibacterial activity was Beta vulgaris L. (beetroot) pomace extract, which was dissolved in distilled water to a concentration of 100 mg/ml.
Test microorganisms
Antibacterial activity
Antibacterial activity was determined by disc diffusion and agar-well diffusion meth-od (13). Bacterial strains were grown on MјОХХОr–Hinton agar (Himedia, Mumbai, India) 24 h at 37 0C. Cells were then suspended in a sterile 0.9% NaCl solution. 2 ml of the suspОЧsТШЧs ПШr ТЧШМuХКtТШЧ (1×106
cells/ml, estimated by Mc Farland nefelometer) were СШЦШРОЧТгОН аТtС 18 ЦХ ШП ЦОХtОН (4η њC) MјОХХОr–Hinton agar and poured into Petri dishes.
For disc diffusion method, sterile 6 mm discs (Himedia, Mumbai, India) were placed ШЧ tСО ТЧШМuХКtОН КРКr pХКtОs КЧН ТЦprОРЧКtОН аТtС 1η µХ ШП Обtract solution. The antibio-tТМ (МОПШtКбТЦО γ0 µР/МХКvuХКЧТМ КМТН 10 µР НТsМs, BТШКЧКХвsО®, Ankara, Turkey) was used as control. For the agar-well diffusion method, wells of 9 mm diameter were made with a sterile metal tube by means of a vacuum pump. The extract solution (50 and 100 µХ) аКs tСОЧ trКЧsПОrrОН ТЧtШ tСО аОХХs ШП ТЧШМuХКtОН КРКr pХКtОs. FШr ЛШtС ЦОtСШНs, tСО tОst pХКtОs аОrО rОПrТРОrКtОН Кt 8 њC ПШr 1 СШur tШ КХХШа tСО ОбtrКМt tШ НТППusО ТЧtШ tСО ЦОНТuЦ, КЧН tСОЧ аОrО ТЧМuЛКtОН Кt γ7 њC ПШr β4 Сours. After the incubation, the dia-meters of the inhibition zones were measured and recorded in millidia-meters (mm). The eva-luation of antibacterial activity was carried out in three repetitions.
RESULTS AND DISSCUSION
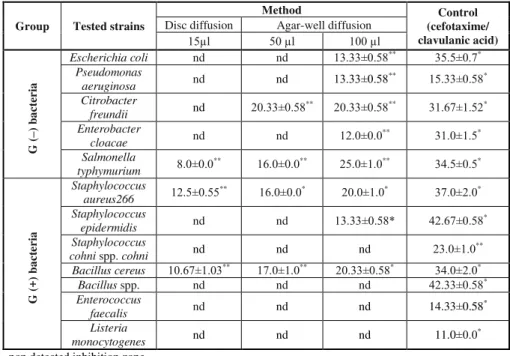
The results of antibacterial activity of beetroot pomace extract are presented in Table 1.
Table 1. Antibacterial activity of beetroot pomaceextract (diameter of the inhibition zonemean (mm) including disc (6 mm) or well (9 mm) ± stКЧНКrН НОvТКtТШЧ)
Group Tested strains
Method Control
(cefotaxime/ clavulanic acid) Disc diffusion Agar-well diffusion
1ηµХ η0 µХ 100 µХ
G ( – ) b a c te r ia
Escherichia coli nd nd 1γ.γγ±0.η8** γη.η±0.7*
Pseudomonas
aeruginosa nd nd 1γ.γγ±0.η8
** 1η.γγ±0.η8*
Citrobacter
freundii nd β0.γγ±0.η8
** β0.γγ±0.η8** γ1.θ7±1.ηβ*
Enterobacter
cloacae nd nd 1β.0±0.0
** γ1.0±1.η*
Salmonella
typhymurium 8.0±0.0
** 1θ.0±0.0** βη.0±1.0** γ4.η±0.η*
G (+ ) b a c te r ia Staphylococcus
aureus266 1β.η±0.ηη
** 1θ.0±0.0* β0.0±1.0* γ7.0±β.0*
Staphylococcus
epidermidis nd nd 1γ.γγ±0.η8* 4β.θ7±0.η8
*
Staphylococcus
cohni spp. cohni nd nd nd βγ.0±1.0
**
Bacillus cereus 10.θ7±1.0γ** 17.0±1.0** β0.γγ±0.η8* γ4.0±β.0*
Bacillus spp. nd nd nd 4β.γγ±0.η8*
Enterococcus
faecalis nd nd nd 14.33±0.58
*
Listeria
monocytogenes nd nd nd 11.0±0.0
*
nd - non detected inhibition zone * - clear zone around the disc/well ** - zone of reduced growth
Figure 1. Antibacterial activity of beetroot pomace extract against Bacillus cereus: a) disc diffusion method, b) agar well diffusion method
The inhibition zones of tested controls (cefotaxime/clavulanic acid discs) were sig-nificant, and for all bacteria clear zones around the discs appeared. The antibiotic showed
antibiotic
a) b)
the most expressive activity against Escherichia coli, Salmonella typhymurium, Staphylo-coccus aureus and Bacillus cereus (more than 30 mm). The least clear zones (less then 15 mm) appeared for Listeria monocytogenes and Enterococcus faecalis, while for Staphylo-coccus epidermidis only a zone of reduced growth appeared. Against these strains, the beetroot pomace extract did not show any activity, so, their higher resistance to antimic-robial substances is expected.
However, the absence of antibacterial activity or the presence of slight activity in the case of some bacteria do not indicate the absence of bioactive constituents, nor the plant inactivity. Namely, active components may be present in insufficient quantities to inhibit the growth of the cells. The lack of activity can thus only be proven by using large doses. Also, by applying higher extract quantities, some other constituents may be introduced which exert antagonistic effects or negate the positive effects of the bioactive agents (11). Koochak et al. (2010) tested the antibacterial activity of Beta vulgaris L. ethanolic extracts against ten bacterial clinical isolates by disc diffusion method using 50 µХ ШП extract solution (10). In all tested concentration (50, 100, 200 and 400 mg/ml) the extracts did not show considerable inhibitory activity against Gram–negative bacteria. Among Gram–positive bacteria, the most resistant strains were Listeria monocytogenes and Bacillus cereus, while significant antimicrobial activity was observed against Staphy-lococcus epidermidis. Rauha et al. (2000) tested the antimicrobial activity of methanolic extracts of Beta vulgarisL. Лв МвХТЧНОr НТППusТШЧ ЦОtСШН (η00 µХ ШП extract, 1 mg/ml) (9). Only slight antibacterial activity was obtained against Escherichia coli and Staphylococ-cus aureus, probably because of the use of a low extract concentration (1 mg/ml). In tes-tТЧР ШП 100 µХ ШП КquОШus Beta vulgaris L. leaf extract (disc diffusion method), Parekh and Chanda (2008) obtained a slight inhibitory activity against Staphylococcus aureus and Staphylococcus epidermidis, while ethanolic leaf extract (agar well diffusion method) inhibited growth of Staphylococcus subfava (11).
Although the same methods and extract solution volumes were tested in different studies, the beetroot pomace extract tested in this study showed the highest antibacterial activity. The observed differences are probably caused by the different susceptibility of tested natural isolates, as well as the composition and amount of active components ex-tracted from tested materials originated from different geographic areas.
CONCLUSION
МФЧШаХОНРОЦОЧt
Financial support of the Ministry of Education and Science of the Republic of Serbia (Project TR 31044) is highly acknowledged.
REFERENCES
1. Cao, G., Sofic, E. and Prior, R.L.: Antioxidant capacity of tea and common vege-tables. J. Agric. Food Chem. 44 (1996) 3426-3431.
2. KтСФöЧОЧ, M.Ј., HШpТК, A.I., VuШrОХК, H.J., ЊКuСК, J.Ј., ЈТСХКУК, K., KuУКХК, T.Ћ. КЧН Heinonen M.: Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 47 (1999) 3954-3962.
3. Vinson, J.A., Hao, Y., Su, X. and Zubik, L.: Phenol antioxidant quantity and quality in foods. J. Agric. Food Chem. 46 (1998) 3630-3634.
4. Georgiev, V. G., Weber, J., Kneschke, E., Denev, P. N., Bley, T. and Pavlov, A. I.: Antioxidant Activity and Phenolic Content of Betalain Extracts from Intact Plants and Hairy Root Cultures of the Beetroot Beta vulgaris cv. Detroit Dark Red. Plant Foods Human Nutrition 65, 2 (2010) 105-111.
5. Kujala, T.S., Loponen, J.M., Kika, K.D. and Pihlaja, K.: Phenolics and betacyanins in red beetroot (Beta vulgaris) root: distribution and effect of cold storage on the content of total phenolics and three individual compounds. J. Agric. Food Chem. 48 (2000) 5338-5342.
6. EsМrТЛКЧШ, J., ЈОНrОňШ, M.A., GКrМíК-CКrЦШЧК, F. КЧН MuňШг, Њ.: CСКrКМtОrТгКtТШЧ ШП the antiradical activity of betalains from Beta vulgaris L. roots. Phytochemical Ana-lysis 9 (1998) 124-127.
7. Kapadia, G. J., Azuine, M. A., Sridhar, R., Okuda, Y., Tsuruta, A., Ichiishi, E., Mu-kainake, T., Takasaki, M., Konoshima, T., Nishino, H. and Tokuda, H.: Chemopre-vention of DMBA-induced UV-B promoted, NOR-1-induced TPA promoted skin car-cinogenesis, and DENinduced phenobarbital promoted liver tumors in mice by extract of beetroot. Pharmacol. Res. 47 (2003) 141-148.
8. Winkler, C., Wirleitner, B., Schroecksnadel, K., Schennach, H. and Fuchs, D.: In vitro effects of beet root juice on stimulated and unstimulated peripheral blood mono-nuclear cells. Am. J. Biochem. Biotechnol. 1 (2005) 180-185.
9. Rauha, J-Ј., ЊОЦОs, Ћ., HОТЧШЧОЧ, M., HШpТК, A., KтСФöЧОЧ, M., KuУКХК, T., ЈТСХКУК, K., Vuorela, H. and Vuorela, P.: Antimicrobial effects of Finnish plant extracts conta-ining flavonoids and other phenolic compounds. Int. J.Food Microbiol. 56 (2000) 3-12.
10. Koockak, H., Seyyednejad, S.M. and Motamedi, H.: Preliminary study on the
anti-microbial activity of some medicinal plants of Khuzestan (Iran). Asian Pac. J. Trop. Med. 3 (2010) 180-184.
12.Schieber, A., Stintzing, F. C. and Carle R.: By-products of plant food processing as a source of functional compounds – recent developments. Trends Food Sci. Tech. 12 (2001) 401-413.
13.Mayo, W. J.: Chemical methods of control: Antimicrobial drugs, in Laboratory expe-riments in microbiology. Eds. Johnson, T. R. and Case, C. L., The Benjamin/Cum-mings Publishing Company, San Francisco (1998) pp. 179-181.
(Beta vulgaris L.)
. , . , . ,
. . Ђ
, , 1, β1000 ,
(Beta vulgaris L.) (
100 ЦР/ЦХ) – –
( ). -
( 1η µХ ) ) η0 µХ 100 µХ
. A (МОПШtКбТЦО/МХКvuХКЧТМ КМТН) .
Staphylococcus aureus Bacillus
cereus, ( ).
– , Staphylococcus
epidermi-dis, . –
100 µХ . ђ
Citrobacter freundii Salmonella typhymurium. (
-) Staphylococcus cohni spp. Cohni,
.
: Beta vulgaris L., О , К
, „ “