_________________________________________________________ Journal of Experimental Biology and Agricultural Sciences
KEYWORDS
Seed-borne
Fungal infections
Germination mortality
Melia volkensii
Seedlings
ABSTRACT
A laboratory study was carried out at the School of Biological Sciences, Chiromo Campus, University of Nairobi, for identification of seed-borne fungi that emerge during germination of M. volkensii seeds. The study sought to determine the incidence, pathological symptoms and effects of the fungi on post-germination survival of seedlings. Mature seeds collected from wild trees in three agro-climatic zones in semi arid eastern Kenya were scarified by nipping and slitting of the testa and divided into two groups. The treatment group received a single pulse pre-treatment consisting of a 30-minute soak in 1% (m/v) Bavistin, while the control group was soaked in tap water. Aspergillus flavus and Rhizopus stolonifer infections emerged in the control group. A Mann-Whitney U test showed the control group as having significantly higher infections with both fungi (range of p values = 0.003 to 0.010) during and after germination than the fungicide-treated group. Post-germination seedling survival was also significantly enhanced by 31.66% in the treated group relative to the control (range of p values = 0.004 to 0.010). A single pre-treatment with the systemic fungicide Bavistin may be recommended for reduction of seedling mortality in M. volkensii during and after germination.
Mulanda ES*, Adero MO, Amugune NO, Akunda E and Kinyamario JI
School of Biological Sciences, University of Nairobi, P.O. Box 30197-00100 Nairobi, Kenya.
Received: July 23, 2013; Revision: August 17, 2013; Accepted: September 03, 2013 Available Online September 20, 2013.
INCIDENCE AND CONTROL OF SEED-BORNE FUNGAL INFECTIONS
ASSOCIATED WITH POST-GERMINATION MORTALITY IN SEEDLINGS OF
Melia
volkensii
GURKE
E-mail: emulanda123@yahoo.com (Mulanda ES)
Peer review under responsibility of Journal of Experimental Biology and Agricultural Sciences.
* Corresponding author
Journal of Experimental Biology and Agricultural Sciences, September - 2013; Volume – 1(4)
Journal of Experimental Biology and Agricultural Sciences
http://www.jebas.org
ISSN No. 2320 – 8694
_________________________________________________________ 1 Introduction
Melia volkensii Gurke (Meliaceae family), a drought-tolerant tree native to East Africa, is the most valuable timber tree species in the arid and semi-arid lands (ASALs) of eastern and coastal Kenya (Mwamburi et al., 2005). Over-exploitation for timber has greatly reduced natural populations of the species in its native habitat, creating urgent need for conservation (Runo et al., 2004; Hanaoka et al., 2012). One of the proposed conservation methods is domestication through on-farm commercial forestry (Muok et al., 2010).
The seeds of M. volkensii have germination difficulties due to mechanical dormancy which requires delicate and tedious physical scarification to break (Orwa et al., 2009). The seeds are listed amongst difficult seeds at the Kew Royal Botanic Gardens (2013). The germination difficulty is compounded by high post-germination mortality due to high susceptibility of seedlings to fungal infections (Kimondo & Kiamba, 2005).
Studies on the fungal pathogens involved and their effects on post-germination mortality of M. volkensii seedlings are very rare. The little knowledge available is at best anecdotal. A preliminary study of M. volkensii fungal diseases in nurseries and plantations was carried out by Njuguna et al., (2005) and reported the presence of leaf blights, shoot blights, powdery mildews, stem cankers, root collar rots (damping off), wilts, tip die back and branches die back diseases by Alternaria species,
Colletrotrichium species, Botryosphaeria species, Fusarium
species, and Phomopsis speciesrespectively. The present study sought to find out the types and incidence of seed-borne fungi that emerge during germination of M.volkensii seeds and their effects on post-germination survival.
The existence of endophytic fungi in woody plants is documented by Bills (1996). Geris et al., (2003) reported the presence of a total of 55 fungal isolates from symptomless, healthy roots, stems and fruits of Melia azedarach of the Meliaceae family. Amongst all the fungal genera Aspergillus
and Penicillium were reported as the most common fungal species for the plant. It is probable that seeds acquire endophytic fungi from the fruits.
2 Materials and Methods
2.1 Plant materials
Mature fruits were collected from wild M. volkensii trees of three native ecotypes i.e. Mavuria in Mbeere South District (upper Eastern Kenya), Katulani in Kitui Central District and Nguutani in Mwingi West District (lower Eastern Kenya). Collection of fruits was done in February 2013. Fruit maturity was determined by the use of two established field indicators of fruit ripening in this species; yellow-green to yellow colour and about 4 cm in length (Orwa et al., 2009).
The fruits were de-pulped to obtain the stony endocarps by thrashing and the endocarps dried in open sun for 7 days. Seeds were stored in the dried endocarps to avoid loss of viability as recommended by Kimondo & Kiamba (2005). Seeds obtained after cracking of endocarps were germinated within seven days of extraction.
2.2 Pre-treatment and germination
Seeds were germinated in sterile (autoclaved) river sand under laboratory conditions. After two days of germination, germinated seedlings were transferred to autoclaved soil, still under laboratory conditions, for more vigorous seedling growth. After ten days seedlings were transferred to the greenhouse of College of Biological Sciences, Chiromo campus, University of Nairobi. The complete study was carried out in stainless steel dishes of 16 cm upper rim diameter and 550 cm3 volumetric capacities.
Before germination all the collected seeds were scarified by nipping at the micropylar ends followed by 2 or 3 longitudinal slits in the testa as described by Muok et al., (2010). The scarified seeds were then soaked in cold water at ambient temperature for 48 hours, during which the water was changed at the interval of 24 hours to get rid of exudates of phenolics and other inhibitors. The treatment group was soaked for 30 minutes in 1% (m/v) of the systemic fungicide Bavistin (Active ingredient: carbendazim 50 DF) and rinsed severally by tap water before the 48 hour cold water pre-germination soak. The control group was soaked for 30 minutes in an equal volume of tap water before the 48 hour cold water soak.
The treatment group had 7 dishes and each dish had 30 pre-treated seeds, giving a total of 210 seeds. The control group had six dishes and each dish had 30 pre-treated seeds to give 180 seeds in total. In the control group there were 2 dishes for each of the three ecotypes while in the treatment group there were two dishes each for Mbeere and Kitui but three for Mwingi.
2.3 Growth conditions
Individual dishes were placed in separate transparent polythene bags of 30.5 cm base x 46 cm height. The bags were tied for trapping of warmth and humidity, as recommended by Kyalo (2005). They were then placed in an improvised polythene-covered growth chamber made up of a wooden framework of 55cm breadth, 57 cm height and 230 cm length mounted on a bench. The bags were arranged in a completely randomized design.
2.4 Measurement of Parameters
Emergence of the radicle was used as a criterion for germination. The germination response was measured 2 days after sowing. The number of seeds continuing to germinate was counted at each 2-day interval for 8 days. Counts and observations of the number of seeds with fungal infections, type of fungi and seedling mortality were also recorded at each 2 day interval for 8 days. Infected seedlings were observed up to 20 days. Identification of the fungi was based on macroscopic and microscopic characteristics, as suggested by Geris et al., (2003).
2.5 Imaging and data analysis
Macroscopic images of infected seeds and shoots were taken using a Sony digital camera (Model DSC-W390). Detailed images of the fungi were taken using a Keyence® (Z35) VHX Digital Scanning Microscope (x 100). Data from the three ecotypes were pooled into two groups of Bavistin-treated and control. The two sets of data were compared using the Mann-Whitney U test in SPSS version 17.0.
3 Results and Discussion
Germination was evident 2 days after sowing and was completed within 10 days, in agreement with the findings of Kimondo & Kiamba (2005). Two types of emergent seed-borne fungi were encountered in this study, Aspergillus flavus
and Rhizopus stolonifer, both with adverse effects on seedling health. A. flavus was the more frequent amongst these two fungi.
The incidences of A. flavus and R. stolonifer infection in fungicide-treated and untreated seeds are shown in Tables 1 and 2. Plates 1 and 3 (a & b) show M. volkensii seeds infected with A. flavus and R. stolonifer respectively. The microscopic structures of the two types of fungi are shown in plates 2 and 4.
Eight days after sowing, the median percentage incidence of A. flavus was 11.67 as compared to 0.00 of the fungicide-treated seeds, which represents a significantly higher incidence of the fungus (p = 0.005) (Table 1). The infection was evident on the seed surfaces from day 4 and then spread to emerging seedlings, with adverse effects on stems and leaves (Plates 1 and 6). A similar systemic spreading of Aspergillus infections through the stem has been reported in corn hybrids (Windham & Williams 2007). Mycock et al., (1990) reported that A. flavus was able to invade internal tissues of maize seedlings. In the present study, pre-treatment of seeds with 1% Bavistin significantly improved (p = 0.01) post-germination survival, with a median percentage survival of 83.33% on day 8 in contrast to 51.67% in the control group (Table 3). Although the difference in incidence of R. stolonifer between the treatment and control groups was smaller than that for A. flavus, it was still significant (p = 0.01) (Table 2). The median percentage incidence of R. stolonifer on day eight (5.00 %) was half of A. flavus (11.67 %), in the control groups (Tables 1 & 2). However, Rhizopus infections spread rapidly from the seeds into the germination substrate due to its dual parasitic and saprotophic modes.
_________________________________________________________
Plate 2 Scanning photomicrograph of Aspergillus flavus showing conidiophores with mature (A) and immature (B) vesicles. (Scale = 1mm).
This resulted in a dense mycelium of the fungus over the entire surface of the germination substrate that engulfed all the seeds and seedlings, with visible soft rot in the cotyledons, hypocotyls and roots (Plates 3a and b).
In the seedlings that managed to withstand the Rhizopus in early stages of germination, the fungus spread to the raised shoots and caused the leaves to fold into a ball (Plate 5). However, the damaging effects of Rhizopus on the seedlings through soft rot were more drastic in the initial stages of Plate 3a Untreated germinating M. volkensii seeds infected with
the seed-borne fungus Rhizopus stolonifer 17 days from date of sowing. Soft rot started in the cotyledons then spread to
hypocotyls and roots.
Plate 3b Sporulated mycelium of the seed-borne fungus Rhizopus stolonifer 20 days from date of sowing. The mycelium has spread to the germination substrate. Seedlings show progressive soft rot.
germination. Rhizopus spp are known to cause soft rot diseases in fresh fruits, flowers, bulbs, tubers and seedlings (Agrios, 2005). Shukla et al., (2006) also reported the first case of inflorescence, fruit and leaf rot by R. stolonifer in Rauvolfia serpentina (Apocynaceae) which presented with symptoms of wet rot leading to premature death of the infected parts.
A previous study of the endophytic fungi of Melia azedarach
L.by Geris et al., (2003) appears to suggest that Aspergillus
may be endophytic in the Melia species. Ruhul-Amin et al., (2009) found prevalences of 1.0 to 8.0% and 0.0 to 12.00% respectively for A. flavus and R. stolonifer on cucumber seeds, with the two fungi being among the most prevalent from a total of seven seed-borne pathogens.
These prevalence rates are in agreement with those encountered in the present study. In addition, Ruhul-Amin et al., (2009) reported that pre-treating the cucumber seeds with garlic tablet suspensions significantly reduced the prevalence of the seed-borne fungi, occurrence of abnormal seedlings and rotten seeds. The garlic-treated cucumber seeds also had a significant enhancement of germination. This supports the idea that pre-germination removal of seed-borne fungi can enhance germination and post-germination seedling survival in
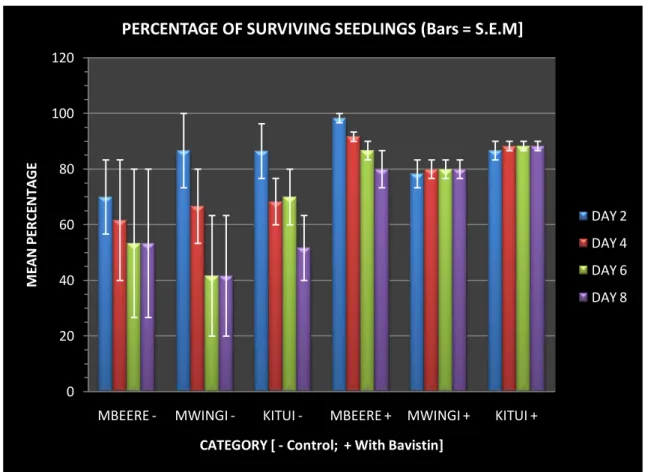
M.volkensii. Use of 1% Bavistin in this study greatly enhanced seedling survival (Table 3 and Figure 1). After of days of germination, the increases in mean percentage seedling survival in the treated seeds relative to controls were 26.67, 38.33 and 36.67 % for Mbeere, Mwingi and Kitui respectively (Figure 1).
Plate 4 Scanning photomicrograph showing sporangiophores and sporangia of Rhizopus stolonifer
from mycelium growing on M. volkensii seeds (scale = 1mm)
Plate 5 14-day old M. volkensii seedlings showing pathological symptoms of Rhizopus stolonifer infection in
the control group. Fungus displayed a dual saprotrophic and parasitic mode.
_________________________________________________________
Table 1 Incidence of A. flavus infections in germinating seeds of M. volkensii. Median percentages of seedlings with visible fungal infection.
Treatment Day 2 Day 4 Day 6 Day 8
Control† 0 5.00 8.34 11.67
Bavistin (1%)†† 0 0 0 0
Mann-Whitney U value 24 4.00 5.00 4.50
p value (6,8) 1.000 0.003 0.007 0.005
Significance N/S Significant Significant Significant
† 6 batches of seeds, 30 seeds per batch (N= 180 seeds). †† 8 batches of seeds, 30 seeds per batch (N = 240 seeds).
Table 2 Incidence of R. stolonifer infections in germinating seeds of M. volkensii. Median percentages of seedlings with visible fungal infection.
Treatment Day 2 Day 4 Day 6 Day 8
Control† 0 3.33 3.33 5.00
Bavistin (1%)†† 0 0 0 3.33
Mann-Whitney U value 16.00 8.00 1.000 3.000
p value (6,8) 0.090 0.010 0.004 0.010
Significance N/S Significant Significant Significant
† 6 batches of seeds, 30 seeds per batch (N= 180 seeds). †† 8 batches of seeds, 30 seeds per batch (N = 240 seeds).
Table 3 Effect of fungicide treatment on median post-germination seedling survival in M. volkensii.
Fungus treatment Day 2 Day 4 Day 6 Day 8
Control† 80.00 68.34 61.67 51.67
Bavistin (1%)†† 96.67 90.00 83.33 83.33
Mann-Whitney U value 9.5 1.000 1.000 3.000
p value (6,7) 0.094 0.004 0.004 0.010
Significance N/S Significant `Significant Significant
† 6 batches of seeds, 30 seeds per batch (N= 180 seeds). †† 7 batches of seeds, 30 seeds per batch (N= 210 seeds).
Conclusions and Recommendations
The seed-borne fungi A. flavus and R. stolonifer were found to be associated with post-germination seedling mortality in M. volkensii. The current practice of using polythene covers to maintain high humidity and warmth for both laboratory and field germination encourages growth of fungal pathogens.
Pre-germination removal of the seed borne fungi can be achieved using a single pre-treatment with 1% Bavistin on scarified seeds. This is a simpler method of controlling the
fungal infections in the field and on farms than soil sterilization and repeated fumigation.
Acknowledgement
Figure 1 Effect of Bavistin treatment on post-germination survival in M. volkensii.
References
Agrios GN (2005) Plant pathology. Elsevier Academic Press, USA.
Bills GF (1996) Isolation and analysis of endophytic fungal communities from woody plants. In: Endophytic fungi in grasses and woody plants: systematic, ecology and evolution. APS Press pp 31- 65.
Geris dos Santos RM, Rodrigues-Fo E, Rocha WC, Teixeira MFS (2003) Endophytic fungi from Melia azedarach. World Journal of Microbiology & Biotechnology. 19: 767 – 770.
Hanaoka S, Muturi GM, Watanabe A (2012) Isolation and characterization of microsatellite markers in Melia volkensii
Gurke. Conservation Genetic Resources 4: 395-398.
Kew Royal Botanic Gardens (2013) Kew’s difficult seeds
project- Melia volkensii.
www.kew.org/science-research-data/kew-in-depth/difficult-seeds/species-profiles/melia-volkensii. Accessed on 10 June 2013.
Kimondo JM, Kiamba K (2005) Overview of natural distribution, propagation and management of Melia volkensii. In: Kamondo BM, Kimondo JM, Mulatya JM, Muturi GM (eds.) Recent Mukau (Melia volkensii Gurke) Research and Development. Proceedings of the First National Workshop, Kenya Forestry Research Institute (KEFRI), Kitui Regional Research Center, November 16 to 19, 2004, pp 7 – 11.
Kyalo E (2005) An overview of Melia volkensii propagation at Tiva nursery, Kitui District. In: Kamondo BM, Kimondo JM, Mulatya JM, Muturi GM (eds.) Recent Mukau (Melia volkensii Gurke) Research and Development. Proceedings of the First National Workshop, Kenya Forestry Research Institute (KEFRI), Kitui Regional Research Center, November 16 to 19, 2004, pp 23 - 24.
Muok B, Mwamburi A, Kyalo E, Auka S (2010) Growing
Melia volkensii- A guide for farmers and tree growers in the drylands. Kenya Forestry Research Institute (KEFRI) Information Bulletin no.3. Nairobi, Kenya. p.20.
Mwamburi A, Kimondo JM, Kyalo E. (2005). Traditional methods used by farmers to break seed dormancy in Melia volkensii in Eastern and Coastal provinces of Kenya. In:
0
20
40
60
80
100
120
MBEERE -
MWINGI -
KITUI -
MBEERE +
MWINGI +
KITUI +
M
E
A
N
P
E
R
C
E
N
T
A
G
E
CATEGORY [ - Control; + With Bavistin]
PERCENTAGE OF SURVIVING SEEDLINGS (Bars = S.E.M]
DAY 2
DAY 4
DAY 6
_________________________________________________________
Kamondo BM, Kimondo JM, Mulatya JM, Muturi GM (eds.) Recent Mukau (Melia volkensii Gurke) Research and Development. Proceedings of the First National Workshop, Kenya Forestry Research Institute (KEFRI), Kitui Regional Research Center, November 16 to 19, 2004, pp 28 – 31.
Mycock DJ, Rijkenberg FH, Berjak P (1990) Infection of maize seedlings by Aspergillus flavusvar columnaris var nov.
Journal Seed Science and Technology18: 693 – 701.
Njuguna JW, Mwangi L, Gibera L (2005) Appraisal of Melia volkensii diseases and their control in Makueni and Kitui districts. In: Kamondo BM, Kimondo JM, Mulatya JM, Muturi GM (eds.) Recent Mukau (Melia volkensii Gurke) Research and Development. Proceedings of the First National Workshop, Kenya Forestry Research Institute (KEFRI), Kitui Regional Research Center, November 16 to 19, 2004, pp 53 – 55.
Orwa C, Mutua A, Kindt R, Jamnadass R, Antony S (2009) Agroforestree Database: a tree reference and selection guide version 4.0. Melia volkensii. World Agroforestry Centre,
Kenya. http://www.worldagroforestry.org/treedb2/AFTPDFS/ Melia_volkensii. pdf. Accessed on 10 June 2013.
Ruhul Amin ABM, Rashid MM, Meah MB (2009) Efficacy of garlic tablet to control seed-borne fungal pathogens of cucumber. Journal of Agricultural Rural Development7: 135-138.
Runo MS, Muluvi GM, Odee DW (2004) Analysis of genetic structure in Melia volkensii (Gurke) populations using random amplified polymorphic DNA. African Journal of Biotechnology 3: 421 -425.
Shukla RS, Alam M, Sattar A, Khaliq A, Singh HN (2006). First report of Rhizopus stolonifer causing inflorescence and fruit rot of Rauvolfia serpentina in India. European and Mediterranean Plant Protection Organisation Bulletin36: 11 – 13.