Vol-7, Special Issue-Number2-April, 2016, pp434-442 http://www.bipublication.com
Research Article
Effect of chronic noise exposure on neuron in the hippocampus
of wistar rats
Rezaei M1 , Sazegar GH2* and Homayoun M3
1
M.Sc Candidate of Anatomical Sciences, Department of Anatomical Sciences and Cell Biology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. E-Mail:
2
* Assistant Professor, Department of Anatomical Sciences and Cellular Biology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. Tel: +98 511 800 2483, Fax: +98 511 800 2484,
3
Phd Candidate of Anatomical Sciences, Department of Anatomical Sciences and Cell Biology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
*Corresponding Author; E-Mail: *SazegarGH@mums.ac.ir, mansure_rezaei@yahoo.com, mansour.homayoun @yahoo.com
ABSTRACT
Chronic noise exposure may lead to neural hippocampus apoptosis. Also stresses may result indark neuron. The aim of this study wasto determines effects of chronic noise on the hippocampus neurons of exposedrats.
Methods: In this study 16 male wistar rats were divided randomly into 2 groups, including exposure (N) and control (C) groups. 30 days after onset of examination, skulls of rats were opened to remove their brains. TUNEL and Toluidine blue techniques were used for examination of samples. The number of TUNEL positive and dark neurons in the hippocampus of both groups was counted and were compared.
Results:In comparison with control group, in hippocampus of exposed rats neural apoptosis and dark neurons were increased considerably (P<0.05).
Conclusion: Apoptosis and dark neuron in hippocampus of rats can be caused by exposure to chronic noise.
Key Words: Chronicnoise,broad band (white) noise, hippocampus, apoptosis, dark neuron.
INTRODUCTION
An inevitable factor that influences the body’s organs is stress [1,2]. According to the studies, noise is the topmost encountered stressor among various environmental stressors that effects animals and humans worldwide [2, 3]. In modern societies quick industrialization is the most important factor for raising of noise pollution that causes several problems such as civic and social health problems [4]. Workplace, traffic, and appliances and countless resources can produce noise [5]. The most basic problem is high levels occupational noise in throughout the world [6]. According to declaration of the National Institute for Occupational Safety and Health (NIOSH, 1998), more than 30 million American workers
are exposed to high risk noise[6]. Also, the World Health Organization (WHO, 1999) guesstimates that about 20% of the European are subjected to above 65 db noise that generated by urban traffic [6]. This level is considered as maximum healthy threshold and might be responsible for several disorders that affect structures belonging to the nervous, endocrine, and cardiovascular systems, in addition to auditory organs [6, 7].
noise increases the risk of physical damages and is considered as a health hazard [5, 9]. The World Health Organization (WHO) document, Guidelines for Community Noise, mentioned that exposure to noise can result in many detrimental effects, including both of auditory and non-auditory effects [4, 10]. In addition to psychological, physiological, or behavioural changes, living in a noisy environment affects sleep, work efficiency, performance, and communication ability of people [8, 11]. Recognizing and discriminating of the sound and its stress levels are the brain’s functions, as the essential organ that translates and responds to stimulants [1,12], It reacts forthwith to help the body to regulate stressful situations by releasing hormones [3]. Researchers revealthe levels of the stress hormones such as corticosterone increase due to noise exposureas a stressful factor [13]. Effects of noise stress are not sudden and catastrophic but gradual and insidious [3].Physiological systems of body involve in combating noise and it may lead to noise-induced impairments [3].
Reports have revealed that exposure to noise stress affects mental and behaviour performances [14].Studies have shown that occupational noise exposure has undesirability influences on the cognitive task performances of workers and it impairs cognitive and motivational functions of children such as attention, problem solving and memory [14, 15]. Researches on animals showed that noise exposure caused the damage of spatial memory [16]. Studies have reveal that exposure to noise alters the biogenic amine levels in the brain [2, 17]. Spatial learning and memory in particular, are tasks of the hippocampus [14].Auditory stimulus excites the hippocampus [18-21] and is affected by noise directly [22]. It has been proven that intense noise result in cell death of granule and pyramidal neurons [23], and it changes the firing patterns of place cells in hippocampus [24].Studies show noise exposure impairs hippocampal neurogenesis in the rat and may affect its neurotransmission [25]. Past studies revealed that hippocampus contents of glutamate increases by exposure to chronic noise
[14] and excitotoxicity of glutamate is associated with apoptosis of neurons[26].Apoptosis occurs in variety of physiological and pathological processes and is a controlled shape of cell death genetically [27]. After exposure to the chronic noise, TUNEL positive or apoptotic neurons were detected in hippocampus, this fact indicates that exposure to chronic noise cause a deleterious effect on the hippocampus neurons [4]. Hippocampus isposed in temporal lobe cortex and is a part of limbic system [27]. In CA1-3 pyramidal cell layer and DG granule cells TUNEL positive neurons were detected [4]. Previously, dark neurons were considered as histological artifacts in neurosurgical biopsies[28- 30], but later, the dark neurons were observed after brain trauma [28, 29, 31]. Recently, it has been reported that dark neurons are sometimes produced without any mechanical forces or trauma [28, 32, 33].Basophilic appearance and morphological changes are known as the dark neurons and might be seen due to hypoglycaemia, ischemia, stress and epilepsy [28, 32, 34, 35, 36]. Toluidine blue technique was used for identification of dark neurons.
This study was conducted to investigate effect of chronic noise exposure on neuron in the hippocampus of wistar rats.
MATERIAL AND METHODS Animal and grouping
Sixteen wistar strain male rats (Lab Animal Centre, Mashhad university of medical science, Iran) weighting 250-300g were used for this study. The animal were kept with relative humidity (50-70%), controlled ambient temperature(23±2°C) with a 12 hours light/dark cycle and a free access to water and food. For adaptation of animals, before onset of experiment they had kept in our laboratory for 5 days. The rats were divided into 2 groups randomly with 8 animals in each group. One group was test group and one group was control group.
intervention(c) the animals of the control group were not exposed to noise.
Noise exposure setup
Broad band (white) noise at 100db A intensity was used for this study. The sound was produced by NCH Tone Generator software and delivered to a pair loud speakers (Axtrom,SP400) fixed above animal plexiglass cage with the same distance from the four sides of square.
Noise level were measured by a sound level meter Norsonic 132 and kept at 100 db A intensity with background noise level below 35db A. The range of main spectrum of noise emission was from 100Hz to 20kHz.
Specimen preparation and histological methods
Animals were anesthetized by chloroform on the 31st day (for avoiding the acute noise effect of 30st day in exposed animals) [37] and their brain were removed immediately, rinsed in normal saline and fixed in 10% formaldehyde for 48 h at room temperature. After fixation the tissue were dehydrated with ethanol 70%, 80%, 90%, 100% (two changes) and cleared by xylene (two changes). For 90 minutes tissues were immerged in paraffin wax with two times changes at 56-58 °C. The paraffin blocks were cut by microtome into 5 µm thick, when the hippocampus was observed, for Toluidine blue staining, 20 slides were prepared.Also 6 sections were chosen and mounted on poly-L-lysine coated slides randomly (3 sections in each slide) for TUNEL technique.
TUNELstaining
For identification of apoptotic cell TUNEL technique was conducted. By using TUNEL reaction, DNA fragmentations in apoptotic cell nuclei were shown[38]In this study is used
TUNEL Kit (Roche, Germany). The tissues on poly-L-lysine coated slides were deparaffinized by xylene (3 changes), rehydrated by ethanol (100%, 90% and 70%), put in distilled water, washed in 0.1 M PBS for 15 min(with two changes) and put in proxidaze( 3% H2O2 in methanol) for 10 min in a dark place. Then they were rinsed in 0.1 M PBS for 15 min(with two changes), treated by 50 µg/ml proteinase K(Roche, Germany) for 20 min. After washing with PBS, specimens were incubated in the labeling reaction mixture containing terminal deoxynucleotidyl transferase and the deoxynucleotide mixture at 4°C overnight [38]. Then specimens were rinsed in PBS and incubated withPOD kit (Roche, Germany) for one hour. They were washed with PBS and treated with diaminobenzidine (DAB)
solution(0.03% diaminobenzidine and 200 µl
H2O2/100 ml PBS) for 15 min. Then tissue sample were washed with running water, put in hematoxylin for 2 min for contrasting. The specimens were washed with running water(3 times) and distilled water, dehydrated with ethanol(70%,90% and 100%), cleared with xylene(3 changes) and coverslip was stuck. Finally the specimens were observed under light microscope. Which dark brown nuclei were considered as apoptotic nuclei. The tissue section was treated with DNase I for positive control (Roche, Germany), and negative control sections were incubated with the labeling mix lacking the TdT enzyme [38].
Quantification of apoptotic cells
The samples were photographed using a light microscope with a 40× objective lens (UPlan FI, Japan), and images were conducted to a computer using a camera (BX51, Japan). TUNEL positive cells counted per unit area in hippocampus by morphometrical methods. The numbers of TUNEL positive cells were enumerated using imageJ software. The mean number of TUNEL positive cells per unit area (NA) was calculated using the following formula in rat’s hippocampus [38,39]:
NA=ΣQa/f. ΣP
In this formula, “ΣQ” is the summation of
sections, “a/f” is the area associated with each
frame of imageJ software , and “ΣP” is the sum
of frame associated points hitting space [38].
Toluidine blue staining
Toluidine blue technique was performed for identification of dark neurons. Using xylen (3 times changes) specimens were deparaffinised and rehydration was done by using ethanol (100%, 90% and 70%) and were rinsed in distilled water for 3 min. Tissue sections were stained with toluidine blue for 30 min, then washed with distilled water, dehydrated by ethanol(70%,90% and 100%), cleared by xylene(3 changes) and coverslips was stuck. Then they were observed under light microscope.
Quantification of dark neurons
The samples were photographed using a light microscope with a 40× objective lens (UPlan FI, Japan), and images were conducted to a computer using a camera (BX51, Japan). Dark neurons counted per unit area in hippocampus by morphometrical methods. The numbers of dark neurons were enumerated using imageJ software. The mean number of dark neurons per unit area (NA) was calculated using the following formula in rat’s hippocampus [38,39]:
NA=ΣQa/f. ΣP
In this formula, “ΣQ” is the summation of counted dark neurons appeared in sections, “a/f” is the area associated with each frame of
imageJ software, and “ΣP” is the sum of frame
associated points hitting space [38].
Statistical analysis
The acquired data from cells counting methods were reported as mean ± SE. Then they was compared among both groups by using t Test with SPSS 21software. Statistical significance set at P<0.05.
RESULT Apoptosis cell
Apoptosis is a phenomenon that control by genes.Itclassify by distinct morphological specifications, such as chromatin condensation, nuclear shrinkage, and oligonucleosomal DNA fragmentation, that can be recognized by
TUNEL method.The stain of apoptotic nuclei is dark brown [38]. There was a markedly increase of apoptotic cells in hippocampus in N group as compared with control (P<0.05, Figs. 1). Consequently, chronic noise exposure cause significant difference between noise and control groups from viewpoint of apoptotic cell in hippocampus of rats.
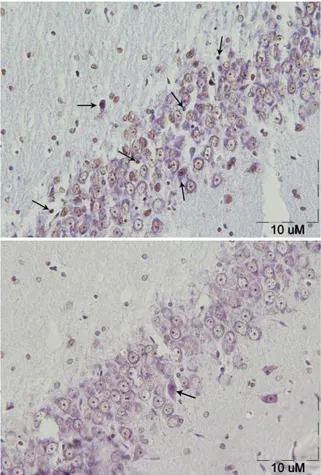
Figure 1. TUNEL staining in rat’s hippocampus of chronic noise exposure group(right photomicrograph) and control group(left photomicrograph). Arrows indicate apoptotic cell (magnification 40×).
chronic noise exposure group and control group(mean±SEM). Apoptotic cell numbers in noise group increased markedly comparing to control group (P<0.05).
Dark neuron
Basophilic appearance and morphological changes are known as the dark neurons and might be seen due to hypoglycaemia, ischemia, stress and epilepsy[28, 32, 34, 35, 36]. Toluidine blue technique was used for identification of dark neurons. There was a markedlyenhancement of dark neurons in hippocampus in N group as compared with control (P<0.05, Figs. 3). Consequently, chronic noise exposure cause significant difference between noise and control groups from viewpoint of dark neuron in hippocampus of rats.
Figure3. Toluidine blue staining in rat’s
hippocampus of chronic noise exposure group(right photomicrograph) and control group(left photomicrograph). Arrows indicate dark neurons (magnification 40×).
Figure 4. Comparison of dark neurons numbers per
unit area in rat's hippocampus of chronic noise exposure group and controlgroup(mean±SEM). Dark neurons numbers in noise group increased markedly comparing to control group (P<0.05).
DISCUSSION
[38]. It may originate either extracellular or intracellular, such as toxins, growth factors, hormones, nitric oxide. One of the most important regulators for apoptosis are members of Bcl-2 family proteins. 15 Bcl-2 family members have been recognized in mammalian cells. They act as a either pro-apoptotic Bax or anti-apoptotic Bcl-2regulators. The amount of anti- and pro-apoptotic proteins assesses how many cells respond to survival or apoptotic signals [27].
In this study, apoptotic neuron were observed
following chronic noise exposure in
hippocampus, suggesting that chronic noise exposure enforces a tortious effect on the neurons of hippocampus(P<0.05). A previous study has revealed that in hippocampus NR2B expression, tau hyperphosphorylation and apoptosis decrease by exposure to chronic noise. Decrement of NR2B expression can have correlation with neurons loss that induced by apoptosis [4]. It was reported that tau expression by activation of the JNK pathway result in promotion of neuronal apaptosis [41]. The Glu-NMDAR system abnormality and tau hyperphosphorylation may cause production of apoptotic cells, which in turn leads to neurocognitive deficit [4].
Seizures have clear relationship with pathological conditions in central nervous systemin both animal and human and some cases lead to progressive functional and structural abnormalities in CNS.Studies have been shown that seizure may causes morphological changes in neurons including dark neurons in brain tissue[42-47]. Previously, dark neurons were considered as histological artifacts in neurosurgical biopsies [28- 30], but later, the dark neurons were observed after brain trauma [28, 29, 31]. Recently, it has been reported that dark neurons are sometimes produced without any mechanical forces or trauma [28, 32, 33]. Basophilic appearance and morphological changes are known as the dark neurons and might be seen due to hypoglycaemia, ischemia, stress and epilepsy [28, 32, 34, 35, 36]. The fact that noise is a stressful stimulus is widely accepted [1, 2].
Noise induces stress in both of animals and humans, and disrupts the activity or balance of life [1,8]. Noise as a stressor agent has a major influence on the human health [1, 8]. Exposure to the noise for long-time increases the probability of physical damages and is mentioned as a health hazard [5, 9]. In this study, dark neuron were behold in hippocampus after chronic noise exposure, suggesting that chronic noise as a stressor may exert a tortious effect on hippocampus. In summary, this study indicates Long-term noise exposure may cause apoptosis and dark neurons in hippocampus of rat. However the mechanisms of chronic noise and its neural damage has not fully understood and it needs further study to clarify its cause and effect.
Acknowledgements
The data presented in this article is from a MSc. student thesis. This study has been supported financially by Vice Chancellor for Research, Mashhad University of Medical Sciences, Mashhad, Iran. Also, the authors would like to thank Mr. A.R. Ebrahimzadeh-Bideskan,Mrs. F. Motejadded and S.A. Moussavi-Najarkola for her excellent technical assistance.
REFERENCES
1. Saida Haider, Fizza Naqvi, Zehra Batool, Saiqa Tabassum, Tahira Perveen, Sadia Saleem, and Darakhshan Jabeen Haleem. Decreased Hippocampal 5-HT and DA Levels Following Sub-Chronic Exposure to Noise Stress: Impairment in both Spatial and Recognition Memory in Male Rats. Sci Pharm. 2012 December; 80(4): 1001– 1011.Published online 2012 October7. 2. Ravindran R, Rathinasamy SD, Samson J,
Senthilvelan M. Noise stress induced brain neurotransmitter changes and the effect of Ocimum sanctum (Linn) treatment in albino rats. J Pharmacol Sci. 2005;98:354–360. 3. Samson J, Sheeladevi R, Ravindran R,
4. Cui B, Wu MQ, Zhu LX, She XJ, Ma Q, Liu HT. Effect of chronic noise exposure on expression of N-methyl-D-aspartic acid receptor 2B and Tau phosphorylation in hippocampus of rats. Biomed Environ Sci. 2013 Mar; 26(3): 163-8.
5. Cui B, Zhu L, She X, Wu M, Ma Q, Wang T, Zhang N, Xu C, Chen X, An G, Liu H. Chronic noise exposure causes persistence of tau hyperphosphorylation and formation of NFT tau in the rat hippocampus and prefrontal cortex. Exp Neurol. 2012 Dec; 238(2): 122-9.
6. Uran SL, Caceres LG, Guelman LR. Effects
of loud noise
on hippocampal and cerebellarrelated behav iors. Role of oxidative state. Brain Res, 2010; 1361, 102-14.
7. Lenzi P., Frenzili G., Gesi M., Ferrucci M., Lazzeri G., Fornai F., Environ. DNA damage associated with ultrastructural alterations in rat myocardium after loud noise exposure. Health Perspect., 2003 Apr;111(4):467-71.
8. Tsai HY, Lu YH, Wu CR, Chen YF. Effects of noise on monoamine levels in the rat brain using in vivo microdialysis. Pflugers Arch. 2005;450:83–87.
9. Ising H, Kruppa B. Health effects caused by noise: evidence in them literature from the past 25 years. Noise Health 2004; 6: 5-13. 10.Brigitta B, Thomas L, Dietrich HS, Eds.
Guidelines for community noise. (Singapore): Institute of environmental epidemiology, Ministry of the Environment; 2000. pp. 25-36.
11.Cohen S. After effects of stress on human performance and social behavior: a review of research and theory. Psychol Bull. 1980;88:2–108.
12.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189.
13.Archana R, Namasivayam A. A comparative study on different crude extracts of Ocimum sanctum on noise stress. Phytother Res. 2002;16:579–580.
14.Cui B, Wu M, She X. Effects of chronic noise exposure on spatial learning and memory of rats in relation to neurotransmitters and NMDAR2B alterations in the Hippocampus. J Occup Health. 2009;51:152–158.
15.Stansfeld SA, Berglund B, Clark C, Lopez-Barrio I, Fischer P, Ohrström E, Haines MM, Head J, Hygge S, van Kamp I, Berry BF, RANCH study team Aircraft and road traffic noise and children’s cognition and health: a cross national study. Lancet. 2005;365:1942–1949.
16.Uygur EE, Arslan M. Effects of chronic stress on cognitive functions and anxiety related behaviors in rats. Acta Physiol Hung. 2010;97:297–306.
17.Samson J, Sheela Devi R, Ravindran R, Senthilvelan M. Biogenic amine changes in brain regions and attenuating action of Ocimumsanctumin noise exposure. Pharmacol Biochem Behav. 2006;83:67–75. 18.Bickford-Wimer PC, Nagamoto H, Johnson
R, Adler LE, Egan M, Rose GM, Freedman R.Auditory sensory gating in hippocampal neurons: a model system in the rat. Biol Psychiatry. 1990 Jan 15; 27(2):183-92. 19.Xi MC, Woody CD, Gruen E. Identification
of short latency auditory responsive neurons in the cat dentate nucleus. Neuroreport.1994 Aug 15; 5(13):1567-70.
20.Ehlers CL, Kaneko WM, Robledo P, Lopez AL.Long-latency event-related potentials in rats: effects of task and stimulus parameters. Neuroscience.1994 Oct; 62(3):759-69. 21.Sakurai Y.Coding of auditory temporal and
pitch information by hippocampal individual cells and cell assemblies in the rat. Neuroscience.2002;115(4):1153-63. 22.Kari Suzanne Kraus, Sucharita Mitra, Zarina
Jimenez, Sneha Hinduja, Dalian Ding, Haiyan Jiang, Li Gray, Edward Lobarinas, Wei Sun, and Richard J Salvi. Noise Trauma Impairs Neurogenesis in the Rat Hippocampus. Neuroscience. 2010 Jun 2;167(4):1216-26.
short-lasting impulse noise causes neuronal c-Jun expression and induction of apoptosis in the adult rat brain. Neurotrauma. 2002 Aug;19(8):985-91.
24.Goble TJ, Møller AR, Thompson LT.
Acutehigh-intensitysoundexposurealtersresponses of placecells in hippocampus.Hear Res. 2009 Jul;253(1-2):52-9.
25.Kraus KS, Mitra S, Jimenez Z, Hinduja S, Ding D, Jiang H, Gray L, Lobarinas E, Sun W, Salvi RJ. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience. 2010;167:1216–1226.
26.Gavrieli Y, Sherman Y, Ben-Sasson SA.
Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol, 1992; 119, 493-501.
27.Saeedi Borujeni MJ, Hami J, Haghir H, Rastin M, SazegarGh. Evaluation of Bax and Bcl-2 Proteins Expression in the Rat Hippocampus due to childhood Febrile Seizure. Iran J Child Neurol. Winter 2016; 10(1):53-60.
28.Mansouri S, Ataei ML, Hosseini M, Bideskan AR. Tamoxifen mimics the effects of endogenous ovarian hormones on repeated seizures induced by pentylenetetrazole in rats. Exp Neurobiol. 2013 Jun;22(2):116-23. doi: 10.5607/en.2013.22.2.116. Epub 2013 Jun 27.
29.Ooigawa H, Nawashiro H, Fukui S, Otani N, Osumi A, Toyooka T, Shima K. The fate of Nissl-stained dark neurons following traumatic brain injury in rats: difference between neocortex and hippocampus regarding survival rate. Acta Neuropathol. 2006;112:471–481.
30.Jortner BS. The return of the dark neuron. A histological artifact complicating contemporary neurotoxicologic evaluation. Neurotoxicology. 2006;27:628–634.
31.Cammermeyer J. Is the solitary dark neuron a manifestation of postmortem trauma to the brain inadequately fixed by perfusion? Histochemistry. 1978;56:97–115.
32.Kherani ZS, Auer RN. Pharmacologic analysis of the mechanism of dark neuron production in cerebral cortex. Acta Neuropathol. 2008;116:447–452.
33.File SE. Tolerance to the anti-pentylenetetrazole effects of diazepam in the mouse. Psychopharmacology (Berl) 1983;79:284–286.
34.Auer RN, Kalimo H, Olsson Y, Siesjö BK. The temporal evolution of hypoglycemic brain damage. I. Lightand electron-microscopic findings in the rat cerebral cortex. Acta Neuropathol. 1985;67:13–24. 35.Baracskay P, Szepesi Z, Orbán G, Juhász G,
Czurkó A. Generalization of seizures parallels the formation of "dark" neurons in the hippocampus and pontine reticular formation after focal-cortical application of 4aminopyridine (4-AP) in the rat. Brain Res. 2008;1228:217–228.
36.Ishida K, Shimizu H, Hida H, Urakawa S, Ida K, Nishino H. Argyrophilic dark neurons represent various states of neuronal damage in brain insults: some come to die and others survive. Neuroscience. 2004;125644.
37.Manikandan S, Srikumar R, Jeya Parthasarathy N, Sheela Devi R.Protectiveeffect of AcoruscalamusLINN on freeradicalscavengers and lipid peroxidation in discrete regions of brain against noise stress exposed rat.Biol Pharm Bull. 2005 Dec;28(12):2327-30.
38.Khordad E, Fazel A, Ebrahimzadeh Bideskan A. Iran Biomed J. The effect of ascorbicacid and garlicadministration on
lead-inducedapoptosis in
ratoffspring'seyeretina. 2013; 17 (4): 206-13.
39.Rajabzadeh AA, Ebrahimzadeh Bideskan AR, Haghir H, Fazel AR. Morphometrical study of polysialylated neural cell adhesion molecule positive cells in rat pups hippocampus following induction of seizure during pregnancy. Iran Biomed J. 2011 Oct;15(4):157–63.
deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J, 1993; 12, 371-7. 41.Matsuzawa A, Ichijo H. Stress-responsive
protein kinases in redox-regulated apoptosis signaling. Antioxid & Redox Signal, 2005; 7, 472-81.
42.Toth Z, Yan XX, Haftoglou S, Ribak CE, Baram TZ. Seizure-induced neuronal injury: vulnerability to febrile seizures in an immature rat model. J Neurosci. 1998;18:4285–4294.
43.Holmes GL. Seizure-induced neuronal injury: animal data. Neurology. 2002;59:S3–S6.
44.Sankar R, Shin DH, Liu H, Mazarati A, Pereira de Vasconcelos A, Wasterlain CG. Patterns of status epilepticus-induced neuronal injury during development and long-term consequences. J Neurosci. 1998;8393.
45.Weniger G, Boucsein K, Irle E. Impaired associative memory in temporal lobe epilepsy subjects after lesions of hippocampus, parahippocampal gyrus, and amygdala. Hippocampus. 2004;14:785–796. 46.Carreño M, Donaire A, Sánchez-Carpintero
R. Cognitive disorders associated with epilepsy: diagnosis and treatment. Neurologist. 2008;14:S26–S34.