Sevdan Yılmaz
Sebahattin
Ergün
Nergiz Soytaş
Dietary supplementation of cumin (Cuminum
cyminum)
preventing
streptococcal
disease
during first-feeding of Mozambique tilapia
(Oreochromis mossambicus)
Authors’ address:
Department of Aquaculture, Faculty of Marine Sciences and Technology, Çanakkale Onsekiz Mart University, 17100 Çanakkale, Turkey.
Correspondence:
Sevdan Yılmaz
Department of Aquaculture, Faculty of Marine Sciences and Technology, Çanakkale Onsekiz Mart University, 17100 Çanakkale, Turkey.
Tel.: +90 286 2180018 ext. 1589 e-mail: sevdanyilmaz@comu.edu.tr
Article info:
Received: 22 January 2013
Accepted: 21 June 2013
ABSTRACT
This study was conducted to investigate the effect of dietary cumin (Cuminum
cyminum) powder (CP) as a feed additive on growth performance and disease
resistance during first-feeding of Mozamique tilapia (Oreochromis mossambicus). Five isonitrogenous (40% crude protein) and isocaloric (18.9 kj g-1) diets were formulated to contain 0 (control), 0.5, 1, 1.5, and 2.0% CP. In a 45-day feeding trial, 15 plastic tanks (21 L) were stocked with 40 fry (0.012 ±0.001 g) each. After feeding experiment, fish were infected with Streptococcus iniae and mortalities were recorded. The second-order polynomial regression indicated that a dietary CP level of 1.14% provided the best survival rate challenge infection with S. iniae, growth performance and feed utilization. In conclusion, CP can be used as growth promoter to improve feed utilization and weight gain in tilapia fry, and it can be also used as an antimicrobial agent during first-feeding of O. mossambicus. Therefore, CP can be suggested as an alternative to antibiotics in controlling streptococcal disease in tilapia culture.
Key words: disease, fish, cumin powder, Streptococcus iniae, tilapia
Introduction
Tilapias are economically important fish for intensive culture in many countries. Their adults and juveniles are more resistant to bacterial, parasitic, fungal and viral diseases compared to other fish species. However, tilapia larvae is often hampered by high mortality rates, and economic loss due to infectious diseases (Bricknell & Dalmo, 2005) like
Streptococcus sp., Flavobacterium columnare, Aeromonas
hydrophila,and Edwarsiella tarda (El Sayed 2006; Amal &
Zamri Saad, 2011).
Streptococcal disease is mainly controlled by antibiotics in tilapia farming (Abutbul et al., 2004). However, their continuous use often leads to the development of drug resistance (Zilberg et al., 2010) and it might leave unwanted toxic residues in their flesh and environment (Chitmanat et al., 2005). In recent years, herbal supplements have been used instead of chemical applications in aquaculture because they are more consumer acceptance and ecofriendly approach in
disease management (Raa, 1996). Medicinal herbs or spices are able to enhance immunity and generate more pathogen resistance (Harikrishnan et al., 2011a). Several studies have also reported that the spices like garlic, ginger, thyme, rosemary and fenugreek improved health status, growth performance and/or disease resistance in Dicentrarchus
labrax and O. mossambicus (Yılmaz & Ergün, 2012; Yılmaz
et al., 2012; Yılmaz et al., 2013).
Varieties of spices have been used traditionally to prevent and treat diseases and are known to improve the immune system (Chauhan et al., 2010). Allspice (Cuminum cyminum,
Apiaceae) has been used as a spice since ancient times
relaxant compound in animals and human beings (Boskabady et al., 2005; Azeez, 2008).
Previous studies have also reported that herbs or spices such as Allium sativum (Aly et al., 2008), Nigella sativa
(Diab et al., 2008), Syzygium aromaticum
(Rattanachaikunsopon & Phumkhachorn, 2009), thyme, rosemary and fenugreek have been successfully used in tilapia culture. On the other hand, information regarding larval culture, especially the effects of herbal supplements on fish performance and disease resistance, are not clear. Hitherto, there was only one report on the effects of herbs (garlic and echinacea) on the growth performance and disease resistance (A. hydrophila and Pseudomonas fluorescens) of tilapia (O. niloticus) fry (Aly et al., 2010). However, no study has been reported in literature with respect to the dietary cumin on the growth rate and disease resistance against S.
iniae in O. mossambicus fry.
The purpose of this study was to investigate the effect of fish diet supplemented with cumin on the growth performance and disease resistance during first-feeding of O.
mossambicus fry.
Materials and Methods
Experimental design and feeding trial
Sixteen days old O. mossambicus fry averaging about 0.012 g were produced in Çanakkale Onsekiz Mart University, Faculty of Marine Sciences and Technology, Çanakkale, Turkey. During the experiment, water quality characteristics (mean ± SE) were as follows: temperature was 28.3±0.1°C, pH was 7.1±0.2, dissolved oxygen was 7.10±0.3 mg.L-1, conductivity was 585±20 uS, total NH
3 was
0.13±0.01 mg.L-1, nitrite was 0.06±0.02 mg.L-1 and nitrate
was 1.1±0.1 mg.L-1
. Fish experiments were performed in accordance with the guidelines for fish research from the animal ethic committees at Çanakkale Onsekiz Mart University, Çanakkale, Turkey.
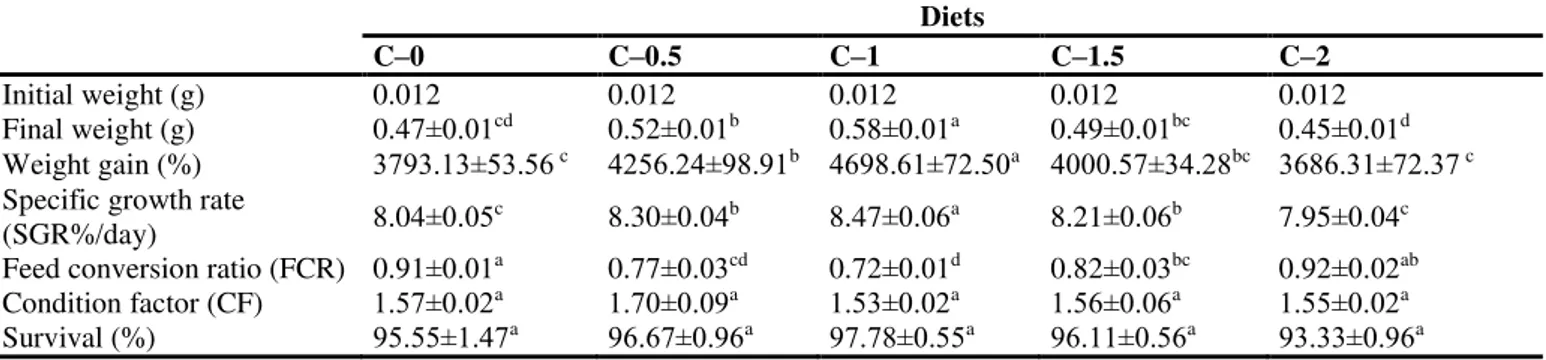
Cumin (Cuminum cyminum) seed meal was obtained from Kotanyi, GmbH, Istanbul, Turkey. Five experimental diets were prepared with the supplementation of cumin at the concentrations of 0, 0.5, 1, 1.5 and 2% for diets C-0, C-0.5, C-1, C-1.5 and C-2, respectively. The feed components of control and experimental diets are presented in Table 1.
Table 1. The proximate composition of the experimental diets supplemented with different concentrations (C-0 to C-2) of cumin.
Experimental diets
C–0 C–0.5 C–1 C–1.5 C–2
Ingredients (%)
Fish meala 30.00 30.00 30.00 30.00 30.00
Soybean meala 33.00 33.00 33.00 33.00 33.00
Wheat floura 15.00 15.00 15.00 15.00 15.00
Fish oila 6.50 6.50 6.50 6.50 6.50
Vitamin–mineral mixb,c 4.0 4.0 4.0 4.0 4.0
Starchd 6.50 6.00 5.50 5.00 4.50
Cumine 0 0.5 1.0 1.5 2.0
Total 100 100 100 100 100
Chemical analyses
Protein (%) 40.30 40.39 40.48 40.57 40.66
Fat (%) 10.43 10.54 10.65 10.76 10.87
Ash (%) 9.98 10.02 10.06 10.10 10.14
Nitrogen-free extractsf 30.80 30.57 30.34 30.11 29.88
Energy (kj.g-1)g 18.87 18.89 18.92 18.94 18.97
The ingredients were mixed in a blender. The pellets (2 mm diameter) were made in a mincing machine, and the pellets were dried in a drying cabinet (40°C) until moisture dropped to around 10%. The pellets were crushed into desirable particle sizes (150-250 μm) and stored at –20°C until use.
The experiment was designed in triplicate for each diet. Fifteen number of 21 L capacity plastic tanks were stocked with 600 fry (40 fry/tank). The fry were fed at the rate of 12% (15 days), 10% (15 days) and 8% (15 days) of body weight per day in three feedings (09:00, 13:00 and 17:00 h) for 45 days. Each tank was provided with sponge filters connected via airline to a Resun GF–120 air pump. During the experiment, water was exchanged daily at a rate of ~10% of the total volume.
Growth trial and proximate chemical analysis of diets
Growth performance and feed utilization were calculated according to the following formulae:
Weight gain (g) = final fish weight – initial fish weight, Weight gain (%) = 100 (final fish weight – initial fish weight) / initial fish weight,
Specific growth rate (SGR, %/day) = 100 (ln final fish weight) – (ln initial fish weight) / experimental days,
Feed conversion ratio (FCR) = feed intake / weight gain, Condition factor (CF)= Fish weight × 100 / total length3
Proximate analyses of the diets were performed using standard methods (AOAC, 1998). Dry matter was analyzed by drying at 105°C in an oven to a constant weight, crude fat by ether extraction, crude protein by the Kjeldahl method, and crude ash by incineration at 525°C in a muffle furnace for 12 h.
Bacteria and challenge experiment
The bacterium (Streptococcus iniae) was previously isolated from diseased tilapia. Specimens were collected aseptically from brain and anterior kidney sites at post-mortem examination. Specimens were cultured directly onto sheep blood agar at 28°C for 24–48 h. Gram-stained positive, beta-hemolytic, catalase negative coccus colonies were subcultured onto blood agar and then identified by APIStrep (Biomerieux). The isolated S. iniae were kept frozen in 15% glycerol, 85% Brain Heart Infusion (BHI) broth, in aliquots, at –70°C until used.
After the growth trial (45 days), each group of fish fry was kept in an aerated 3 L glass jar (30 fish/jar) at 28°C
(Wiedenmayer et al., 2006). The bacterial culture was prepared by inoculating 250 ml of BHI broth in 500 mL culture flask with a thawed 1 mL aliquot of the frozen S.
iniae isolate and incubating it for 24 h at 28°C. The broth
culture was centrifuged at 5000 rpm at 15°C for 10 min (Abutbul et al., 2004). Then, the pellet was resuspended in phosphate buffer saline (PBS), and each dilution was added to a glass jar containing 3L of water. Each dilution trial was conducted in triplicates, and mortalities were recorded daily for 6 days. Fresh dead fish were cultured brain and anterior kidney on blood agar to confirm S. iniae.
Statistics
Each value was expressed as mean ± SEM for each of the measured variables. Statistical significance (P < 0.05) determined by ANOVA followed by a TUKEY post hoc multiple comparison test with SPSS 17.0 (SPSS Inc., Chicago, IL, USA) software (Logan, 2010). The second-order polynomial regression analysis was introduced to determine the optimum cumin requirement of tilapia fry. The survival of tilapia in each challenge treatment group was estimated using Kaplan-Meier analysis and differences between the groups were assessed with the Log-Rank (MantelCox) test for pairwise comparisons.
Results
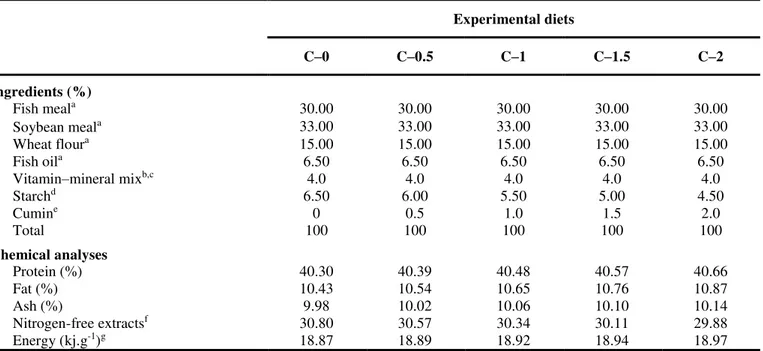
The five diets were equally accepted by the fish and there was no disease in any treatment. There were no significant differences (P > 0.05) in survival rate and condition factor among the experimental diets (Table 2). The C–2 diets were not significantly (P > 0.05) caused the change in tilapia growth performance, average weight, and average length. However, final weight and weight gain (Table 2), and average weight and average length (Figure 1) of tilapia fry fed the C–0.5 and C–1 diets were significantly (P <0.05) greater than that of tilapia fry fed the C–0 diets (Table 2). It is founded that the highest and the lowest FCR were obtained at C–0 and C–1 diets (0.72±0.01 and 0.91±0.01, respectively). Moreover, SGR values increased significantly at cumin-supplemented diets (C–0.5, C–1, and C–1.5) and its highest value was obtained with C–1 diet (8.47±0.06, Table 2).
Table 2. Growth performance and feed utilization of O. mossambicus fry fed diets containing different concentrations
(C-0 to C-2) of cumin for 45 days. 1
Diets
C–0 C–0.5 C–1 C–1.5 C–2
Initial weight (g) 0.012 0.012 0.012 0.012 0.012
Final weight (g) 0.47±0.01cd 0.52±0.01b 0.58±0.01a 0.49±0.01bc 0.45±0.01d Weight gain (%) 3793.13±53.56 c 4256.24±98.91b 4698.61±72.50a 4000.57±34.28bc 3686.31±72.37 c Specific growth rate
(SGR%/day) 8.04±0.05
c 8.30±0.04b 8.47±0.06a
8.21±0.06b 7.95±0.04c Feed conversion ratio (FCR) 0.91±0.01a 0.77±0.03cd 0.72±0.01d
0.82±0.03bc 0.92±0.02ab Condition factor (CF) 1.57±0.02a 1.70±0.09a 1.53±0.02a 1.56±0.06a 1.55±0.02a Survival (%) 95.55±1.47a 96.67±0.96a 97.78±0.55a 96.11±0.56a 93.33±0.96a
Legend: 1 Values are means ± SEM (n=3). Different letters in same line indicate significant differences within groups (P < 0.05).
Figure 1. Effect of cumin on average body weight (g) and length (cm) of O. mossambicus fry. Significant different (P<0.05) between the experimental diets are indicated by different letters.
Figure 2.The relationships between dietary cumin levels and final weight of O. mossambicus fry.
Figure 3. The relationships between dietary cumin levels and SGR of O. mossambicus fry.
Figure 4. The relationships between dietary cumin levels and FCR of O. mossambicus fry.
d bc
a b
cd
d bc
a ab
cd
0.00 0.20 0.40 0.60 0.80
C-0 C-0.5 C-1 C-1.5 C-2
Experimental diets
A
ve
ra
ge
w
e
ight
(
g)
a
2.90 3.10 3.30 3.50 3.70 3.90
A
ve
ra
ge
l
e
ngt
h (
c
m
)
a
Average weight (g) Average length (cm)
y = -0.0924x2 + 0.1735x + 0.4555
R2 = 0.7664
0.40 0.50 0.60
0 0.5 1 1.5 2
F
ina
l w
e
ight
(
g)
aa
y = -0.4227x2 + 0.7981x + 8.0265 R2 = 0.8958
7.5 8 8.5 9
0 0.5 1 1.5 2
S
G
R
aa
y = 0.1796x2 - 0.3464x + 0.9018 R2 = 0.8445
0.6 0.8 1
0 0.5 1 1.5 2
F
C
R
aa
Cumin meal (%)
Cumin meal (%)
Figure 5. Kaplan-Meier survivorship curves of control (C–0) and in groups fed with cumin supplemented in diets for C-0.5 to C-2 following challenge with S. iniae.
In the challenge experiment (Figure 5), a significant increas (P<0.05) in the survival rate of S. iniae infected tilapia fry fed the C–1 (84%), C–1.5 (72%), and C–2 (61%) diets, while the survival rates of tilapia fry fed the C–0.5 (45%) diets did not significantly (P>0.05) change in survival rate compared that of the tilapia fry fed the C–0 (43%) diets.
Discussion
The present study shows that the highest fish growth and feed utilization were obtained at 1% of cumin diet. There are some similar plants used as supplementary diet ingredients. For example, Ahmad & Tawwab (2011) conducted an experiment with Nile tilapia fingerlings fed a basal diet containing 0, 5, 10, 15, or 20 g.kg-1 diet caraway seed, Carum
carvi (belonging to family Apiaceae as cumin) for 12 weeks,
and they found that the use of 12.5 g.kg-1caraway seed meal
improved fish performance compared to the fingerlings fed the control diet (0 g.kg-1). Abd El Hakim et al. (2010) also
evaluated the use of fennel, Foeniculum vulgare (Apiaceae) in a diet for Nile tilapia fingerlings for 14 weeks and they stated that 1.0% fennel produced the maximum fish performance. On the other hand, there are negative effects of dietary herbal additives on fish growth also. For instance, Yılmaz et al. (2006) investigated the effects of four inclusion levels of Ferula coskunii (Apiaceae) (0, 1.5, 3, 3.5 and 4%) on growth and feed utilization in carp. In fish fed the F.
coskunii supplemented diets, final weight, weight gain, FCR,
and SGR were negatively affected at each inclusion levels compared with fish fed the control diet (0 g.kg-1). These
(Bandaranayake, 2006). This is in agreement with the findings in the present study and those previously reported (Shalaby et al., 2006; Diab et al., 2008), where the growth rates in O. niloticus fed over the optimum concentration levels of the spice (garlic and black seed), decreased drastically compared to control diet and/or other supplemented spice diets.
As cumin is able to cause O. mossambicus to enhance nutrient digestibility leading to improved nutrient utilization (fat and nitrogen), which in turn it could also explain the better growth. Srinivasan (2005) reported that different active compounds of spices or herbs stimulated digestion with an enhanced bile acid concentration and stimulated the pancreas and increased secretion of digestive enzyme activities (lipases, amylases and proteases). Ali et al. (2011) determined that the use of low protein or low protein low energy diets increased FCR, and decreased weight gain and nitrogen retention in Cobb broiler chicks, while supplementing low protein low energy diet with cumin (0.2%), citric acid (0.2%) and anhydrous sodium sulphate (0.5%) together improved weight gain, FCR and nitrogen retention percentage by 7.21, 6.16 and 16.69%, respectively. These results agree with those obtained by Jang (2010) who found that the addition of 2% cumin to the diet of Ross–308 broiler chicks improved their weight and FCR. The another study showed that heat stress decreased body weight gain, feed intake, FCR, carcass percentage, nitrogen retention, and ash retention in chicks (Ali et al., 2010). However, the addition of 0.2% cumin in the diet can recover the negative effect of heat stress on growth performance, nitrogen retention and ash retention. All of these results confirmed that oral administration of cumin results in the stimulation of digestive enzyme secretion and nutrient digestibility improvement in the intestine.
Moreover, these findings about the effects of cumin on growth, has two or more possible explanations. The first explanation can be illustrated with its attractant characteristic. Harada (1990) found that cumin was a strong attractant of spice for yellowtail. Some early studies also reported that olfactory feed ingredients like spices (basil, caraway, etc.) were found to enhance growth through their ability to act as feeding enhancers (El Dakar et al., 2008; Ahmad & Tawwab 2011). The second explanation may be because it contains vital compounds such as aromatic oils, essential fatty acids, vitamins, minerals, etc. (Azeez, 2008), and these compounds may have important effects on growth. Also research in animals indicates that cumin stimulate the secretion of
pancreatic enzymes, important factors in nutrient digestion and assimilation (Bhosale et al., 2010).
Cumin have a high antimicrobial and antioxidant activity with major compounds of limonene (21.5), α–pinene (29.1%) and 1,8–cineole (17.9%) (Gachkar et al., 2007; Singh et al., 2002). In addition, cumin fruit contains two sesquiterpenoid glucosides, cuminoside A and B, and two alkyl glycosides isolated together with five known compounds (Takayanagi et al., 2003). These compounds are responsible for its immunomodulatory effects. Chauhan et al. (2010) reported that orally administered cumin (100 and 200 mg.kg-1) has the
potential to stimulate the T lymphocytes expression, secretion of Th1 cytokines and suppression of elevated corticosterone levels in normal (non-induced) and cyclosporine-A induced immune-suppressed swiss albino mice, which can be attributed to flavonoid glycoside, 7-(1-O-β -D-galacturonide)-4'-(1-O-β-glucopyranosyl)-3'4',5,7-tetrahydroxyflavone. Flavonoids are natural products present in considerable amounts in spices (Chauhan et al., 2010). A variety of in vitro
and in vivo experiments have shown that flavonoids possess
immunomodulatory activity with different modes of action (Chauhan et al., 2010). The plant constituents may directly initiate activation of the innate defense mechanisms acting on receptors and triggering intracellular gene activation that may result in production of anti-microbial molecules (Bricknell & Dalmo, 2005).
Harikrishnan et al. (2011b) reported that the use of green tea contains large amounts catechins (flavonoid) in a diet for kelp grouper (Epinephelus bruneus) for 15 days and they found that 0.01 and 0.1% level of green tea positively enhances the non-specific humoral and cellular immune responses and disease resistance. A different study showed that the administration of 1.0% spices as rosemary, thyme or fenugreek, which are rich in flavonoids in a diet for O.
mossambicus fry for 45 days and they improved disease
resistance against S. iniae (Yılmaz et al., 2013). In this report,
we demonstrated that the protective effect of cumin in reduced mortality when O. mossambicus fry fed with 1, 1.5 and 2% doses supplementation diet for 45 days against S.
iniae infection. Probably, the enhancement of immune system
by an cumin supplemented diet is possibly an important factor in protecting O. mossambicus against bacterial challenge and in decreasing their percentage mortality.
promoter and an immunostimulant during first-feeding of O.
mossambicus fry. Furthermore, it can be suggested as an
alternative to antibiotics in controlling streptococcal disease in tilapia culture.
Acknowledgement
We would like to thanks Dr. Chanagun Chitmanat for reviewing the manuscript.
References
Abd El Hakim NF, Ahmad MH, Azab ES, Lashien MS, Baghdady ES. 2010. Response of Nile tilapia, Oreochromis niloticus to diets supplemented with different levels of fennel seeds meal (Foeniculum vulgare). Abbassa Int. J. Aquac., 3: 215-230. Abutbul S, Golan–Goldhirsh A, Barazani O, Zilberg D. 2004. Use
of Rosmarinus officinalis as a treatment against Streptococcus iniae in tilapia (Oreochromis sp.). Aquaculture, 238(1–4): 97-105.
Ahmad MA, Tawwab M. 2011. The use of caraway seed meal as a feed additive in fish diets: growth performance, feed utilization, and whole–body composition of Nile Tilapia, Oreochromis niloticus (L.) fingerlings. Aquaculture, 314(1–4): 110-114.
Ali MN, Moustafa KEKME, Shabaan M, Radwan AM, Sayed MA. 2011. Effect of using Cuminum cyminum l, citric acid and sodium sulphate for improving the utilization of low protein low energy broiler diets. Int. J. Poult. Sci., 10(7): 514–522.
Ali MN, Qota EMA, Hassan RA. 2010. Recovery from adverse effects of heat stress on slow–growing chicks using natural antioxidants without or with sulphate. Int. J. Poult. Sci., 9(2): 109-117.
Aly SM, Attı NMA, Mohamed FM. 2008. Effect of garlic on the survival, growth, resistance and quality of Oreochromis niloticus. 8th Int. Symp. Tilap. Aquac., 277–295.
Aly SM, El Naggar GO, Mohamed FM, Mohamed WE. 2010. Effect of garlic, echinacea, organic green and vet–yeast on survival, weight gain, and bacterial challenge of overwintered Nile tilapia fry (Orechromis niloticus). J. Appl. Aquac., 22(3): 210-215. Amal MNA, Zamri Saad M. 2011. Streptococcosis in tilapia
(Oreochromis niloticus): a review. J. Trop. Agric. Sci., 34(2): 195–206.
Amin Gh. 2001. Cumin. – In: Peter KV (ed), Handbook of Herbs and Spices. Woodhead Publishing, Boca Raton, p. 164–167. AOAC. 1998. Official methods of analysis of AOAC international.
VA: Association of Official Analytical Chemists, Gaithersburg.
Azeez S. 2008. Cumin. – In: Parthasarathy VA, Chempakam B, & Zachariah TJ. (eds.), Chemistry of Spices. CAB International, Oxfordshire, p. 242–259.
Bandaranayake WM. 2006. Quality control, screening, toxicity, and regulation of herbal drugs. – In: Ahmad I, Aqil F, & Owais M. (eds.), Modern Phytomedicine: Turning Medicinal Plants into Drugs. John Wiley and Sons, Weinheim, p. 25–57.
Bhosale SV, Bhilave MP, Nadaf SB. 2010. Formulation of fish feed using ingredients from plant sources. Res. J. Agric. Sci., 1(3): 284-287.
Boskabady M, Kiani S, Azizi H. 2005. Relaxant effect of Cuminum cyminum on guinea pig tracheal chains and its possible mechanism. Indian J. Pharmacol., 37(2): 111-115.
Bricknell I, Dalmo RA. 2005. The use of immunostimulants in fish larval aquaculture. Fish Shellfish Immun., 19(5): 457-472. Chauhan PS, Satti NK, Suri KA, Amina M, Bani S. 2010.
Stimulatory effects of Cuminum cyminum and flavonoid glycoside on Cyclosporine–A and restraint stress induced immune–suppression in Swiss albino mice. Chem–Biol. Interac., 185(1): 66-72.
Chitmanat C, Tongdonmuan K, Khanom P, Pachontis P, Nunsong W. 2005. Antiparasitic, antibacterial, and antifungal activities derived from a Terminalia catappa solution against some tilapia (Oreochromis niloticus) pathogens. Songklanakarin J. Sci. Technol., 27: 359-364.
Diab AS, Aly SM, John G, Abde–Hadi Y, Mohamed MF. 2008. Effect of garlic, black seed and biogen as immunostimulants on the growth and survival of Nile tilapia, Oreochromis niloticus
(Teleostei: Cichlidae), and their response to artificial infection with Pseudomonas fluorescens. Afr. J. Aquat. Sci., 33(1): 63-68. El Dakar AY, Hassanien GD, Gad SS, Sakr SE. 2008. Use of dried basil leaves as a feeding attractant for hybrid tilapia,
Oreochromis niloticus X Oreochromis aureus, fingerlings. Med. Aquacult. J.,1(1): 35–44.
El Sayed A-FM. 2006. Tilapia culture. CABI, Wallingford.
Gachkar L, Yadegari D, Rezaei MB, Taghizadeh M, Astaneh SA, Rasooli I. 2007. Chemical and biological characteristics of
Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chem., 102(3): 898-904.
Harada K. 1990. Attraction activities of spices for oriental weatherfish (Misgurnus anguillicaudatus) and yellowtail (Seriola quinqueradiata). Bull. Japan. Soc. Sci. Fish., 56(12): 2029-2033.
Harikrishnan R, Balasundaram C, Heo MS. 2011a. Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture, 317(1–4): 1-15.
Harikrishnan R, Balasundaram C, Heo MS. 2011b. Influence of diet enriched with green tea on innate humoral and cellular immune response of kelp grouper (Epinephelus bruneus) to Vibrio carchariae infection. Fish Shellfish Immun.,30(3): 972-979. Jang JP. 2010. Comparison of effect of Cuminum cyminum and
probiotic on performance and serum composition of broiler chickens. Annals Biol. Res., 2(6): 630-634.
Logan M. 2010. Biostatistical design and analysis using r: a practical guide. Wiley–Blackwell, London.
Raa J. 1996. The use of immunostimulatory substances in fish and shellfish farming. Rev. Fish. Sci., 4(3): 229-288.
Rattanachaikunsopon P, Phumkhachorn P. 2009. Protective effect of clove oil–supplemented fish diets on experimental Lactococcus garvieae infection in tilapia. J. Biosci. Bioeng., 73(9): 2085-2089.
Shalaby AM, Khattab YA, Abdel Rahman AM. 2006. Effects of garlic (Allium sativum) and chloramphenicol on growth performance, physiological parameters and survival of Nile tilapia (Oreochromis niloticus). J. Venom. Anim. Toxins, 12(2): 172-201.
Srinivasan K. 2005. Spices as influencers of body metabolism: an overview of three decades of research. Food Res. Int., 38(1): 77– 86.
Takayanagi T, Ishikawa T, Kitazima J. 2003. Sesquiterpene lactone glucosides and alkyl glucosides from the fruit of cumin. Phytochemistry, 63(4): 479-484.
Wiedenmayer AA, Evans JJ, Klesius PH. 2006. Experimental
Edwardsiella tarda infection in nonabraded channel catfish
Ictalurus punctatus by immersion. Fisheries Sci., 72(5): 1124-1126.
Yılmaz E, Genc MA, Çek Ş, Mazlum Y, Genc E. 2006. Effects of orally administered Ferula coskunii (Apiceae) on growth, body composition and histology of common carp, Cyprinus carpio. J. Anim. Vet. Adv., 5(12): 1236-1238.
Yılmaz S, Ergün S, Soytaş N. 2013. Herbal supplements are useful for preventing streptococcal disease during first–feeding of tilapia fry, Oreochromis mossambicus. The Israeli Journal of Aquaculture – Bamidgeh, IJA_64.2013.833.
Yılmaz S, Ergün S, Çelik EŞ. 2012. Effects of herbal supplements on growth performance of sea bass (Dicentrarchus labrax): Change in body composition and some blood parameters. J. BioSci. Biotech., 1(3): 217-222.
Yılmaz S, Ergün S. 2012. Effects of garlic and ginger oils on hematological and biochemical parameters of sea bass,
Dicentrarchus labrax. J. Aquat. Anim. Health, 24(4): 219-224. Zilberg D, Tal A, Froyman N, Abutbul S, Dudai N, Goldhirsh AG.
2010. Dried leaves of Rosmarinus officinalis as a treatment for