Universidade Federal do Rio Grande do Norte Centro de Biociências
Programa de Pós-Graduação em Ecologia (PPgECO)
Causas e consequências da onivoria de peixes em ecossistemas
aquáticos.
Danyhelton Douglas Farias Dantas
Orientador: Prof. Dr. José Luiz Attayde, UFRN Co-orientador: Prof. Dr. Ronaldo Angelini, UFRN
Causas e consequências da onivoria de peixes em ecossistemas
aquáticos.
Danyhelton Douglas Farias Dantas
Natal - RN
2015
Tese apresentada ao programa de Pós-graduação em ecologia da Universidade Federal do Rio Grande do Norte, como parte dos requisitos necessários para a obtenção do grau de doutor Ecologia.
Orientador: Prof. Dr. José Luiz de Attayde
Catalogação da Publicação na Fonte. UFRN / Biblioteca Setorial do Centro de Biociências
Dantas, Danyhelton Douglas Farias.
Causas e consequências da onivoria de peixes em ecossistemas aquáticos / Danyhelton Douglas Farias Dantas. – Natal, RN, 2015.
79 f.: il.
Orientador: Prof. Dr. José Luiz de Attayde. Coorientador: Prof. Dr. Ronaldo Angelini.
Tese (Doutorado) – Universidade Federal do Rio Grande do Norte. Centro de Biociências. Programa de Pós-Graduação em Ecologia.
1. Onivoria – Tese. 2. Metabolismo. – Tese. 3. Estrutura trófica. – Tese. I. Attayde, José Luiz de. II. Angelini, Ronaldo. III. Universidade Federal do Rio Grande do Norte. IV. Título.
Causas e consequências da onivoria de peixes em ecossistemas
aquáticos.
Danyhelton Douglas Farias Dantas
Rosemberg Menezes, Dr.
Kermal Ali Ger, Dr.
Prof. Hugo Sarmento, Dr.
Prof. Rafael Guariento, Dr.
Prof. José Luiz de Attayde, Dr.
Natal - RN 2015
Tese apresentada ao programa de Pós-graduação em ecologia da Universidade Federal do Rio Grande do Norte, como parte dos requisitos necessários para a obtenção do grau de doutor Ecologia.
Orientador: Prof. Dr. José Luiz de Attayde
E pt ou i d.
Be formless, shapeless, like water. Now, you put water into a cup, it
becomes the cup. If you put water into a bottle, it
becomes the bottle. If you put water into a tea pot, it
becomes the tea pot. Now, water can flow or it can
crash. Be water my frie d.
(Bruce Lee).
Results were fo d through direct e peri e tatio = We pla ed
arou d with it u til it worked .
(PhD Comics)
Te ho os se tidos já dor e tes, O corpo quer a alma entende. Esta é terra de ninguém. Sei que devo resistir Eu quero a espada em minhas mãos.
Sou metal, raio relâmpago e trovão Sou metal, eu sou o ouro em seu brasão Sou metal, quem sabe o sobro do dragão.
Não me entrego sem lutar Tenho ainda coração. Não aprendi a me render Que caia o inimigo então.
E nossa história não estará pelo avesso Assim, sem final feliz Teremos coisas bonitas.
E até lá, vamos viver Temos muito ainda por fazer.
Não olhe pra trás. Apenas começamos. O mundo começa agora.
Apenas começamos.
Agradecimentos
Agradeço a meus pais pelo exemplo e as orientações que fizeram com que eu me tornasse quem sou hoje. Durante muito tempo eles foram “as costas que vi” enquanto trilhava o meu caminho.
Mais uma vez (e com certeza nunca a última) agradeço aos meus amigos e orientadores, José Luiz de Attayde (tio Coca) e Ronaldo Angelini, pelas ajudas e conversas profissionais, muitas vezes pessoais. Exemplos que vou sempre seguir. Obrigado por sempre estarem por perto.
Aos professores Adriano Calliman, Luciana Carneiro, Sergio Maia, Vanessa Becker, Adriana Carvalho, Michael Vanni :) e Maria González, por toda a ajuda e orientação nesses últimos anos, contribuindo diretamente para meu crescimento intelectual e profissional. Aos doutores Rosemberg Menezes (amigo de longas datas), Kermal Ali Ger, Hugo Sarmento, e Rafael Guariento, por aceitarem fazer parte da minha banca examinadora cedendo seu tempo e conhecimento para contribuir com o crescimento destes trabalhos.
Aos muitos amigos de trabalho que compõem nossa base de pesquisa pela ajuda e apoio, em especial aos alunos e profissionais do LEA (Laboratório de ecologia aquática), LARHISA (Laboratório de Recursos Hídricos e Saneamento Ambiental) e do Laboratório de Limnologia. A todos os amigos gringos (e os pseudogringos) que fizeram do curto tempo em Oxford uma experiência fascinante e enriquecedora.
Um muito obrigado (!!!!!!) mais que especial à Mariana (Maricota), Pablo (Ped...fofim), Leonardo (Carcará), Gabi (Gabeixon), Fabiana (Binha) e Maria (Marcolina). Sem dúvida o melhor time de amigos que uma pessoa pode querer. Melhor até que a Liga da Justiça/Vingadores. Espero que nos próximos anos possamos impulsionar, mais ainda, a nossa produção acadêmica. Força galera!!!!.
A coordenação do curso de Pós-Graduação em Ecologia da UFRN pelo apoio. A CAPES pela concessão das bolsas de doutorado e doutorado sanduíche.
Aos meus irmãos Diogo, Dagon e Lucas, por continuarem a me aturar nesses últimos anos marcados por tantas dificuldades e alegrias; “Passamos por tudo, que venham mais”. Mesmo um pouco distantes ainda estaremos perto. Sem vocês minha vida não teria graça ou sentido, Obrigado por me deixarem estar perto de vocês.
i
Resumo
A onivoria é uma estratégia alimentar comum entre os peixes tropicais, mas pouco se conhece sobre as possíveis causas e consequências deste padrão de comportamento alimentar. Neste trabalho, levantamos a hipótese de que os peixes tropicais tendem a se alimentar mais baixo nas redes alimentares para compensar a maior demanda energética, a qual aumenta com a temperatura da água e o tamanho do corpo do animal. A análise dos dados de 8172 espécies de peixes marinhos e de água doce do mundo, de regiões tropicais e temperadas, demonstrou que a posição trófica dos peixes não carnívoros diminui com o aumento do tamanho do corpo em regiões tropicais, mas não em regiões temperadas. Este padrão sugere que a maior demanda energética dos peixes tropicais maiores deve ter exercido uma pressão seletiva para a evolução da onivoria. Como consequência, a dinâmica trófica dos ecossistemas aquáticos tropicais deve apresentar padrões distintos aos observados em ambientes temperados, com implicações importantes para o manejo da qualidade da água e a restauração de ecossistemas aquáticos eutrofizados. Outra hipótese deste trabalho, é que os efeitos de peixes planctívoros onívoros sobre comunidades planctônicas tropicais dependem da composição estequiométrica dos produtores primários que, por sua vez depende da disponibilidade relativa de luz e nutrientes. Um experimento em mesocosmos manipulando a disponibilidade de luz e a presença de peixes planctívoros confirmou a hipótese de trabalho, sugerindo que a composição estequiométrica e consequentemente a qualidade dos recursos alimentares determinam a estrutura trófica das redes alimentares pelágicas em lagos tropicais. Finalmente, outro experimento em mesocosmos sugere que a remoção de peixes onívoros bentívoros deve ser mais eficaz do que a remoção de peixes onívoros planctívoros para a melhoria da qualidade da água de lagos e reservatórios tropicais. Este último experimento demonstrou que os peixes planctívoros onívoros aumentam a biomassa fitoplanctônica através do mecanismo de cascata trófica sem aumentar as concentrações de nutrientes na coluna d´água. Por outro lado, os peixes bentívoros onívoros, se alimentando de detritos e outros recursos bentônicos e excretando nutrientes na água, translocam nutrientes do sedimento para a coluna d´água, aumentando o aporte interno de fósforo e a biomassa fitoplanctônica através da sua interação com o sedimento. Portanto, o aporte interno de fósforo pode ser reduzido e a qualidade da água de lagos tropicais eutrofizados pode ser melhorada através da remoção de peixes bentívoros onívoros.
ii
Abstract
Omnivory is a predominant feeding strategy among tropical fishes, but knowledge about its causes and consequences of this pattern is scarce. In this study we hypothesized that tropical fish feed lower in food web as a way to compensate a higher energetic demand, which increases with increasing water temperature and body size. Information about 8172 freshwater and marine fish species from whole world, from tropical and temperate ecosystems, showed that the trophic position of non-carnivore fish decreases with increasing body size in tropical but not in temperate ecosystems. This result indicates that the higher energetic demand of large-bodied tropical fish should exert a selective force in favor of omnivory. As a consequence, trophic dynamics in tropical freshwater ecosystems should have different patterns comparing to temperate ones, with major implications for water management and restoration of eutrophic aquatic ecosystems. Another hypothesis of this work was that effects of tropical omnivorous planktivorous fish on planktonic communities depend of primary producers stoichiometric composition, which depends of light availability relative to nutrients ratios. A mesocosm experiment, manipulating light availability and planktivorous fish presence, confirmed our hypothesis indicating that resource stoichiometric composition (consequently nutritional quality), determine trophic structure of pelagic food webs in tropical lakes. Finally another mesocosm experiment indicated that the removal of omnivorous benthivorous fish should be more efficient than removal of omnivorous planktivorus fish, as a way to improve water quality in tropical lakes and reservoirs. This last experiment showed that omnivorous planktivorous fish increase phytoplankton biomass due to trophic cascade interactions, without increasing nutrient concentrations in the water column. On the other hand, omnivorous benthivorous fish, feeding on detritus and other benthonic food sources and excreting nutrients in the water column, are responsible for translocate nutrient from sediments to the water column, increasing phosphorus pool and phytoplankton biomass. Thereby, internal phosphorus supply should be reduced and water quality of eutrophicated lakes could be improved by removing omnivorous benthivorous fish.
1
Índice
Resumo i
Abstract ii
Introdução Geral 2
Capítulo I: "Temperature affects fish body size-trophic position relationship
in freshwater and marine ecosystems." 10
Capítulo II: "Ontogenetic development of omnivorous predator interacts
with resource stoichiometry to affect pelagic food webs." 22 Capítulo III: "Biomanipulation of lakes and reservoir should target the
removal of benthivorous instead of planktivorous fish." 39
Discussão Geral 54
2
Introdução Geral
Entender a importância das relações alimentares como um elo de ligação das populações dentro da comunidade foi um marco nos estudos ecológicos. A noção de cadeia e teia alimentares desenvolvidas por Charles Elton (1927) mostrou uma maneira simples de representar as conexões entre os indivíduos e espécies que compõem a comunidade. Esse conhecimento foi posteriormente otimizado pela ecologia energética proposta por Lindeman (1942) e ainda hoje intriga e desafia pesquisadores.
Desde meados dos anos 60 (Hairston et al., 1960) o interesse por compreender a natureza dos fatores que estruturam e regulam o fluxo de energia e matéria nas teias alimentares tem crescido (Power, 1992; Hairston & Hairston, 1993; Moore et al., 2004; Gonzalez-Bergonzoni et al., 2014; Özen et al., 2014). Ideias como a regulação das teias alimentares por vias ascendentes (por exemplo, nutrientes) e descendentes (ex. predadores) (Leibold et al. 1997, Persson, 1999, Polis, 1999, Chase, 2000, Oksanen & Oksanen, 2000; Borer et al., 2006; Gruner et al., 2008; Shurin et al., 2012; Majdi et al., 2013) foram alvo de muitos estudos e são ainda bastante usuais.
3 Essas três ideias serviram de alicerce para a construção da teoria de cascata trófica, a qual sugere que mudanças em um determinado nível trófico afetam negativamente a abundância e/ou comportamento de organismos em níveis tróficos adjacentes, geralmente resultando, indiretamente, em respostas inversas na abundância ou biomassa de níveis tróficos inferiores não adjacentes (Carpenter et al., 1985; Pace et al., 1999; Polis et al., 2000). Um dos primeiros autores a utilizar o termo "cascata trófica" foi Paine (1980), muito embora, o pensamento por trás dessa ideia já houvesse sido trabalhado por Hairston e colaboradores (1960) quando eles usaram a abstração de que o mundo é verde porque os carnívoros mantém os herbívoros em baixas densidades, o que permite que as plantas cresçam livres da pressão dos herbívoros. Um exemplo claro desse efeito de cascata trófica pode ser visto ao se observar que a presença de peixes piscívoros pode influenciar a abundância de peixes planctívoros, que por sua vez podem afetar a abundância, estrutura de tamanho e a produtividade da comunidade planctônica (Brooks & Dodson, 1965; Carpenter & Kitchell, 1993). Carpenter e colaboradores (1985 e 1987) em uma série de estudos clássicos comprovaram, através da manipulação da estrutura trófica de lagos com características semelhantes, que, ambientes cujo aporte externo de nutrientes é similar, podem apresentar diferenças significativas em sua biomassa e produtividade primária (fitoplanctônica) devido a variações nas estruturas de suas teias alimentares. Essa teoria tem estimulado pesquisas em diferentes áreas da ecologia, principalmente como ferramenta de manejo com o objetivo de restaurar ambientes eutrofizados (Carpenter et al., 1985; Carpenter et al., 1987; Hansson et al., 1998; Mehner et al., 2002; Albright et al., 2004; Moss et al., 2004).
4 um predador em um determinado ecossistema podem gerar efeitos indiretos em outro ecossistema. Um exemplo claro disso pode ser descrito ao pensarmos em um simples lago (Knight et al., 2005, ver figura 1 do artigo), onde os peixes se alimentam de larvas de insetos aquáticos, insetos esses que na sua fase adulta se alimentam de espécies polinizadoras. Desse modo os peixes podem ser responsáveis por, indiretamente, facilitarem a reprodução de plantas terrestres, uma vez que eles, por meio de um efeito de cascata trófica, reduzem a pressão de competição sobre os polinizadores. Dessa maneira efeitos diretos dos indivíduos com histórico de vida complexo podem ser responsáveis por efeitos em cascata que vão alem dos limites do ecossistema.
Embora existam muitas evidências empíricas que dão suporte a essa teoria (Brett & Goldman, 1996; Brett & Goldman, 1997), e trabalhos demonstrando a prevalência e ocorrência de cascatas tróficas em ambientes naturais (Vanni et al., 1990; Kauffman et al., 2010; Dobson, 2014), a ideia de manipulação dos níveis superiores a fim de controlar as cadeias tróficas sofreu com algumas criticas, principalmente por assumir que as interações têm uma natureza estática do ponto de vista espacial e temporal (Chase, 2003) ou mesmo com relação à maneira como é utilizado o termo "cascata trófica" (Polis et al., 2000). A teoria de cascata trófica chegou a ser contestada devido ao número de estudos que não apresentavam impactos estatisticamente significativos de peixes piscívoros ou zooplanctívoros sobre a estrutura das cadeias alimentares em ambientes de água doce (DeMelo et al., 1992). Isso se deve em parte ao fato de que a estrutura e a dinâmica trófica dos ambientes naturais são mais complexas do que se presumiu inicialmente na teoria de cadeias alimentares (Polis & Strong, 1996; Finke & Denno, 2004; Hillebrand & Cardinale, 2004; Finke & Denno, 2005; Edwards et al., 2010) o que torna a magnitude dos efeitos de cascata trófica muito mais variáveis (Borer et al., 2005).
5 tamanho e composição da comunidade fitoplanctônica, sem, entretanto, afetar a biomassa total do fitoplâncton (Okun et al., 2008). A esse tipo de interação eles atribuíram o termo cascata trófica "críptica" (Tessier & Woodruff, 2002).
Interações de cascata trófica são comumente identificadas em estudos utilizando peixes piscívoros e zooplanctívoros (e.g. Brooks & Dodson, 1965; Carpenter et al., 1987; Carpenter & Kitchell, 1993; Brett & Goldman, 1996), isso se deve ao fato que hoje já se possui bastante conhecimento acerca dos efeitos desses peixes sobre a comunidade planctônica (Brooks & Dodson, 1965). Entretanto, a ideia da onivoria altera a força da cascata trófica. Isso ocorre porque peixes onívoros podem, por diferentes modos, afetar, direta e indiretamente, diferentes níveis dentro da cadeia trófica, uma vez que é característica desta guilda utilizar recursos provenientes de mais de um nível trófico (Pimm & Lawton, 1978). Assim, diferente das espécies piscívoras e zooplanctívoras, os impactos causados pela presença de peixes onívoros em ecossistemas aquáticos são mais complexos e isso têm desperto o interesse científico nos últimos anos (Carneiro, 2008). Aliado a isso existe o fato de que nos ambientes aquáticos a onivoria é uma estratégia alimentar bem difundida entre os peixes, principalmente nos ecossistemas tropicais de água doce (Fernando, 1994; Gonzalez-Bergonzoni et al., 2012). A simples presença da onivoria pode ser responsável por mudar alguns conceitos bem estabelecidos na ecologia. Por exemplo, é consenso que, em peixes, o tamanho corporal do indivíduo está diretamente relacionado com sua posição trófica (os maiores indivíduos estão no topo da cadeia) (Pauly, 1998; Romanuk et al., 2011), sendo este padrão principalmente determinado por uma limitação morfológica associada ao tamanho da abertura da boca dos predadores para ingerir suas presas.
6 al., 2007). Uma dessas estratégias seria o forrageio nos níveis mais baixos da cadeia alimentar, tais como a maior expressão da detritivoria, herbivoria e/ou onivoria. Portanto, a onivoria surgiria como uma possibilidade de contornar essa possível limitação energética a qual os indivíduos de grande porte sofrem nos ambientes aquáticos tropicais.
Grande parte dos impactos causados pelos peixes onívoros sobre a estrutura da teia alimentar passa diretamente pela seleção do recurso a ser explorado. Essa seletividade depende de uma gama de fatores como, a qualidade nutricional do recurso, estágio de vida do consumidor, abundância e disponibilidade do recurso. Por exemplo, embora peixes onívoros filtradores, como a Tilápia do Nilo (Oreochromis niloticus), possam se alimentar tanto do fito quanto do zooplâncton, um aumento na densidade ou na qualidade nutricional do fitoplâncton pode ser responsável por aumentar o grau de herbivoria do peixe. Uma vez que a as razões estequiométricas do fitoplâncton variam bem mais que as razões do zooplâncton (Darchambeau et al., 2005) esse tipo de mudança pode acontecer nos ambientes e, neste caso, a herbivoria seria uma estratégia alimentar mais eficiente. A qualidade nutricional do fitoplâncton, por sua vez, está intimamente relacionada com a concentração de nutrientes presentes na composição dos mesmos, ex. concentrações e razões celulares de C, N, e P. As razões desses elementos podem variar em resposta a disponibilidade de nutrientes e da radiação no ambiente (Sternet & Elser 2002). A hipótese da luz e nutrientes (HLN) embasa essa mudança na estequiometria dos produtores, e prediz que um aumento na razão luz:nutriente aumenta a razão C:nutriente da alga (reduzindo a qualidade nutricional da mesma) (Sterner et al. 1997).
7
(<≈60mm) são predadores visuais, e provavelmente selecionam o zooplâncton de grande porte como presa (com exceção das larvas muito pequenas, as quais são limitadas pelo tamanho da boca), similar a diversos outros peixes. Estes indivíduos podem indiretamente favorecer o crescimento fitoplanctônico via efeito de cascata trófica. Entretanto, indivíduos grandes podem optar por serem filtradores, alimentando-se tanto de fitoplâncton como também do zooplâncton, e muito provavelmente de detritos bentônicos. Dessa maneira eles podem afetar a biomassa fitoplanctônica diretamente via herbivoria e indiretamente através da redução da densidade do zooplâncton, pelo mecanismo de cascata trófica. Além disso, ambos os estágios de vida podem diretamente favorecer o crescimento fitoplanctônico via excreção de nutrientes. Dessa maneira onívoros filtradores podem afetar as comunidades fitoplanctônicas e zooplanctônicas, através de mecanismos diretos e indiretos e o seu real efeito será dependente do estágio de vida do organismo.
Desta maneira o segundo capítulo desta tese avaliou a influência da estequiometria do recurso sobre o efeito de peixes planctívoros sobre as teias tróficas pelágicas. A ideia que estimulou esse trabalho foi que o balanço entre luz e nutrientes controla a composição estequiométrica dos produtores (Sterner et al., 1997), os quais por sua vez, influenciam a produção secundária, a ciclagem de nutrientes e a eficiência na transferência de energia dentro das teias tróficas aquáticas (Sterner & Elser, 2002; Dickman et al., 2008). Dessa maneira é esperado que em ambientes com alta disponibilidade de luz relativa a fósforo, efeitos de cascata trófica sejam enfraquecidos no nível do herbívoro, uma vez que, a qualidade nutricional do alimento é baixa o desenvolvimento do herbívoro seja limitado, reduzindo sua capacidade de responder a uma redução na pressão de predação pelo peixe planctívoro.
8 os autores observaram a capacidade de um peixe onívoro (Dorosoma cepedianum) translocar nutrientes do habitat bentônico para o habitat pelágico.
Essa translocação ocorre durante a interação do peixe com o sedimento do lago, e pode causar um processo conhecido como bioturbação, que seria a mistura e a ressuspensão de parte do sedimento causando um aumento da turbidez (Breukelaar et al.,1994; Croel & Kneitel, 2011) e da concentração de nutrientes (Schaus & Vanni, 2000; Adamek & Marsalek, 2013) na coluna de água. Ou seja, além da parcela de nutrientes oriundos da reciclagem de nutrientes pelos peixes (excreção), que tem um papel fundamental na estruturação dos ambientes aquáticos (Vanni & Layne, 1997; Attayde & Hansson, 2001, Torres & Vanni, 2007), surge uma “nova” fonte de nutrientes, pois é diferente daquela originada pelo padrão convencional de reciclagem (Caraco et al., 1992), que é dependente do acoplamento de habitats mediado pelo acesso do peixe ao sedimento. Esse aporte alternativo de nutrientes pode resultar em um aumento na disponibilidade de nutrientes para os produtores primários, levando a mudanças na biomassa e na estrutura da composição da comunidade planctônica (Schaus & Vanni, 2000). Esse impacto de peixes que apresentam um hábito bentônico mediado pelo seu acesso ao sedimento, sobre a dinâmica de nutrientes no ecossistema aquático é tema do terceiro capítulo dessa tese.
Dessa maneira o terceiro capítulo desta tese avaliou e quantificou, através de dois experimentos em escala de mesocosmo (o primeiro realizado com o peixe bentívoro Prochilodus brevis e o segundo com o onívoro filtrador Oreochromis niloticus), o efeito
10
Capítulo I
Temperature affects fish body size-trophic position relationship in
freshwater but not in marine ecosystems.
Danyhelton D. F. Dantas1, Ronaldo Angelini2, Adriano Caliman1, Luciana S. Carneiro1, Sergio M. Q. Lima3, Pablo A. Martinez4 & José L. Attayde1.
1
Departamento de Ecologia, Universidade Federal do Rio Grande do Norte, Natal 59078-900, Brasil.
2Departamento de Engenharia Civil, Universidade Federal do Rio Grande do Norte, Natal 59078-900,
Brasil.
3Departamento de Botânica, Ecologia e Zoologia, Universidade Federal do Rio Grande do Norte, Natal
59078-900, Brasil.
4
11
Abstract
The trophic position of fish species in aquatic food webs is constrained by their energetic demand, which scales positively with fish body size and water temperature. We hypothesized that larger fish species with higher energetic demand would tend to become more energy limited and forage lower in the food web with increasing water temperature. We tested the hypothesis that the trophic position of larger fish species would be lower in tropical than in temperate waters. Using the FishBase dataset, we analyzed the relationship between the maximum length (body size) and trophic position of 7,587 ray-finned fish species, categorized according to ecosystem type (freshwater or marine) and climatic region (tropical or temperate). Linear mixed-effects models (LMMs), with controlled nested effect for phylogeny (i.e., order, family, and genus), confirmed the prediction for the entire dataset and for freshwater but not for marine fish species. The results suggest that fish species compensate for the energetic constraints of a larger body size by increasing omnivory in tropical freshwaters. On the other hand, the larger size of marine ecosystems may compensate for energy limitation and fulfill the greater energetic demand of larger fish in tropical marine waters. Our results suggest that global warming may affect the fish trophic position and food-chain length in freshwater but not in marine ecosystems.
12
Introduction
Energy flows in ecosystems supports life and are guided by the laws of thermodynamics. As energy moves up through a food web, much of it is lost as heat of respiration, with a consequent decrease in energy availability to top predators (Elton 1927). The absolute rate of respiration depends on the energetic demand of the species and individuals, which increases with increasing body size (Peters 1983, McNab 2002) and temperature (Gillooly et al. 2001, Brown et al. 2004). If no other ecological mechanism compensate for energy limitation, the expected increase in absolute individual’s energetic demand with increasing body size and temperature, requires an increase in individual’s energy consumption to compensate for its higher absolute energetic demand (Arim et al. 2007). Corollary, as individuals become more energy limited with increasing body size and temperature, they would tend to be smaller (Atkinson 1994, Atkinson & Sibly 1997, Blackburn et al. 1999) and/or forage lower in food webs in warmer temperatures (Arim et al. 2007).
Furthermore, fish body size and trophic position are expected to be positively correlated (Romanuk et al. 2011). Fish gape-size is a function of fish body size and puts an upper limit to the size of the prey a predator can consume creating a hierarchy of trophic connections in which large animals consume small ones (Arim et al. 2007). Larger individuals have the morphological ability to occupy higher trophic positions in the food web but also have higher absolute energetic demands and should be limited by the amount of energy available to them. Therefore, energetic constraints limit the trophic position of larger animal individuals if no other ecological processes (i.e. reduction in abundance) compensate for energy limitation (Arim et al. 2007).
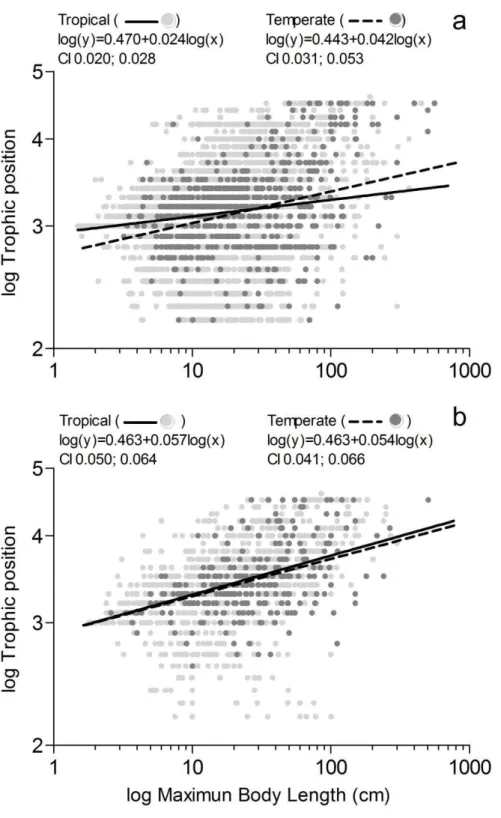
13 Figure 1: Hypothesized relationships between fish trophic position and body size in tropical (solid line)
and temperate (dashed line) ecosystems. Warmer temperature is expected to decrease trophic position for larger more than for smaller ray-finned fish.
Methods
14
family and genera of the species . Trophic position was the response variable, ecosystem type (freshwater or marine), climatic zones (tropical or temperate) and maximum body length were included as fixed variables and phylogenetic resolution such as order, family and genus were included as nested random variables.
Defined as the longest individual recorded for a given species, maximum total body length was used as a metric of body size (Froese & Pauly, 2013). Although mass, not length per se, is the most relevant size metric for exploring energetic constraints, maximum total body length was used as a proxy of maximum body mass because these two variables are highly positively correlated (r2= 0.94, p< 0.001 see Romanuk et al., 2011) and data on maximum body mass was not available for many species. Trophic position is calculated in FishBase by adding 1 to the mean trophic position, weighted by relative abundance, of all food items consumed by a species (Froese & Pauly, 2013). For a given consumer j, trophic position is defined as:
Trophj =1+ DCij
i=1
S
å
*Trophi15 Species that have their preferred upper depth range below 200 m were excluded from our analysis because they are classified as cold-water regardless of their climatic zone. Species classified as subtropical were also excluded from our analysis because many cosmopolitan fishes that occur in both tropical and temperate zones are included into this category. For ecosystem type classification, since some freshwater fishes enter brackish and marine water and vice versa, FishBase assign the three yes-no categories Saltwater, Brackish, Freshwater on the basis of occurrence and tolerance reported in the literature. For this paper we only considered species with one classification. Fishbase considers as a primary freshwater (or a primary marine) species a fish that has evolved in freshwater (or saltwater) and spends all their life time there. Data on maximum total body length and trophic position were log10 transformed prior to the analysis to meet the
assumptions of the statistical analysis.
The LMM fitted using the function “lmer” from the package “lme4” (Bates et al., 2014) in
R. For model selection we used the Akaike’s Information.
Results
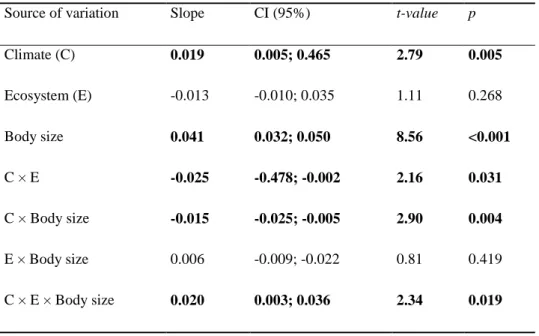
16 Table 1: Results of the Linear Mixed-Models for all 7587 species of Actinopterygii used in this study. In
this analysis, phylogeny did not affect the results, once taxonomic categories (order, family, and genus) were used as nested random factors to control for the effect of nonindependence of species traits. The nested random factors explain 82% of variance. The slope of the relation with 95% confidence intervals (CI), t-value, and p-value are shown. Bold values are significant (p < 0.05).
Source of variation Slope CI (95%) t-value p
Climate (C) 0.019 0.005; 0.465 2.79 0.005
Ecosystem (E) -0.013 -0.010; 0.035 1.11 0.268 Body size 0.041 0.032; 0.050 8.56 <0.001
C × E -0.025 -0.478; -0.002 2.16 0.031
C × Body size -0.015 -0.025; -0.005 2.90 0.004
E × Body size 0.006 -0.009; -0.022 0.81 0.419 C × E × Body size 0.020 0.003; 0.036 2.34 0.019
17 Figure 2: Trophic position as a linear function of the maximum total body length for all actinopterygian
18 Figure 3: Trophic position as a linear function of the maximum total body length for Actinopterygii
19
Discussion
Our results show that larger freshwater fish forage lower in the food web in warmer tropical climate compared to cooler temperate climate, indicating that the support of our hypothesis is dependent on ecosystem type. Food availability increases as we go down the food web, implying that the energetic demands of larger animals are more easily met for herbivores, detritivores and omnivores than for carnivores. Therefore, feeding on primary producers and detritus may compensate for energy limitation and fulfill the greater energetic demand of larger fish in warmer freshwaters. Recent studies have demonstrated shorter food chain length (Doi et al. 2012) and greater catabolic demand (Amado et al. 2013) for freshwater communities in warmer climates. Furthermore, a recent meta-analysis shows a consistent increasing trend in the relative richness of omnivorous fish with decreasing latitudes for both freshwaters and marine ecosystems, but omnivore richness was higher in freshwaters than in marine ecosystems (González-Bergonzoni et al. 2012) a pattern also observed in our study (S1). Combined with the fact that our results were unaffected by phylogeny, these aforementioned body of evidences may indicate that the structure and topology of freshwater food webs were more affected by climate-dependent mechanisms that constraints energy flow than marine food webs.
The larger size of marine ecosystems may compensate the energy limitation and fulfill the greater energetic demand of larger fish in warmer marine waters. Ecosystem size is positively correlated to food chain length (Post et al. 2000; Post 2002, Takimoto & Post 2013). Larger ecosystems may have greater total resources available at the base of food webs (Schoener 1989) and higher functional trophic diversity (Cohen & Newman 1991; Post et al. 2000) to maintain longer food chains. Moreover, spatial processes in larger ecosystems may enhance the persistence and stabilize predator-prey interactions, promoting long food chains (Wilson et al. 1998; Holt 2002; Takimoto et al. 2012).
21
Supplementary material.
S 1: Table showing relative frequency of omnivorous and carnivorous for all classifications applied in this work.
% omnivorous % carnivorous
Tropical 12.61 87.39
Temperate 6.55 93.45
Freshwater Tropical 13.89 86.11
Freshwater Temperate 8.66 91.34
Marine Tropical 7.52 92.48
Marine Temperate 1.34 98.66
S 2: Results of linear mixed-effect model of climate (tropical and temperate), ecosystem (freshwater and
marine), body size (cm), and their interactions on trophic position. Akaike’s Information Criterion (AIC),
the log-Likelihood (logLik), Chi-Square test (Chisq), Chi-Square probabilities (p(>Chisq)), and degree of freedom (DF) are listed for each model.
Model DF Chisq p(>Chisq) logLik AIC ∆AIC
Trofic~Climate 6 6.53 0.01 8596.4 -17181 354
Trofic~Ecosistem 6 0 1 8593.7 -17175 360
Trofic~Climate*Ecosistem 8 6,41 0.04 8596.9 -17178 357
22
Capítulo II
Ontogenetic development of omnivorous predator interacts with
resource stoichiometry to affect pelagic food webs.
Danyhelton D. F. Dantas1, Jennifer M. Newell2, Jandeson B. Dias3, Renata F. Panosso1, Maria J. González2, Michael J. Vanni2, Jose L. Attayde1.
1
Departamento de Ecologia, Universidade Federal do Rio Grande do Norte, Natal 59078-900, Brasil.
2Department of Zoology, Miami University, Oxford, Ohio.
23
Abstract
The balance between light and nutrients controls the stoichiometric composition of
phytoplankton, which is known to influence secondary production and nutrient cycling by
aquatic consumers. Therefore, we hypothesize that the stoichiometric composition of
phytoplankton may influence the predation and excretion effects of planktivorous fish on
pelagic food webs. To test this hypothesis we performed a mesocosm experiment with a 2 x 3
factorial design, where two levels of light (low and high) were combined with three levels of
planktivorous fish (no fish, with zooplanktivorous fish, with omnivorous filter-feeding fish).
The zooplanktivorous larvae and omnivorous juvenile stages of the planktivorous fish species
Oreochromis niloticus was used in our experiment to manipulate the planktivore type while
standardizing the fish species. The six treatments were replicated four times and randomly
allocated in 24 mesocosms (250L). The experiment lasted 21 days and each mesocosm was
monitored, at the beginning and at the end of the experiment, for the stoichiometric composition
of organic particulate matter (seston) and the abundance and composition of phytoplankton and
zooplankton communities. Nutrient excretion by fish was also measured at the end of the
experiment. Results of a two-way ANOVA show that fish increased seston C:P and N:P ratios
and that this effect was stronger when light was high relative to nutrient availability. Total
phytoplankton biovolume increased with increasing light availability in the absence of fish,
while in treatments with fish no change in total phytoplankton biovolume was observed in
response to light availability. Fish increased the proportion of cyanobacteria at high light levels.
Results of the contrasts among the three levels of fish show that juvenile fish account for most
of the significant fish effect on seston C:P and N:P ratios and on phytoplankton biovolume and
composition. A significant interaction was observed between light and fish effects on total
zooplankton density, as fish larvae increased total zooplankton density but only at high light
levels. On the other hand, both fish stages increased the proportion of small grazers,
independent of light levels. The above results suggest that when phytoplankton is carbon rich
and phosphorus poor, planktivorous fish release less phosphorus back to phytoplankton,
increasing phosphorus limitation and favoring cyanobacteria species with high affinity for
phosphorus. This effect is stronger in juvenile stages for which P demand for fish growth is
higher. We conclude that resource stoichiometry interacts with the ontogenetic development of
planktivorous fish to influence their effects on pelagic food webs.
24 Introduction
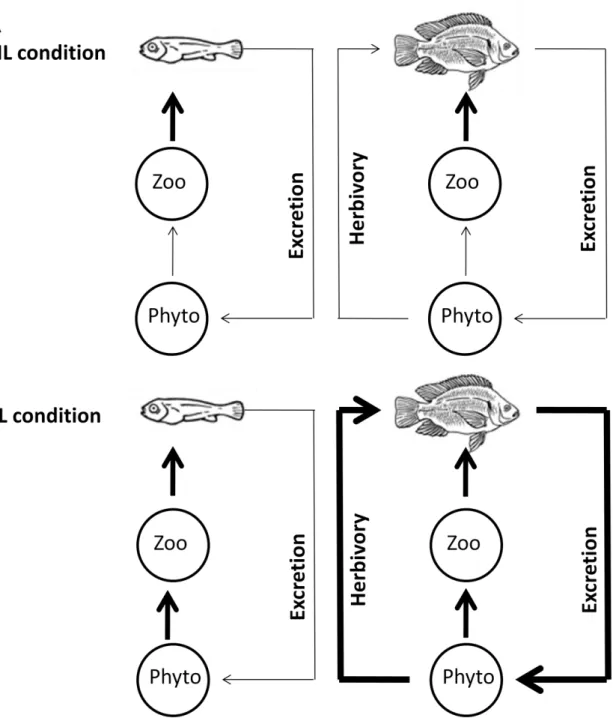
Ecological stoichiometry theory predicts that the balance between light and nutrients influences the stoichiometric composition of primary producers (Sterner et al., 1997), which is known to influence secondary production, nutrient recycling and the efficiency of energy transfer in aquatic food webs (Sterner & Elser, 2002; Dickman et al., 2008). According to this theory, as light relative to phosphorus availability increases, lake ecosystems become more carbon rich and phosphorus poor, causing direct P limitation of the growth of multiple trophic levels, weakening the strength of trophic cascades and diminishing P recycling by consumers (Sterner et al., 1997). In lakes with high light to phosphorus ratios, trophic cascades would be dampened at the herbivore level because nutritional constraints on herbivore growth would decrease their ability to respond to reduced predation pressure by planktivorous fish (Frost et al., 2006). In such lakes, P-limited consumers would have low rates of mass-specific P release as they would increase the efficiency of P uptake and retention. On the other hand, in lakes with low light to phosphorus ratios opposite patterns would be expected.
The effects of planktivorous fish on the structure and dynamics of planktonic communities have been well documented in the last five decades (Carpenter & Kitchell, 1993). It has been recognized that planktivorous fish not only affects plankton through size-selective predation on zooplankton, but they can also affects plankton through nutrient recycling (Vanni et al., 1997; Attayde & Hansson, 2001; Vanni, 2002). In tropical lakes some planktivorous fish are filter-feeding omnivores and can also affects plankton through grazing on phytoplankton (Lazzaro 1987; Menezes et al., 2010). Although these effects of planktivorous fish on plankton communities have been extensively investigated, the influence of resource stoichiometry on such effects have been largely neglected (but see Higgins et al., 2006; Dickman et al., 2008).
26 Figure 1: Conceptual model showing expected interactions in (A) high light condition (HL) and (B) low
27
Methods
Study site and Experimental design
We conducted a mesocosm experiment in a fish hatchery station located at the UFRN campus in Macaíba, Rio Grande do Norte, Brazil (5°53'7"S; 35°21'38"W). We used a 2x3 factorial design with two light levels (low and high) crossed with three fish level (no fish, with larvae or juvenile of tilapia). These six treatments were replicated four times and randomly allocated to 24 mesocosm (250 L). Mesocosms were filled with a mixture of water and plankton from a mesotrophic reservoir and a eutrophic fish pond of the hatchery station. This was done to provide an inoculum of plankton species from both mesotrophic and eutrophic systems.
On 27th March mesocosms were filled and water and plankton samples were taken from six randomly chosen mesocosms to check for similar initial conditions. On the same date, half of the mesocosms was covered with a fine screen while the other half was covered with a gross screen to manipulate the two light levels. The two screens, respectively, reduced 10% and 90% the radiation on the water surface of the mesocosms. Fish used in the experiment were collected from the fish ponds and added to the mesocosms on 8th April when the experiment started. In the treatments with juvenile fish, three individual were added per tank (77-95 mm SL), while in the treatments with fish larvae, we added 12 individuals per tank (20-45mm SL) in order to achieve similar metabolic rates in all fish treatments (assuming metabolic rate scales with the ¾ power of fish mass). Samples were taken on 8th and 29th April at the start and end of the experiment, respectively.
Sampling and analysis
28 Instruments; Stainton et al., 1977). Seston C, N, and P concentrations were used to calculate C : N, C : P, and N : P ratios, which are expressed here in molar units.
Subsamples for phytoplankton analysis were fixed with lugol’s solution. Phytoplankton was quantified according to Utermöhl’s method (Wetzel & Likens, 2000) under an inverted microscope. Before counting, subsamples were allowed to sediment for 3h for each centimeter height of the chamber. The individuals (cells, colonies and filaments) were enumerated in random fields as proposed by Uhelinger (1964), with an error smaller than 20% and a confidence interval of 95% (Lund et al., 1958). To estimate the phytoplankton biovolume at least 25 individuals from each species were measured by applying approximations to similar geometric solids.
For zooplankton analysis a subsample of 30L were filtered through a plankton net with 68µm mesh size and preserved with 4% formaldehyde. Zooplankton organisms were counted under a microscope in a 1mL Sedwick-Rafter chamber. Zooplankton was divided in two size classes: large (cladoceran and adults copepods) and small (nauplii copepod and rotifer) zooplankton.
We also quantified nutrient excretion rates of fish at the end of the experiment. We randomly removed one single individual from each mesocosm of the juvenile fish treatments and four individuals from each mesocosms of the fish larvae treatments and put them separately in plastic bags filled with distilled water to release nutrients for one hour. Water samples were then collected from each plastic bag and used to quantify phosphorus and nitrogen concentration excreted by fish.
Prior to all statistical analyses, when necessary, data was transformed to homogenize variances. To analyze the effects of light availability, planktivorous fish and their interactions we conducted a two-way ANOVA.
Results
29 light availability in the absence of fish, while in the presence of fish no change in total phytoplankton biovolume was observed in response to light availability (Figure 3a). Fish increased the proportion of cyanobacteria at high light levels, while increased the proportion of chlorophytes at low light levels (Figure 3b, c). Both light and fish decreased the proportion of diatoms, but no significant interaction between their effects on diatoms was found (Figure 3d). Results of the contrasts between the three levels of fish show that juvenile fish accounted for most of the fish effects on seston C:P and N:P ratios and on phytoplankton biovolume and composition (data not shown).
30 Figure 2: Effects of light and fish levels and their interactions on seston C:N (A), C:P (B) and N:P (C)
31 Figure 3: Effects of light and fish levels and their interactions on total phytoplankton biovolume (A), the
32 Figure 4: Effects of light and fish levels and their interactions on total zooplankton density (A) and the
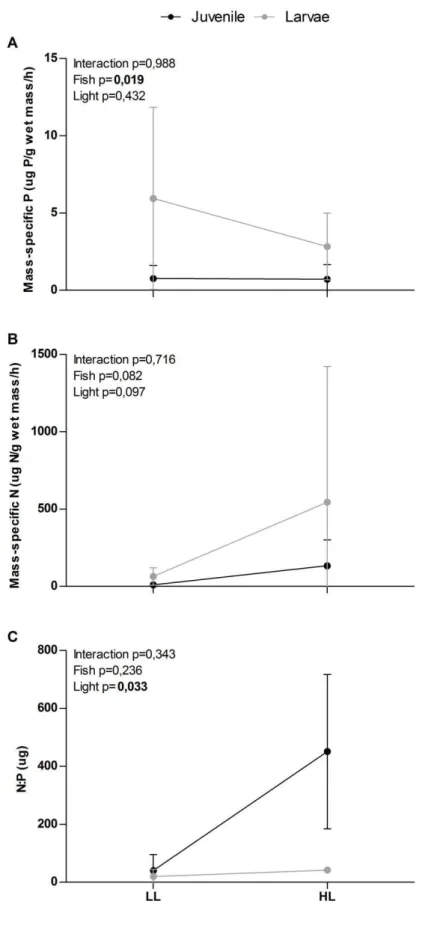
33 Figure 5: Mass-specific nutrient excretion rates for (a) phosphorus, (b) nitrogen and (c) N:P excretion
34
Discussion
In this work we showed that the effects of planktivorous fish on plankton communities depend on the relative availability of light and nutrients. Our results confirmed the assumption of increasing seston carbon relative to nutrient ratios with increasing light availability (Sterner et al., 1997), but also show that the effects of light on seston stoichiometry depends on planktivorous fish. At high light to phosphorus availability, planktivorous fish shoud be limited by phosphorus and should release nutrients at high C:P and N:P ratios. However, only juvenile fish released nutrients at higher N:P ratios with increasing light availability and this was caused by an increase in N instead of a decrease in P release rates with increasing light availability. Therefore, the effects of fish on seston stoichiometry can only partially be explained by fish-mediated nutrient recycling and our hypothesis that mass-specific P release rates would decrease with increasing light availability was not confirmed.
We predicted that variation in primary producers’ nutritional quality would be responsible for changes in the effects of planktivorous fish on plankton communities. In particular we expected that trophic cascades by visual-feeding zooplanktivorous fish would be stronger at low light to phosphorus ratios. On the other hand, we hypothesized that grazing on phytoplankton by filter-feeding omnivorous fish would be higher at low light to phosphorus ratio, compensating the effects of stronger trophic cascades and resulting in weak response of total phytoplankton biomass to filter-feeding planktivorous fish.
We expected that the strength of trophic cascade effects of both planktivores types would decrease with increasing light availability, but we found only weak evidence supporting our hypothesis. We also expected that omnivorous filter-feeding juveniles would have weaker trophic cascade effects on phytoplankton than zooplanktivorous larvae at low light to nutrient ratios. This is because tilapia grazing would be stronger under this condition, when seston quality is higher.
35 At high light condition omnivore’s food choice apparently was more influenced by quantity than quality of food. We used results of fish nutrient excretion experiment to evaluate fish food choice. Since fish nutrient excretion rates are expected to reflect stoichiometry of food source we expected that if large tilapia feed heavily on phytoplankton under low light conditions, P excretion rates should be relatively high (because algal C:P will be low), and we predict that they will excrete at low N:P. If these individuals feed (partly or wholly) on phytoplankton under high light, they should excrete less P (and at higher N:P) than at low light. N:P excretion ratio across light treatments may be especially pronounced if P assimilation efficiency is lower on P-deficient phytoplankton, as has been show for others organisms (Higgins et al., 2006; Frost et al., 2004).
36 Moreover, the mass-specific nutrient excretion ratios of phosphorus and nitrogen showed a higher excretion rate for larvae fish, probably this difference could be related to differences in individual body N:P ratios. Because the elemental content of an individual’s body and diet often change during development, the ecological stoichiometry of an individual should also vary ontogenetically. For example, larger and more developed individuals, as our juvenile fish, often have higher calcium and phosphorus contents, likely due to bone formation (Davis & Boyd, 1978; Hendrixson et al., 2007; Pilati & Vanni, 2007). In addition, larger individuals often having greater fat stores and higher carbon contents than smaller individuals. The relationship between ontogenetic diet shifts and a consumer’s body composition (and needs) may be quite complex, depending on the nature of the diet shifts. However, we are confident that variations in fish nutrients excretion rates among treatments are due to food nutritional quality, since the positive association between nutritional quality of ingested food and nutrient excretion rates are consistent with the prediction that nutrients releases rates increase with food nutrient content.
37 Plankton community composition was affected by treatments. Although we did not found interactive effect between treatments on zooplankton density or community structure, fish presence was responsible for the major impact on zooplankton community structure. Filter-feeder omnivores (juvenile fish) and zooplanktivores (larvae fish) had similar effects on zooplankton community. Apparently both fish size predate on large zooplankton (cladoceran and copepod) and create a scenario in favor of small size zooplankton (rotifer, copepodite and nauplii). As commented before this kind of impact could generate implications for whole ecosystem, since in tropical shallow lakes are often dominated by inedible phytoplankton, which are supposed to not be grazed by small body zooplankton, as rotifer (Fernando, 1994; Lazzaro, 1987; Jeppesen et al., 2007).
Treatments interactively affect phytoplankton community composition. Particular response of phytoplankton communities to light and fish suggest that even abroad taxonomic groups, phytoplankton taxa are differently adapted to environmental conditions (Reynolds et al., 1993). Phytoplankton ability to adapted to differential resource and grazers regimes are diverse and include morphological, chemical and behavior attributes, these attributes could be responsible for some community-level responses (Lichman & Klausmeier, 2008). Half of our treatments present singular phytoplankton community composition. In high light-juvenile fish phytoplankton community was dominated by cyanobacteria, low light-juvenile fish treatment showed chlorophytes dominance, and in high light-larvae fish phytoplankton community composition showed a codominance scenario between chlorophytes and diatoms. Other treatments showed a diatoms dominance. Probably fish nutrient excretion rates were determinant for phytoplankton community structure, since our mesocosms did not have any input of nutrient, fish nutrient excretion assume the main role as nutrient source.
39
Capítulo III
Biomanipulation of lakes and reservoir should target the removal of
benthivorous instead of planktivorous fish
Danyhelton D. F. Dantas
1
, Caroline G. B. Moura, Pablo L. Rubim
1
, Leonardo Teixeira
1
, Mariana C. Amaral
1
, Fabiana Araújo
1
, José L. Attayde
1
.
40
Abstract
The removal of benthivorous and planktivorous fish have been extensively used as a management tool to improve the water quality of temperate lakes and reservoirs. However, the extent to which such biomanipulation can be equally successful in tropical systems remains a controversial issue. This study aimed to evaluate the effects of a benthivorous (Prochilodus brevis) and a planktivorous (Oreochromis niloticus) fish on the water quality of a tropical eutrophic man-made lake. Because both fish species can bioturbate lake sediment, we also investigated whether their effects on water quality are mediated by their interactions with the sediment. Two consecutive experiments with a 2x2 factorial design were carried out in 20 mesocosms (6 m3), where fish presence/absence were manipulated in combination with the presence/absence of a fish cage (4 m3) preventing fish access to the sediment. Nutrients (N and P) and chlorophyll a concentrations as well as water transparency were measured in all treatments at the
beginning. middle and end of the experiments. The benthivorous fish increased total phosphorus and chlorophyll a concentration and decreased water transparency only when they had access to the sediment. However, no differences were found in the excretion rates of benthivorous fish with and without access to the sediment, suggesting that their effects were caused by bioturbation of the sediment and not by nutrient translocation through bottom feeding and excretion in the water column. On the other hand, the planktivorous fish increased phytoplankton biomass without changing the concentrations of nutrients and the water transparency. Moreover, their positive effect on phytoplankton biomass was independent of their access to the sediment, suggesting that they increased phytoplankton biomass through a trophic cascading effect. We conclude that biomanipulation of eutrophic lakes in the tropics should target the removal of benthivorous fish, because they increase the internal load of phosphorus to the water column stimulating phytoplankton growth in tropical as in temperate lakes. Removing planktivorous fish may also reduce phytoplankton biomass and improve water quality, but this would depend on the strength of the trophic cascading effects, which should be lower in tropical than in temperate lakes.
41
Introduction
Fish play a key role in the resilience of the turbid state of eutrophic shallow lakes because they can control the abundance of zooplankton herbivores, thus increasing phytoplankton biomass (Scheffer, 1998). Moreover, they can resuspend sediments in search for benthic food, increasing turbidity (Zambrano et al., 2001; Scheffer et al., 2003), and also translocate nutrients from benthic to pelagic habitats, enhancing phytoplankton growth (Vanni, 2002). Therefore, a strong reduction of planktivorous and/or benthivorous fish biomass is often needed to bring the lake back to a clear water state after reduction of external nutrient loading (Scheffer, 1998). The effects of such biomanipulation have been extensively investigated in temperate lakes and have been reviewed by many authors in the last two decades (e.g. Hansson et al., 1998; Mehner et al., 2002; Cooke et al., 2005; Søndergaard et al., 2008; Jeppesen et al., 2012).
On the other hand, very few studies have investigated the applicability of biomanipulation as a tool for water quality management of tropical and subtropical lakes (Jeppesen et al., 2012). It has been suggested that (sub) tropical lakes may not respond to planktivorous fish manipulation as trophic cascade theory predicts because zooplankton grazing pressure on phytoplankton should be weak in these systems (Jeppesen et al., 2007). In such warm lakes, zooplankton is often dominated by small-sized grazers (Lacerot, 2010; Iglesias et al., 2011) while phytoplankton is often dominated by large colonial or filamentous cyanobacteria (Kosten et al., 2012), which cannot be efficiently grazed by such small-sized herbivores (Carpenter & Kitchell, 1993).
42 2010). Based on such prediction, any reduction in planktivorous fish biomass may either increase or decrease phytoplankton biomass, depending on the initial fish biomass and the relative strength of the direct and indirect effects of filter-feeding fish on phytoplankton (Attayde et al., 2010).
On the other hand, the effects of benthivorous fish on the water quality of tropical shallow lakes should be similar to those observed in temperate lakes. Benthivorous fish enhance phytoplankton via nutrient cycling resulting from two functionally distinct processes: i) nutrient release by excretion and egestion and ii) bioturbation of the sediment during their foraging activities. Benthivorous fish feeds on benthic resources, and release nutrients into the water column, translocating nutrients from benthic to pelagic habitats and transforming them from particulate to dissolved forms (Vanni, 2002). Nutrient translocation by benthivorous fish is different from nutrient recycling by planktivorous fish, which release nutrients within the same pelagic habitat in which food is ingested. Thus, nutrient translocation by benthivorous fish brings “new” nutrients to the euphotic zone, increasing the total mass of nutrients and stimulating “new primary production” in the pelagic habitat (Vanni, 2002). In contrast, nutrient recycling by planktivorous fish cannot increase the mass of nutrients in the pelagic habitat, but rather it sustains “recycled production” (Vanni, 2002).
43 In this study we examined the effects of a benthivorous (Prochilodus brevis) and a planktivorous (Oreochromis niloticus) fish on the water quality of a tropical eutrophic man-made lake. Because both fish species can bioturbate lake sediment, we also investigated whether their effects on water quality are mediated by their interactions with the sediment. P. brevis is a continuous bioturber, feeding on the sediment surface, while O. niloticus is an occasional bioturber, digging site-specific holes during nest building activities. Both species are common and economically important in the inland water fisheries of the semi-arid region of Brazil.
We conducted two experiments in mesocosm scale with a 2x2 factorial design, manipulating presence of omnivorous fish (Fish/No Fish) and its access to sediment (Access/No Access), to test the hypothesis that benthivorous fish has stronger negative effects on the water quality of tropical lakes and reservoirs due to their nutrient translocation from the sediment to the water column.
Methods
Experimental design
44 To prevent fish access to the sediment inside the mesocosms, fish were placed inside a 4 m3 cage (4 m2 area x 1 m depth) with 2 cm mesh size. Although the cage limited fish interaction with the sediment, it allowed all other chemical and biological interactions inside the mesocosms. In both experiments fish were caught in the lake and added just after the initial water samples were taken. In the benthivorous fish experiment, four individuals (335,9 ± 35,9 g mean weight) were added while in the planktivorous fish experiment, three individuals (482,5 ± 66,7g mean weight) were added to each mesocosm of the fish treatments.
Sampling and analyses
Water samples were taken from each mesocosm just before fish additions and after 15 and 30 days from the beginning of the experiments. Oxygen concentration and water temperature were measured in situ with a Instrutherm MO - 900 probe. Water transparency was measured with a Secchi disc. Water samples were collected at different points of each mesocosm with a PVC tube (1.5 m) and integrated into a single representative sample of each mesocosm, from which subsamples were taken to quantify chlorophyll a [Chl a], total phosphorous [TP] and total dissolved nitrogen [TDN]. The same procedure was done at two different points of the lake, to monitor any variation in the lake that could be misinterpreted as a treatment effect.
Chlorophyll a was determined in spectrophotometer after water filtration onto a 1.2 µm glass fiber filter (VWR 696) and pigment extraction with ethanol (Jespersen & Christoffersen, 1988). Total phosphorus (TP) concentration was measured by the acid ascorbic method after persulfate digestion (Valderrama, 1981). Filtered water (1.2 µm glass fiber filter - VWR 696) were used to determinate soluble reactive phosphorous (SRP) according to Murphy & Riley (1962), while total dissolved nitrogen (TDN) was analyzed using a carbon analyzer TOC-V Shimadzu.
45 and end of the experiments. For all analyses p values were considered significant if < 0.05.
Results
In both experiments omnivorous fish presence increased phytoplankton biomass, but the mechanisms responsible for this increment were different for benthivorous and planktivorous fish. Although both fish species are potentially bioturbers, only the effect of the benthivorous P. brevis was dependent on its access to sediment.
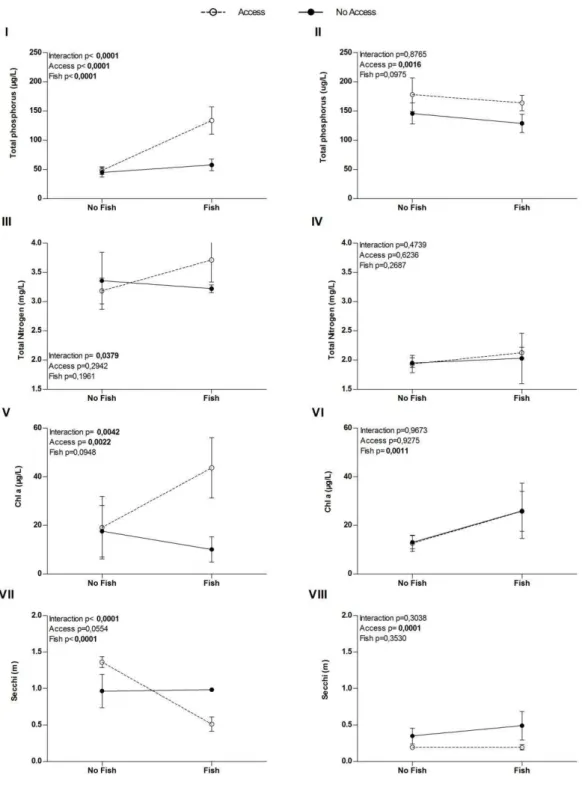
46 Figure 1: Results of the two-way ANOVA for total phosphorous (I, II), total nitrogen (III, IV), Chl a (V,
47 P. brevis weight significantly decreased in both fish treatments (Figure. 2). In both
cases, the difference between initial and final weights were near 15%. On the other hand, tilapia did not lose or gain weight during the experiment in both treatments (Figure 2).
Figure 2: Wet weight of P. brevis (left) and O. niloticus (right) at the beginning and end of the experiments in the fish treatments with and without access to the sediment. Results of the t-tests are given on the top of the bars being compared. Significant p values (< 0.05) are in bold. Bars represent standard deviation.
Discussion
The most important result of this paper was to show that omnivorous fish can affect plankton community via different ways and that interaction with sediment can influence this effect. In both experiments fish presence increased phytoplankton biomass, but mechanisms responsible for this increase were apparently different for each fish species, and access to sediment was determinant only for benthic-feeding omnivorous.
48 In recent years some studies have associated bioturbers role as ecosystem engineers, highlighting their abilities to modify ecosystem features (Meysman et al., 2006). However, impacts of a potential bioturber fish in aquatic ecosystems could be variable, and one of the features that determine this impact is the way that a species interacts with the sediment (Adamek & Marsalek, 2013). Although our results have shown a similar pattern, an increase in phytoplankton biomass, in both experiments, it happens in different conditions for each one: for P. brevis, (benthic-feeding) phytoplankton increase in a condition in which fish had access to sediment. So, fish was able to feed on sediment promoting sediment resuspension and nutrient translocation from benthic to pelagic habitat. This increase in nutrient availability for phytoplankton in the water column (Fig.1 I and III) was probably the cause of high values of Chl a (Fig. 1 V). Others studies support the idea that bioturbers fish can significantly contribute to a increase in nutrient concentration and turbidity at the water column (Schaus & Vanni, 2000; Adamek & Marsalek, 2013). Sometimes this source of nutrients was about double that of external loading (Braband et al., 1990). Shostell and Bukaveckas (2004) conducted a study in which they quantified the proportion of phytoplankton nutrient demand sustained by gizzard shad, (the same bioturber omnivorous fish used for Schaus & Vanni (2000), they found that nutrient excretion by gizzard shad supplied 14-20% of P uptake and 31-58% of N uptake by plankton. This example highlight the importance the nutrient cycling by a bioturber in a aquatic ecosystem.
Besides nutrient translocation, bioturbers organisms can affect biomass and community structure of phytoplankton due to mechanical resuspension of settled algal cells (Misson at al., 2011). This could represent other positive impact of bioturbers on phytoplankton biomass, since sedimentation of viable phytoplankton cells appears as one of the most significant process whereby biomass reduces (Reynolds & Wiseman, 1982; Reynolds et al., 1982). In a recent work Roozen et al. (2007) used a combination of field and laboratory approaches to show that benthivorous fish, through bioturbation activity, are able to resuspend settled algal cells and enhance phytoplankton biomass and community composition.
Different from the latter case in which fish access to sediment was determinant for positive effects on phytoplankton biomass, the experiment with the filter-feeding, O. niloticus, showed an increase in phytoplankton biomass that was associated with fish
49 showing variable effects for omnivorous filter-feeding fish on phytoplankton biomass (Diana et al., 1991; Elhigzi et al., 1995; Starling et al., 1998; Figueredo & Giani, 2005; Attayde & Menezes, 2008; Okun et al., 2008; Rondel et al., 2008; Menezes et al., 2010), our results shows evidence of a classic trophic cascade mediated by this omnivorous fish, which by filtering activity probably reduced zooplankton density and released phytoplankton from grazing pressure.
This positive effect of O. niloticos on phytoplankton biomass is reported in other studies (Starling et al., 2002; Menezes et al., 2010). Unlike P. brevis experiment, O. niloticus was not responsible for increase nutrient concentration or decrease water transparency in the water column, even in the control treatment, both effects indicators of bioturbation process (Croel and Kneitel, 2011; Adamek & Marsalek, 2013). This lack in bioturbation evidence could be explained by the fact that omnivorous filter-feeders as O. niloticus just feed on benthic habitat in specific conditions and the main interaction
with sediment occurs for nest build activity (Joyni et al., 2011). Possibly, the ways that fish species interact with the sediment was a determinant to explain mechanisms responsible for an increase in phytoplankton biomass. Data of zooplankton and phytoplankton community composition are still in analysis phase and will be important to clarify mechanisms behind this positive effect of omnivorous filter-feeding on plankton community.
We compared fish weight at the beginning and at the end of the experiment and expect that both fish species' enhanced weight in the control treatment due to the presence of more food options in this treatment. However P. brevis, that showed strong evidences of interaction with sediment, lose weight (≈15% of initial weight) in both control and no access treatment. We could attribute this reduction as a mesocosm effect, since in control treatment fish was able to feed on sediment. O. niloticus showed a stability of weight in both control and no access treatments, probably due to their filter-feeding strategy that permits fish good conditions for feeding in both treatments, regardless of access condition.