Jaguar Densities across Human-Dominated
Landscapes in Colombia: The Contribution of
Unprotected Areas to Long Term
Conservation
Valeria Boron1
*, Joseph Tzanopoulos1, Jenny Gallo2, Jorge Barragan3, Laura
Jaimes-Rodriguez4, George Schaller5,6, Esteban Payán2
1Durrell Institute of Conservation and Ecology, University of Kent, Canterbury, United Kingdom,2Panthera Colombia, Bogotá, Colombia,3La Aurora Civil Society Nature Reserve, Hato Corozal, Colombia,4Escuela de Biologia, Universidad Industrial de Santander, Bucaramanga, Colombia,5Panthera, New York, New York, United States of America,6Wildlife Conservation Society, Bronx, New York, United States of America
*vb210@kent.ac.uk
Abstract
Large carnivores such as jaguars (Panthera onca) are species of conservation concern
because they are suffering population declines and are keystone species in their ecosys-tems. Their large area requirements imply that unprotected and ever-increasing agricul-tural regions can be important habitats as they allow connectivity and dispersal among core protected areas. Yet information on jaguar densities across unprotected landscapes it is still scarce and crucially needed to assist management and range-wide conservation strategies. Our study provides the first jaguar density estimates of Colombia in agricultural regions which included cattle ranching, the main land use in the country, and oil palm culti-vation, an increasing land use across the Neotropics. We used camera trapping across two agricultural landscapes located in the Magdalena River valley and in the Colombian lla-nos (47–53 stations respectively;>2000 trap nights at both sites) and classic and spatially explicit capture-recapture models with the sex of individuals as a covariate. Density esti-mates were 2.52±0.46–3.15±1.08 adults/100 km2in the Magdalena valley, whereas 1.12 ±0.13–2.19±0.99 adults/100 km2in the Colombian llanos, depending on analysis used. We suggest that jaguars are able to live across unprotected human-use areas and co-exist with agricultural landscapes including oil-palm plantations if natural areas and riparian hab-itats persist in the landscape and hunting of both jaguar and prey is limited. In the face of an expanding agriculture across the tropics we recommend land-use planning, adequate incentives, regulations, and good agricultural practices for range-wide jaguar connectivity and survival.
a11111
OPEN ACCESS
Citation:Boron V, Tzanopoulos J, Gallo J, Barragan J, Jaimes-Rodriguez L, Schaller G, et al. (2016) Jaguar Densities across Human-Dominated Landscapes in Colombia: The Contribution of Unprotected Areas to Long Term Conservation. PLoS ONE 11(5): e0153973. doi:10.1371/journal. pone.0153973
Editor:Christian Andrew Hagen, Oregon State University, UNITED STATES
Received:September 25, 2015
Accepted:April 6, 2016
Published:May 4, 2016
Copyright:© 2016 Boron et al. This is an open access article distributed under the terms of the
Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability Statement:All relevant data are within the paper and its Supporting Information files. Spatial data on water courses and water areas of Colombia are available at the open data repository Diva-gis.org (http://www.diva-gis.org/datadown), by selecting "Colombia" under "Country" and "Inland water" under "Subject".
Introduction
Due to their charisma and functional role in maintaining ecosystem integrity and services [1,2] large carnivores such as the big cats have been a focus of conservation research and action [3]. However, despite conservation efforts, their populations are still declining and their range con-tracting with important ecological consequences [1,4]. Habitat loss driven by agricultural expansion is the main cause of biodiversity decline globally [5,6] and constitutes a severe threat for large carnivores because they occur at low densities, have slow population growth rates, require large areas and sufficient prey [7–9], all of which make them particularly vulnerable to extinction. Their prey requirements also make them susceptible to conflict with humans and retaliatory killing, further increasing their vulnerability [10,11].
Abundance, density, and distribution estimates are key information for conservation and management strategies, and when they refer to modified areas they can provide valuable infor-mation on species tolerance limits [12]. Because of large carnivores’cryptic nature and large ranges it is inherently difficult to assess their population status, hindering conservation efforts, particularly across unprotected areas. Spatial requirements of large carnivores imply that most protected areas alone are not viable for their survival [13,14] and that they have to be integrated with increasing human modified areas into wider connectivity landscapes [15–18]. There is evidence on the role of unprotected areas for carnivore conservation: species like cheetahs, wolves (Canis lupus), pumas (Puma concolor), leopards (Panthera pardus), and jaguars are able to live in human use landscapes [12,19–21].
The jaguar is the only living representative of the genus Panthera found in the New World and it is the largest cat existing in the Americas [22]. It ranges from Mexico to Argentina and it has been lost from over 50% of its historical range [15]. Jaguars are keystone species [1] and they are considered Near Threatened by the IUCN. They are a species of conservation concern due to habitat loss, poaching of its prey, and retaliatory killing following predation of livestock [23].
As for the other large carnivores, protected areas are too few in number for long-term jaguar conservation, which requires a landscape approach with both protected and unprotected lands [15,24]. However the latter have been neglected, and only 15% (N = 12) of the jaguar popula-tion density estimates available [25] refer to areas that are completely unprotected. Therefore it is crucial to obtain more estimates across such areas as agricultural and oil palm (Elaeis gui-neensis) landscapes. The latter are particularly of concern as a driver of impoverished habitat with unknown survival value for jaguars [25,26,27,28].
Colombia is extremely important for range-wide jaguar conservation and connectivity due to its position between Central and South America [24]. In Colombia, jaguars inhabit the Ama-zon and Llanos regions, the Pacific coast, inter-Andean valleys, and the northern area along the Caribbean coast, yet only two jaguar densities estimate are available and they were both in the Amazon [29]. Here we use both SECR and CR models to produce the first jaguar density estimates of Colombia outside the Amazon: across an oil palm landscape in the Magdalena watershed and in an extensive cattle ranch in the llanos ecosystem. These data illustrates the complementary conservation role of unprotected areas to wide ranging large carnivores such as the jaguar.
Methods
Ethics statement
The study was conducted across different private properties in the Departments of Santander and Casanare, Colombia. It did not involve manipulation or handling of any living organism as
Rufford Small Grant #14968-1 (http://www.rufford.org/ projects/valeria_boron); The Explorers Club, Exploration Fund grant (https://explorers.org/ expeditions/funding/expedition_grants); and Idea Wild. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
we used non-invasive methods for data collection. Following regulations from the Colombian Government, a study of such nature does not require permits or approval from an Institutional Animal Care and Use Committee or equivalent committee. Each landowner was consulted and personally granted us permission to access and collect data on his or her property.
Study Areas
We conducted the study at two sites in Colombia (Fig 1). Site-I is located in the central part of the Magdalena River inter-Andean valley (7.3751981N -73.8841707E to 7.5404397N -73.7117879E) in the Department of Santander. The region is characterized by humid tropical forests and wetlands [30], however most has been converted into cattle ranches and oil-palm plantations while the remaining natural habitats are threatened by further agricultural and oil palm conversion [31,32]. The climate is tropical with mean annual temperature of 27°C and bimodal rainfall of 2100–2600 mm annually [30]. Land tenure consists mainly of private prop-erties; there are no protected areas; and land cover types comprise secondary forest, shrub, wet-lands, pastures, crops, oil-palm plantations, and urban areas.
Site-II is located in the Orinoco River basin in the llanos region and in the Department of Casanare (5.9552203N -71.4833672E to 6.0812814N -71.2976157E). This area is naturally characterised by seasonally flooded tropical savannahs bisected by riparian forests, and the dominant land use is extensive cattle ranching with introduced grasses [30]. Mean annual tem-perature is 27°C and average rainfall is between 1000 and 3000 mm with a very marked wet season between April and November [30]. The area is part of the Llanos Amazon Jaguar Con-servation Unit (JCU) [15] (Fig 1) and hosts most of its biodiversity richness along water bodies [34]. Land tenure consists mainly of private properties and land cover types include natural and secondary forest, natural and introduced grasslands, and wetlands.
Jaguar prey species have been historically hunted at both sites and hunting still occurs for subsistence and commercial reasons [35]. Killing of jaguars is rare at Site-I [36] while more fre-quent at Site-II, although no exact data is available [37], there has been an estimate based on historical records of killings of 1 individual every 250 km2per year [38]. Widespread extensive cattle ranching at Site-II favours the occurrence of jaguar predation on livestock and conse-quent persecution from ranchers [21,39].
Camera trapping
Camera trapping surveys were done between April and August 2014 at Site-I and in April-May 2014 at Site-II. We employed a camera design which is recommended for jaguar studies [25,40,41] and meets capture recapture models’assumptions, i.e. the population is closed and all individuals have at least some probability of being captured [42,43]. We conducted surveys<120 days and we placed cameras at a distance of 1.6 ± 0.2 km to meet the
assump-tions of the models.
We employed paired stations and a block design of 47 stations at Site-I, covering an area of 154.8 km2(minimum convex polygon), while a continuous design and 53 station across 151.3 km2at Site-II (Fig 1). We used Cuddeback Attack and Ambush, and Panthera series 3 and 4 cameras and set them at a height of 35 cm. Paired stations ensure photographs of both flanks of each passing individual for complete identification purposes.
Data processing and capture-recapture analysis
[44] and have the advantage of not requiring arbitrary buffers to estimate the Effective Trap-ping Areas (ETAs) and hence density values [45,46]. They use the individuals’spatial locations to determine their activity centres or home range centres and then estimate the density of home range centres across a polygon which contains the trap grid [45,46]. SECR models also assume that home ranges are circular and stable during the survey, individuals activity centres are randomly distributed (as a Poisson process), and the encounter rate of an individual with a trap decreases with increasing distance from the activity centre following a predefined function [45,46].
The most commonly used function and the one we also used is the half-normal detection function, which describes the probability of capture (P) of an individual i at a trap j as a func-tion of distance (d) from the activity centre of the individual to the trap as follow: Pij = g0 exp (- dij2/(2σ2), where g0 is the probability of capture when the trap is located exactly at the centre
of the home range, and sigma (σ) is a spatial parameter related to home range size [45]. One
model that is most relevant to camera trapping studies is the Bernoulli or binomial encounter Fig 1. Study areas.Location of the sites in regard to the Jaguar Corridor and Jaguar Conservation Units (JCUs) in Colombia [15,24,33], and map of the study sites with camera locations. Site-1 is part of the Magdalena River valley, while Site-2 is located in the Orinoco River basin. Both sites were surveyed in 2014.
model, under which an individual can be recorded at different camera stations during one sam-pling occasion but only once at each station [41,47]. The models can be fitted in a maximum-likelihood framework [48,49] or in a Bayesian framework using data augmentation [46,50]. We chose maximum likelihood because it gives comparable results to the Bayesian framework [25,51] with quicker computation times and used the package secr in R [52].
We included the exact number of days that each station was active and allowed both param-eters g0 andσto vary with sex of the individuals [25,44,53]. We compared four models using
the Akaike Information Criterion (AIC) [54]:“SECR.0”(null model),“SECR.sex.g0”(g0 varies between males and females),“SECR.sex.σ”(σvaries between males and females), and“SECR.
sex”(both g0 andσvary between males and females). Including individuals sex as a covariate is
important because jaguar populations have unequal ranging patterns between sexes, which would affect capture probabilities [40,43,55].
For non-spatial capture recapture analysis we converted the capture histories of each indi-vidual into a 1 and 0 matrix and we grouped 6 survey days into one sampling occasion [41,44]. Following we analysed the data with the full likelihood closed captures models in program MARK [56] and compared three models that differ in assumed sources of variation in capture probability (p) using AIC:“Mo”(null model),“Mh”(p varies between individuals), and
“Msex”(p varies between males and females). Following, we estimated the effective trapping areas by adding a buffer to the cameras polygon equal to the Mean Maximum Distance Moved (MMDM). The MMDM is calculated by taking the average of the maximum distances between capture locations for all individuals [55]. Finally we calculated density as: D = N/ETA. We fur-ther included densities estimated with program Capture, the Jacknife estimator and both MMDM and 1/2MMDM inS1 Appendix.
Prey capture rates
We calculated capture rates for jaguar prey species at the two sites using the total number of independent capture events of each species divided by the number of trap-nights and expressed as records per 100 trap nights [57,58]. Independent capture events were defined as consecutive photographs of individuals of the same species taken more than 12 hrs apart for gregarious spe-cies (i.e. capybaras,Hydrochoerus sp.; collared peccaries,Pecari tajacu; and white-tailed deer, Odocoileus virginianus), and more than 30 min apart for all other species [58]. A species was considered prey if reported in jaguar diet studies [39,59–61]. It is debatable whether capture rates actually reflect abundance [57,62] hence we do not report them to make inferences about population sizes but for descriptive purposes.
Results
We recorded seven females (49 events) and three males (39 events) at Site-I and two females (8 events), three males (57 events), and one adult individual of unknown sex at Site-II (Table 1). Four of ten individuals recorded at Site-I have been recorded in the area since 2012. The aver-age number of captures per individual was lower for females than males at both sites: 7 (1–13) vs. 13 (3–26) at Site-I and 4 (3–5) vs. 19 (12–28) at Site-II. Captures of multiple individuals at the same camera stations were common and up to six individuals were recorded at one station in Site-I.
The best CR model for Site-I was Mh (AIC = 130.2), but M0 (AIC = 130.6) was also strongly supported (ΔAIC<2); whereas for Site-II the best CR model was Msex (AIC = 75.4) followed
by Mh (AIC = 76.6), which also had strong support (ΔAIC<2) (Table 2). Both supported
(N/100 km2) at Site-I while N = 6.00 ± 0.00 (6.00–6.00) and density = 1.12 ± 0.13 (95% CI: 0.86–1.38) (N/100 km2) at Site-II.
The best SECR model (AIC = 924.2) for Site-I allowed g0 to vary with sex but had a fixedσ
(SECR.sex.g0), while for Site-II the best model (AIC = 612.5) allowed both parameters to vary with sex (SECR.sex). However, SECR.sex and SECR.sex.g0 also had strong support (ΔAIC<2)
for Site-I and Site-II respectively (Table 2).
Therefore we report density estimates and parameters for both SECR models at both sites (Table 3). Under the secr.sex model g0 resulted much lower for females at both sites (0.051 vs. 0.813 at Site-I; 0.009 vs. 0.118 at Site-II), whereasσwas smaller for females at Site-I while for
males at Site II (Table 3). This led to females home ranges estimates of 42.7 km2and 102.1 km2 at Site-I and Site-II respectively, and to male home range estimates of 52.8 km2at Site-I and 38.3 km2at Site-II
We recorded 12 prey species at Site-I and 16 at Site-II with Central American agouti ( Dasy-procta punctuata) and black agouti (Dasyprocta fuliginosa) being the most frequently captured species at Site-I and 2 respectively (S2 Appendix).
Table 1. Parameters and survey features for Site-I and Site-II.
Site-I Site-II
Location Magdalena River valley Orinoco River basin
Survey period April-August 2014 April-May 2014
Traps active 47 52
Trap nights 2251 2457
Minimum Convex Camera polygon (km2) 154.8 151.3
N recorded 10 6
MMDM (km) 4.2 5.7
Effective sampled area (km2) 396.2 537.2
N = Number of individuals; MMDM = Mean Maximum Distance Moved
doi:10.1371/journal.pone.0153973.t001
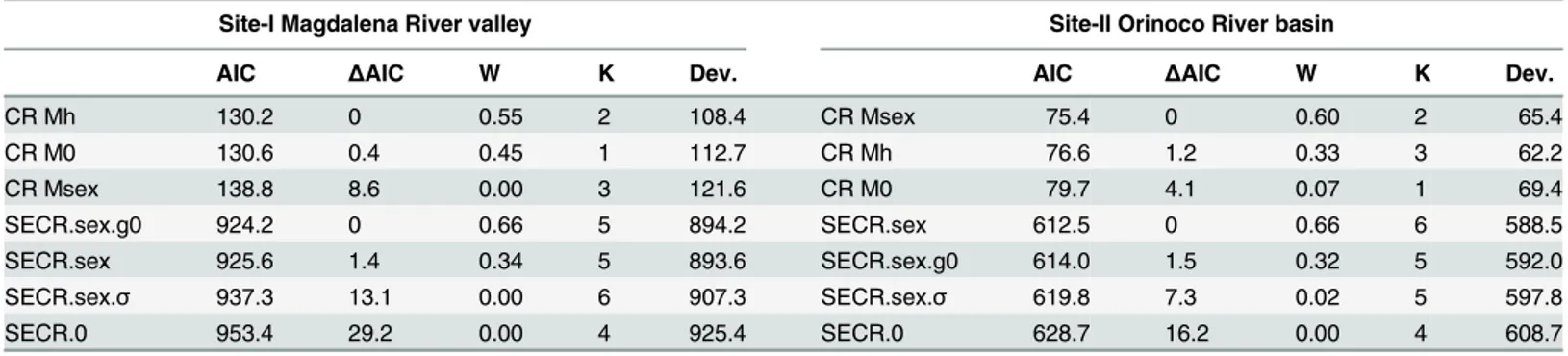
Table 2. Model selection parameters for both Capture-Recapture (CR) and Spatially Explicit Capture Recapture (SECR) models at Site-I and Site-II.
Site-I Magdalena River valley Site-II Orinoco River basin
AIC ΔAIC W K Dev. AIC ΔAIC W K Dev.
CR Mh 130.2 0 0.55 2 108.4 CR Msex 75.4 0 0.60 2 65.4
CR M0 130.6 0.4 0.45 1 112.7 CR Mh 76.6 1.2 0.33 3 62.2
CR Msex 138.8 8.6 0.00 3 121.6 CR M0 79.7 4.1 0.07 1 69.4
SECR.sex.g0 924.2 0 0.66 5 894.2 SECR.sex 612.5 0 0.66 6 588.5
SECR.sex 925.6 1.4 0.34 5 893.6 SECR.sex.g0 614.0 1.5 0.32 5 592.0
SECR.sex.σ 937.3 13.1 0.00 6 907.3 SECR.sex.σ 619.8 7.3 0.02 5 597.8
SECR.0 953.4 29.2 0.00 4 925.4 SECR.0 628.7 16.2 0.00 4 608.7
AIC = Akaike Information Criterion;ΔAIC = difference in AIC values between each model and the model with the lowest AIC; W = AIC model weights;
K = number of model parameters; Dev. = Model Deviances. Mh: capture probability varies between individuals; M0: null model, Msex: capture probability varies between males and females. g0 = probability of capture at the home range centre,σ= spatial parameter related to home range size; SECR.sex.g0: g0 varies between males and females; SECR.sex: both g0 andσvary between males and females; SECR.sex.σ:σvaries between males and females;
SECR.0: null model.
Discussion
It has been recognised that protected areas are inadequate for the long-term conservation of jaguars [18,22]. Therefore, estimating their population size and density in increasingly modi-fied landscapes helps understanding the extent to which jaguar can persist in human areas and informs conservation planning. We provided the first jaguar density estimates of Colombia outside of the Amazon forest [29] and in agricultural landscapes. Cattle ranching is the primary land use in the country and oil palm cultivation is an emerging land use across the Neotropics [28,63].
Jaguar densities
Our results at both sites show that unprotected and productive areas with remaining natural habitats can be important for jaguar populations. Protected areas should always be considered core refuges and they can have a direct effect on population size [29], but large-scale landscape connectivity is also essential. National Parks such as Iguazu and Emas can only harbour small jaguar populations if surrounded by matrices of converted habitat and poaching, and jaguar densities were estimated as low as 0.5–0.9 and 0.3 at those parks respectively [44,64].
Jaguar densities tend to be greater in wetter and prey-rich habitats such as lowland tropical forests [40,53,65] or in the flooded plains of the Pantanal [66] and lower in drier habitats such as the Gran Chaco [67] and Cerrado [44] (S3 Appendix). Densities are also affected by the level of human use: they can be high in productive lands such as cattle ranches in the Pantanal [66], and forestry concessions in the Cerrado [68] and the Amazon [53], but they become low across highly degraded habitats such as Brazilian Atlantic forest [64] or heavily hunted regions [69].
Site-I is within the tropical forest biome and has abundant wetlands and seasonal flooded areas [30], hence it is part of the wetter habitats of the jaguar range. However the SECR density values we obtained at the site (3.0±1.0–3.1±1.1) are lower than similar habitats (S3 Appendix). Tobler et al. [53] report an average jaguar density of 4.4 ± 0.7 across the South Western Ama-zon when using SECR models, while in the Pantanal densities were estimated as high as 6.7 ± 1.1 using a reliable buffer obtained with telemetry [66]. Our lower estimates may have resulted from much of the region being converted to agriculture, including oil palm Table 3. Density and parameters estimated by the two best Spatially Explicit Capture Recapture (SECR) models, i.e. SECR.sex and SECR.sex.g0, at Site-I and Site-II.
Site-I Magdalena River valley Site-II Orinoco River basin
Value SE 95% LCI 95% UCI CV Value SE 95% LCI 95% UCI CV
g0 females SECR.sex 0.051 0.020 0.024 0.106 39% 0.009 0.005 0.003 0.024 56%
g0 males SECR.sex 0.813 0.556 0.003 1.000 68% 0.118 0.025 0.077 0.176 21%
σfemales (km) SECR.sex 1.507 0.147 1.245 1.822 10% 2.327 0.693 1.315 4119 30%
σmales (km) SECR.sex 1.674 0.174 1.366 2.051 10% 1.426 0.129 1.195 1.701 9%
D (N/100km2) SECR.sex 3.15 1.08 1.64 6.05 34% 1.88 0.87 0.79 4.48 46%
g0 females SECR.sex.g0 0.046 0.016 0.023 0.088 35% 0.013 0.006 0.006 0.030 46%
g0 males SECR.secr.g0 0.999 0.000 0.999 0.999 0% 0.108 0.022 0.071 0.159 20%
σ(km) SECR.sex.g0 1.617 0.042 1.537 1.701 3% 1533 133 129 1818 9%
D (N/100km2) SECR.sex.g0 3.04 1.02 1.60 5.78 34% 2.19 0.99 0.93 5.13 45%
SE = Standard error; LCI and UCI = lower and upper confidence intervals respectively; CV = Coefficient of Variation; D = Density. Density values are in bold. g0 = probability of capture at the home range centre,σ= spatial parameter related to home range size; SECR.sex.g0: g0 varies between males and
females; SECR.sex: both g0 andσvary between males and females; SECR.sex.σ: onlyσvaries between males and females; SECR.0: null model.
plantations. However, they are higher than we expected given the extensive habitat conversion. These densities may have resulted from remaining wetlands and existing connectivity with the San Lucas JCU towards the West of the study area as a source for the population (Fig 1). The importance of wetlands for jaguars in the study area is further confirmed by the fact that jaguar were recorded mainly at camera stations situated in wetland habitats and never in oil palm hab-itats. Connectivity between this area and the Catatumbo and Llanos-Amazon JCUs towards the East and South East (Fig 1) is uncertain and should be assessed.
Carnivore densities are highly dependent on the prey base available [9,70] and levels of hunting of both prey and carnivores themselves [69]. Killing of jaguars at Site-I is rare [36] but larger prey species such as deer, tapirs(Tapitus terrestris), peccaries, giant anteaters ( Mymeco-phaga tridactyla), and capybaras on which jaguar depend in other regions [59–61] were absent or infrequent, likely due to both habitat loss and hunting. These species are regularly hunted for subsistence and commercial purposes in Colombia [35]. It is therefore possible that jaguars complement their terrestrial prey base with aquatic species such as caimans (Caiman crocodi-lus) and turtles (PodocnemisandTrachemys sp) as found elsewhere [71].
Site-II is part of the Llanos-Amazon JCU (Fig 1), indicating that jaguars at this site are part of a larger population in a connectivity landscape. The llanos’biome (i.e. seasonally flooded grasslands [30]) is similar to the Pantanal but with some important differences. There is more prey biomass in the Pantanal [72] and flooding is one quarter of the year longer than in the lla-nos, thus limiting productive human land use. Furthermore, the llanos also were colonized 200 years earlier than the Pantanal and display much higher human density and hunting levels. Finally, jaguar densities in the Pantanal were estimated across ranches without hunting in the past 15 years and with extremely low human density. All these factors could explain the lower jaguar density (1.9 ± 0.9–2.2 ± 1.0) we obtained.
Lower jaguar numbers in the llanos could also be due to retaliatory killing following live-stock predation. Incidents of jaguar predation on livelive-stock do occur [37,73] however, currently there is a paucity of data regarding human persecution of jaguar. Past systematic hunting of jaguars for the spotted pelt trade could also explain low population numbers [74] but again, that would assume little to no recovery.
Usually more males than females are recorded in camera trap studies because males tend to move more and have larger home ranges [75]. This is in accordance to what we obtained at Site-II, however the sex ratio was skewed to females (2.3:1) at Site-I, where we even recorded mating events and cubs. This, in addition to recording resident jaguars (since 2012), suggests that the area is important for jaguar conservation and possibly constitutes a breeding refuge [75].
Methodological considerations and sex specific parameters
Our survey effort (47–53 camera stations) was more comprehensive than most jaguar studies, as only 15% of jaguar studies reviewed by Tobler and Powell [25] used>40 camera stations.
Density estimates become unbiased and precision increases if the camera polygon is asymmet-rical [76] and encompasses several home ranges [25,77] which is logistically challenging when sampling wide- ranging species like jaguars. However, even if we assume large home ranges (400 km2) and low detection probabilities at home range center (g0 = 0.01) the density bias for polygons like ours, ca. 150km2, is less than 10% [25].
Female home ranges are usually smaller than those of males’[53,80,83]. We observed the oppo-site pattern at Site-II and could be an artefact of sample size. SECR models assume circular home ranges, and that may have been violated in our landscapes where jaguars move along watercourses and riparian galleries.
Because of sex-specific detection probabilities and home range sizes, including sex as a covariate reduces the bias in density estimates and produced better SECR models at both sites. However the best CR models at Site-1 did not include sex as a covariate and it could be because CR models do not include spatial behaviour, hence reducing differences between the sexes. Ultimately, with small sample sizes, partitioning the data into sex specific group is a trade-off between bias and precision. We also recommend larger camera polygons than ours to increase the number of individuals captured and achieve more accurate density estimates.
We concur with other authors [25,44,53], and recommend using SECR models over CR ones when estimating densities because they are not biased by arbitrary buffers, are robust even with smaller grids [76], and can account for larger numbers of individual and site based covari-ates, producing more reliable estimates and addressing many issues outlined by [86]. Obtaining reliable and comparable estimates is key to avoid biased population statuses, underestimation of threats, and delayed conservation interventions, exposing the species at greater risk of decline. Lastly, we may have under-detected some prey species as all our cameras were placed on roads and trails and might have ignored micro-habitats that are important for certain prey species, however placing cameras on trails is still considered the best option to optimize detec-tion of multiple (forest) mammals at once [57,87,88].
Conclusion
In the case of wide-ranging species such as large carnivores, human-use areas are important habitats for connectivity and dispersal between core protected areas as well as for resident and breeding populations [12,19,89]. Therefore it is essential to study these species in unprotected and modified areas to understand the limits to their tolerance and survival [12]. Our results provide additional evidence on the role of unprotected areas for carnivore conservation, advance current understanding of jaguars in agricultural areas, and provide the first jaguar density estimates in both the llanos ecosystem and in an oil palm landscape. They also indicate that productive areas with extensive cattle ranching and oil palm cultivation can be important for jaguar conservation as long as natural habitats such as wetlands, forests, and riparian galler-ies persist in the landscape. Natural areas in human-dominated regions are crucial for the sur-vival of landscape species worldwide allowing them to disperse and thrive beyond protected areas [15–17,90].
As agriculture and oil palm cultivation continue to expand across the tropics they need to be integrated into range-wide jaguar conservation strategies. For long-term jaguar conservation it is key to engage landowners, implement land-use plans in both regions to maintain natural habitats in the landscape, and establishing further oil palm plantations in already disturbed areas, as identified by García-Ulloa et al. [91]. Across cattle ranching regions it is also crucial to adopt optimal livestock management practices to ensure low predation and low levels of human-jaguar conflict [92,93].
Supporting Information
S1 Appendix. Density results Capture & Mh.
S2 Appendix. Independent capture events and capture rates of jaguars and their prey spe-cies at both sites.
(DOCX)
S3 Appendix. Jaguar (Panthera onca) density estimates from camera trap surveys, modified from Tobler and Powell (2013).
(DOCX)
S4 Appendix. Data, Site-I.
(DOCX)
S5 Appendix. Data, Site-II.
(DOCX)
Acknowledgments
We would like to thank Angela Mejia for helping with the study maps and the Panthera office staff in Bogotá for helping with general logistics. Thanks to A. Quiñones Guerrero, J. Murillo and Cabildo Verde in the Magdalena and the entire Barragán family in the llanos for their local support and their help during fieldwork. Thanks also to our great field guides, to the landown-ers and worklandown-ers for allowing us to work in their properties. Finally, we are extremely grateful to Mathias Tobler for significantly contributing to refining the analysis of the present data.
Author Contributions
Conceived and designed the experiments: VB EP. Performed the experiments: VB JG JB LJ GS EP. Analyzed the data: VB. Contributed reagents/materials/analysis tools: VB JT JG LJ EP. Wrote the paper: VB JT JG JB LJ GS EP.
References
1. Estes J, Terborgh J, Brashares J, Power M, Berger J, Bond W, et al. Trophic downgrading of planet Earth. Science. 2011; 333: 301–306. doi:10.1126/science.1205106PMID:21764740
2. Ripple WJ, Estes JA, Beschta RL, Wilmers CC, Ritchie EG, Hebblewhite M, et al. Status and Ecological Effects of the World’s Largest Carnivores. Science. 2014; 343: 1241484. doi:10.1126/science. 1241484PMID:24408439
3. Brodie JF. Is research effort allocated efficiently for conservation? Felidae as a global case study. Biodi-vers Conserv. 2009; 18: 2927–2939. doi:10.1007/s10531-009-9617-3
4. Macdonald DW, Loveridge AJ, Nowell K. Dramatis personae: an introduction to the wild felids. In: Mac-donald DW, Loveridge AJ, editors. Biology and Conservation of Wild Felids. Oxford: Oxford University Press; 2010. pp. 3–58.
5. Fahrig L. Effects of Habitat Fragmentation on Biodiversity. Annu Rev Ecol Evol Syst. 2003; 34: 487–
515. doi:10.1146/annurev.ecolsys.34.011802.132419
6. Foley J a, Defries R, Asner GP, Barford C, Bonan G, Carpenter SR, et al. Global consequences of land use. Science. 2005; 309: 570–4. doi:10.1126/science.1111772PMID:16040698
7. Crooks KR, Burdett CL, Theobald DM, Rondinini C, Boitani L. Global patterns of fragmentation and con-nectivity of mammalian carnivore habitat. Philos Trans R Soc Lond B Biol Sci. 2011; 366: 2642–51. doi: 10.1098/rstb.2011.0120PMID:21844043
8. Cardillo M, Mace GM, Jones KE, Bielby J, Bininda-emonds ORP, Sechrest W, et al. Multiple Causes of High Extinction Risk in Large Mammal Species. 2005; 1067: 1239–1241.
9. Carbone C, Pettorelli N, Stephens P a. The bigger they come, the harder they fall: body size and prey abundance influence predator-prey ratios. Biol Lett. 2011; 7: 312–315. doi:10.1098/rsbl.2010.0996 PMID:21106569
11. Inskip C, Zimmermann A. Human-felid conflict: a review of patterns and priorities worldwide. Oryx. 2009; 43: 18. doi:10.1017/S003060530899030X
12. Athreya V, Odden M, Linnell JDC, Krishnaswamy J, Karanth U. Big cats in our backyards: persistence of large carnivores in a human dominated landscape in India. PLoS One. 2013; 8: e57872. doi:10. 1371/journal.pone.0057872PMID:23483933
13. Woodroffe R., Ginsberb JR. Edge Effects and the Extinction of Populations Inside Protected Areas. Sci-ence. 1998; 280: 2126–2128. doi:10.1126/science.280.5372.2126PMID:9641920
14. Parks SA, Harcourt AH. Reserve Size, Local Human Density, and Mammalian Extinctions in U.S. Pro-tected Areas. Conserv Biol. 2002; 16: 800–808. doi:10.1046/j.1523-1739.2002.00288.x
15. Sanderson EW, Redford KH, Chetkiewicz CB, Medellin RA, Rabinowitz AR, Robinson JG, et al. Plan-ning to Save a Species: the Jaguar as a Model. 2002; 16: 58–72.
16. Sanderson EW, Redford KH, Vedder A, Coppolillo PB, Ward SE. A conceptual model for conservation planning based on landscape species requirements. Landsc Urban Plan. 2002; 58: 41–56. doi:10. 1016/S0169-2046(01)00231-6
17. Wikramanayake E, McKnight M, Dinerstein E, Joshi A, Gurung B, Smith D. Designing a Conservation Landscape for Tigers in Human-Dominated Environments. Conserv Biol. 2004; 18: 839–844. doi:10. 1111/j.1523-1739.2004.00145.x
18. Crooks KR, Sanjayan MA. Connectivity Conservation. Cambridge, UK: Cambridge University Press; 2006.
19. Mech LD, Boitani L. Wolves. Behavioural Ecology and Conservation. Chicago: The University of Chi-cago Press; 2003.
20. Marker LL, Dickman AJ, Mills MGL, Jeo RM, Macdonald DW. Spatial ecology of cheetahs on north-cen-tral Namibian farmlands. J Zool. 2008; 274: 226–238. doi:10.1111/j.1469-7998.2007.00375.x
21. Payan E, Carbone C, Durant S, Homewood K, Quigley H, Paemelaere E. Where will Jaguars roam? The importance of survival in unprotected lands. Molecular Population Genetics, Evolutionary Biology and Biological Conservation of Neotropical Carnivores. 2013. pp. 603–627.
22. Nowell K, Jackson P. Wild Cats: Status Survey and Conservation Action Plan. IUCN; 1996.
23. Caso A, Lopez-Gonzalez C, Payan E, Eizirik E, de Oliveira T, Leite-Pitman R, et al. Panthera onca. The IUCN Red List of Threatened Species. Version 2015.1 [Internet]. [cited 19 Jun 2015]. Available:http:// www.iucnredlist.org/details/15953/0
24. Rabinowitz A, Zeller K a. A range-wide model of landscape connectivity and conservation for the jag-uar, Panthera onca. Biol Conserv. 2010; 143: 939–945. doi:10.1016/j.biocon.2010.01.002
25. Tobler MW, Powell GVN. Estimating jaguar densities with camera traps: Problems with current designs and recommendations for future studies. Biol Conserv. 2013; 159: 109–118. doi:10.1016/j.biocon. 2012.12.009
26. Fitzherbert EB, Struebig MJ, Morel A, Danielsen F, Donald PF, Phalan B. How will oil palm expansion affect biodiversity? Trends Ecol Evol. 2008; doi:10.1016/j.tree.2008.06.012
27. Danielsen F, Beukema H, Burgess N, Parish F, Bruehl C, Donald P, et al. Biofuel plantations on for-ested lands: double jeopardy for biodiversity and climate. Conserv Biol. 2009; 23: 348–358. doi:10. 1111/j.1523-1739.2008.01096.xPMID:19040648
28. Pacheco P. Soybean and oil palm expansion in South America: a review of main trends and implica-tions. Working paper 90. Bogor; 2012.
29. Payan E. Jaguars, ocelots, and prey ecology across sites with different hunting pressure in Colombian Amazonia. University College London and Institute of Zoology, Zoological Society of London. 2009.
30. IDEAM, IGAC, IAVH, INVEMAR, SINCHI, IIAP. Ecosistemas continentales, costeros y marinos de Colombia. Bogota: Imprenta Nacional de Colombia; 2007.
31. Etter A, van Wyngaarden W. Patterns of Landscape Transformation in Colombia, with Emphasis in the Andean Region. AMBIO A J Hum Environ. 2000; 29: 432–439. doi:10.1579/0044-7447-29.7.432
32. Castiblanco C, Etter A, Aide TM. Oil palm plantations in Colombia: a model of future expansion. Environ Sci Policy. 2013; 27: 172–183. doi:10.1016/j.envsci.2013.01.003
33. Panthera. Jaguar Corridor [Internet]. 2012 [cited 20 Jan 2016]. Available:https://www.panthera.org/ cms/sites/default/files/Jaguar_Corridor_March2012_NoText_0.pdf
34. Payán E, Soto C, Diaz-Pulido A, Nijhawan S, Hoogestein R. El corredor jaguar: una oportunidad para asegurar la conectividad de la biodiversidad en la cuenca del Orinoco. In: Lasso CA, Rial A, Matallana C, Ramírez W, Señaris J, Díaz-Pulido A, editors. Biodiversidad de la Cuenca del Orinoco II Areas
Colombia, Fundación Omacha, Fundación La Salle de Ciencias Naturales e Instituto de Estudios de la Orinoquia (Univers; 2011. pp. 235–247.
35. Rodriíguez-Mahecha JV, Alberico M, Trujillo F, Jorgenson J. Libro Rojo de los Mamiíferos de Colombia. Serie Libros Rojos de Especies Amenazadas de Colombia. Rodriíguez-Mahecha JV, Alberico M, Truji-llo F, Jorgenson J, editors. Bogota: Conservacioón Internacional Colombia, Ministerio de Ambiente, Vivienda y desarrollo Territorial.; 2006.
36. Boron V. Are Jaguars Paying the Price of Our Thirst for Palm Oil? Imperial College London. 2012.
37. Garrote G. Depredación del jaguar (Panthera onca) sobre el ganado en los llanos orientales de Colom-bia. Mastozool Neotrop. 2012; 19: 139–145.
38. Payan E. Jaguar Conservation in the Colombian Llanos: presence, local perceptions and the livestock conflict. New York; 2006.
39. Polisar J, Maxit I, Scognamillo D, Farrell L, Sunquist ME, Eisenberg JF. Jaguars, pumas, their prey base, and cattle ranching : ecological interpretations of a management problem. 2003; 109: 297–310.
40. Silver SC, Ostro LET, Marsh LK, Maffei L, Noss AJ, Kelly MJ, et al. The use of camera traps for estimat-ing jaguar Panthera onca abundance and density usestimat-ing capture/recapture analysis. Oryx. 2004; 38: 148–154. doi:10.1017/S0030605304000286
41. Noss A, Polisar J, Maffei L, García R, Silver S. Evaluating jaguar densities with camera traps. New York; 2013 Jan.
42. Otis D, Burnham K, White G, Anderson D. Statistical inference from capture data on closed animal pop-ulations. Wildlife monographs. 1978.
43. White GC. Capture-recapture and removal methods for sampling closed populations. Los Alamos National Laboratory; 1982.
44. Sollmann R, Furtado MM, Gardner B, Hofer H, Jácomo AT a., Tôrres NM, et al. Improving density esti-mates for elusive carnivores: Accounting for sex-specific detection and movements using spatial cap-ture–recapture models for jaguars in central Brazil. Biol Conserv. 2011; 144: 1017–1024. doi:10.1016/ j.biocon.2010.12.011
45. Efford M. Density estimation in live-trapping studies. Oikos. 2004; 106: 598–610.
46. Royle J, Young K. A hierarchical model for spatial capture-recapture data. Ecology. 2008; 89: 2281–
2289. PMID:18724738
47. Royle J, Nichols J, Karanth K, Gopalaswamy A. A hierarchical model for estimating density in camera-trap studies. J Appl Ecol. 2009; 1: 118–127.
48. Borchers D, Efford M. Spatially explicit maximum likelihood methods for capture–recapture studies. Biometrics. 2008; 64: 377–385. PMID:17970815
49. Efford MG, Dawson DK, Borchers DL. Population density estimated from locations of individuals on a passive detector array. Ecology. Ecological Society of America; 2009; 90: 2676–2682.
50. Royle JA, Gardner B. Hierarchical spatial capture–recapture models for estimating density from trap-ping arrays. In: O’Connell A, Nichols JD, Karanth KU, editors. Camera Traps in Animal Ecology: Meth-ods and Analyses. New York: Springer; 2011. pp. 163–190.
51. Noss AJ, Gardner B, Maffei L, Cuéllar E, Montaño R, Romero-Muñoz A, et al. Comparison of density estimation methods for mammal populations with camera traps in the Kaa-Iya del Gran Chaco land-scape. Anim Conserv. 2012; 15: 527–535. doi:10.1111/j.1469-1795.2012.00545.x
52. Efford MG. secr: Spatially explicit capture-recapture models. R package. 2015.
53. Tobler MW, Carrillo-Percastegui SE, Zúñiga Hartley A, Powell GVN. High jaguar densities and large
population sizes in the core habitat of the southwestern Amazon. Biol Conserv. 2013; 159: 375–381. doi:10.1016/j.biocon.2012.12.012
54. Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-theo-retic Approach. 2nd ed. New York: Springer; 2002.
55. Karanth U, Nichols J. Estimation of Tiger Densities in India Using Photographic Captures and Recap-tures. Ecology. 1998; 79: 2852–2862.
56. White GC, Burnham KP. Program MARK: survival estimation from populations of marked animals. Bird Study. 1999; 46: S120–S139. doi:10.1080/00063659909477239
57. Carbone C, Christie S, Conforti K, Coulson T, Franklin N, Ginsberg JR, et al. The use of photographic rates to estimate densities of tigers and other cryptic mammals. Anim Conserv. Cambridge University Press; 2001; 4: 75–79. doi:10.1017/S1367943001001081
58. O’Brien TG, Kinnaird MF, Wibisono HT. Crouching tigers, hidden prey: Sumatran tiger and prey popula-tions in a tropical forest landscape. Anim Conserv. 2003; 6: 131–139. doi:10.1017/
59. Foster RJ, Harmsen BJ, Valdes B, Pomilla C, Doncaster CP. Food habits of sympatric jaguars and pumas across a gradient of human disturbance. J Zool. 2010; 280: 309–318. doi:10.1111/j.1469-7998. 2009.00663.x
60. Novack AJ, Main MB, Sunquist ME, Labisky RF. Foraging ecology of jaguar (Panthera onca) and puma (Puma concolor) in hunted and non-hunted sites within the Maya Biosphere Reserve, Guatemala. J Zool. 2005; 267: 167. doi:10.1017/S0952836905007338
61. Weckel M, Giuliano W, Silver S. Jaguar (Panthera onca) feeding ecology: distribution of predator and prey through time and space. J Zool. 2006; 270: 25–30. doi:10.1111/j.1469-7998.2006.00106.x
62. Sollmann R, Mohamed A, Samejima H, Wilting A. Risky business or simple solution—Relative abun-dance indices from camera-trapping. Biol Conserv. 2013; 159: 405–412. doi:10.1016/j.biocon.2012. 12.025
63. Etter A, McAlpine C, Wilson K, Phinn S, Possingham H. Regional patterns of agricultural land use and deforestation in Colombia. Agric Ecosyst Environ. 2006; 114: 369–386. doi:10.1016/j.agee.2005.11. 013
64. Paviolo A, Angelo C De, Blanco Y Di, Bitetti M Di. Jaguar Panthera onca population decline in the upper Paraná Atlantic forest of Argentina and Brazil. Oryx. 2008; 42: 554–561.
65. Harmsen BJ. The use of camera traps for estimating the abundanceand studying the ecology of jaguars (Panthera onca). University of Southampton. 2006.
66. Soisalo MK, Cavalcanti SMC. Estimating the density of a jaguar population in the Brazilian Pantanal using camera-traps and capture–recapture sampling in combination with GPS radio-telemetry. Biol Conserv. 2006; 129: 487–496. doi:10.1016/j.biocon.2005.11.023
67. Maffei L, Cullar E, Noss A. One thousand jaguars (Panthera onca) in Bolivias Chaco? Camera trapping in the Kaa-Iya National Park. J Zool. 2004; 262: 295–304. doi:10.1017/S0952836903004655
68. Arispe R, Rumiz D, Venegas C. Censo de jaguares (Panthera onca) y otros mamíferos con trampas cámara en la Concesión Forestal El Encanto. Santa Cruz, Bolivia.; 2007.
69. Quiroga VA, Boaglio GI, Noss AJ, Di Bitetti MS. Critical population status of the jaguar Panthera onca in the Argentine Chaco: camera-trap surveys suggest recent collapse and imminent regional extinction. Oryx. 2013; 48: 1–8. doi:10.1017/S0030605312000944
70. Carbone C, Gittleman J. A common rule for the scaling of carnivore density. Science. 2002; 295: 2273–
2276. doi:10.1126/science.1067994PMID:11910114
71. Azevedo FCC, Verdade LM. Predator-prey interactions: Jaguar predation on caiman in a floodplain for-est. J Zool. 2012; 286: 200–207. doi:10.1111/j.1469-7998.2011.00867.x
72. Schaller GB. Mammals and their biomass on a Brazilian ranch. Arq Zool. 1983; 31: 1–36.
73. Payán E, Ruiz-García M, Franco C. Distribución de jaguares y el conflicto por depredación como ame-naza para su conservación, en la Orinoquía colombiana. In: Romero MH, Maldonado J. A, Bogota JD, Usma JS, Umaña AM, Alvarez MP, et al., editors. Informe sobre el estado de la biodiversidad en
Colombia 2007–2008. Bogotá: Instituto Alexander von Humboldt; 2009. pp. 103–109.
74. Payán E, Trujillo LA. The Tigrilladas in Colombia. Cat News. 2006; 44: 25–28.
75. Maffei L, Noss AJ, Silver SC, Kelly MJ. Abundance/Density Case Study: Jaguars in the Americas. In: O’Connell AF, Nichol JD, Karanth KU, editors. Camera Traps in Animal Ecology: Methods and Analy-ses. New York: Springer; 2011. pp. 163–190.
76. Sollmann R, Gardner B, Belant JL. How does spatial study design influence density estimates from spatial capture-recapture models? PLoS One. 2012; 7: 1–8. doi:10.1371/journal.pone.0034575
77. Maffei L, Noss AJ. How small is too small? Camera trap survey areas and density estimates for ocelots in the Bolivian Chaco. Biotropica. 2008; 40: 71–75. doi:10.1111/j.1744-7429.2007.00341.x
78. Schaller GB, Crawshaw PG Jr. Movement patterns of jaguar. Biotropica. 1980; 161–168.
79. Rabinowitz A, Nottingham B. Ecology and behaviour of the jaguar (Panthera orca) in Belize, Central America. J Zool. 1986; 210: 149–159.
80. Crawshaw PG, Mähler JK, Indrusiak C, Cavalcanti SMC, Leite-Pitman MRP, Silvius KM. Ecology and Conservation of the Jaguar (Panthera onca) in Iguaçu National Park. In: Silvius KM, Bodmer RE, Fra-goso JM V., editors. People in nature: wildlife conservation in South and Central America. New York: Columbia University Press; 2004. pp. 271–285.
81. Ceballos G, Chavez C, Rivera A, Manterola C, Wall B. Tamaño poblacional y conservación del jaguar en la reserva de la biosefera Calakmul, Campeche, México. In: Medellín RA, Equihua C, Chetkiewicz C, Crawshaw P, Rabinowitz A, Redford K, et al., editors. El jaguar en el nuevo milenio. Mexico City: Fondo de Cultura Económica; 2002. pp. 403–417.
83. Cavalcanti S, Gese E. Spatial ecology and social interactions of jaguars (Panthera onca) in the south-ern Pantanal, Brazil. J Mammal. 2009; 90: 935–945.
84. Figueroa A. The ecology and Conservation of jaguars (Panthera onca) in Central Belize: conservation status, diet, movement patterns, and habitat use. University of Florida. 2013.
85. Scognamillo D, Maxit IE, Sunquist M, Polisar J. Coexistence of jaguar (Panthera onca) and puma (Puma concolor) in a mosaic landscape in the Venezuelan llanos. J Zool. London: Published by the Society at Oxford University Press; 2003; 259: 269–279. doi:10.1017/S0952836902003230
86. Foster RJ, Harmsen BJ. A critique of density estimation from camera-trap data. J Wildl Manage. 2012; 76: 224–236. doi:10.1002/jwmg.275
87. Rovero F, Martin E, Rosa M, Ahumada J a., Spitale D. Estimating Species Richness and Modelling Habitat Preferences of Tropical Forest Mammals from Camera Trap Data. Russo D, editor. PLoS One. 2014; 9: e103300. doi:10.1371/journal.pone.0103300PMID:25054806
88. Cusack JJ, Dickman AJ, Rowcliffe JM, Carbone C. Random versus Game Trail-Based Camera Trap Placement Strategy for Monitoring Terrestrial Mammal Communities. PLoS One. 2015; 1–14. doi:10. 5061/dryad.br86d
89. Linnell J, Swenson J, Anderson R. Predators and people: conservation of large carnivores is possible at high human densities if management policy is favourable. Anim Conserv. 2001; 4: 345–349.
90. Thorbjarnarson J, Mazzotti F, Sanderson E, Buitrago F, Lazcano M, Minkowski K, et al. Regional habi-tat conservation priorities for the American crocodile. Biol Conserv. 2006; 128: 25–36. doi:10.1016/j. biocon.2005.09.013
91. Garcia-Ulloa J, Sloan S, Pacheco P, Ghazoul J, Koh LP. Lowering environmental costs of oil-palm expansion in Colombia. Conserv Lett. 2012; 5: 366–375. doi:10.1111/j.1755-263X.2012.00254.x
92. Quigley HB, Crawshaw PG Jr. A conservation Plan for the jaguar Panthera onca in the Pantanal region of Brazil. Biol Conserv. 1992; 61: 149–157.
![Fig 1. Study areas. Location of the sites in regard to the Jaguar Corridor and Jaguar Conservation Units (JCUs) in Colombia [15,24,33], and map of the study sites with camera locations](https://thumb-eu.123doks.com/thumbv2/123dok_br/16451573.197557/4.918.59.868.116.684/location-regard-jaguar-corridor-jaguar-conservation-colombia-locations.webp)