ContentslistsavailableatScienceDirect
Veterinary
Parasitology
j o ur na l ho me p ag e :w w w . e l s e v i e r . c o m / l o c a t e / v e t p a r
The
anthelmintic
effect
of
plant
extracts
on
Haemonchus
contortus
and
Strongyloides
venezuelensis
Camila
O.
Carvalho
a,∗,
Ana
Carolina
S.
Chagas
b,
Fernando
Cotinguiba
c,
Maysa
Furlan
c,
Luciana
G.
Brito
d,
Francisco
C.M.
Chaves
e,
Marília
P.
Stephan
f,
Humberto
R.
Bizzo
f,
Alessandro
F.T.
Amarante
aaUNESP–UniversidadeEstadualPaulista,DepartamentodeParasitologia,InstitutodeBiociências,CaixaPostal510,Botucatu,SP,CEP18618-000,Brazil bEmbrapaPecuáriaSudeste,SãoCarlos,SP,Brazil
cUNESP– UniversidadeEstadualPaulista,DepartamentodeQuímicaOrgânica,InstitutodeQuímica,Araraquara,SP,Brazil dEmbrapaRondônia,PortoVelho,RO,Brazil
eEmbrapaAmazôniaOcidental,Manaus,AM,Brazil fEmbrapaAgroindústriadeAlimentos,RiodeJaneiro,RJ,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received10January2011
Receivedinrevisedform22June2011 Accepted30July2011
Keywords:
Gastrointestinalnematodes Folkmedicine
Plantextracts Control
a
b
s
t
r
a
c
t
Theindiscriminateuseofanthelminticshasresultedintheestablishmentofparasite resis-tance.Thus,thisstudyaimedtoevaluatetheinvitroantiparasiticeffectofplantextractson HaemonchuscontortusinsheepandtheinvivoeffectonStrongyloidesvenezuelensisinRattus norvegicus.TheplantextractsfromPipertuberculatum,Lippiasidoides,Menthapiperita,Hura crepitansandCarapaguianensis,producedatdifferentresearchinstitutions,werechemically analyzedandevaluatedthroughtheegghatchtest(EHT)andlarvaldevelopmenttest(LDT) inH.contortus.P.tuberculatum(150and250mgkg−1ofbodyweight)wasevaluatedforits
anthelminticactiononR.norvegicusexperimentallyinfectedwithS.venezuelensis.Inthe EHT,theLC50andLC90oftheextractswererespectivelyasfollows:0.031and0.09mgmL−1
forP.tuberculatum,0.04and0.13mgmL−1forL.sidoides,0.037and0.10mgmL−1 forM.
piperita,2.16and17.13mgmL−1forH.crepitansand2.03×10−6and1.22×10−12mgmL−1
forC.guianensis.IntheLDT,theLC50andLC90wererespectively:0.02and0.031mgmL−1
forP.tuberculatum,0.002and0.04mgmL−1forL.sidoides,0.018and0.03mgmL−1forM.
piperita,0.36and0.91mgmL−1forH.crepitansand17.65and1890mgmL−1forC. guia-nensis.TheextractofP.tuberculatumshowedthefollowingsubstances:piperamidesas (Z)-piplartine,(E)-piplartine,8,9-dihydropiplartine,piperine,10,11-dihydropiperine,5,6 dihydropiperlongumineandpellitorine.Themajorcompoundsoftheoilswerethymol (76.6%)forL.sidoides,menthol(27.5%)forM.piperitaandoleicacid(46.8%)forC. guia-nensis.Regardingtheinvivotest,neitherdoseofP.tuberculatumcausedanysignificant reduction(P>0.05)inwormburdenandfecaleggcountscomparedwiththecontrolgroup. WeconcludethattheextractsofP.tuberculatum,L.sidoidesandM.piperitahaveeffective activitywhentestedinvitro,butthedosesoftheextractofP.tuberculatumhavenoeffect whenemployedininvivotests.
© 2011 Elsevier B.V. All rights reserved.
∗ Correspondingauthor.Tel.:+551438116239;fax:+551438153744.
E-mailaddresses:cocarvalho@ibb.unesp.br,cocarvalhobio@yahoo.com.br(C.O.Carvalho).
1. Introduction
Widelydistributedaroundtheworld,Haemonchus con-tortus,agastrointestinalnematodeusuallyfoundinsmall ruminants,causeslargeeconomiclossestolivestock breed-ers by causing appetite depression, damages in gastric functionandalterationsin totalproteincontent,energy andmineralmetabolism(Fox,1993).Themain prophylac-ticmethodusedagainstthisparasitehasbeenanthelmintic treatments.However,thewidespreadandindiscriminate administrationof anthelminticshasresulted in parasite resistance.Thefirstcaseofresistancetoanthelminticswas accuratelydescribedbyDrudgeetal.(1964).Thereafter, manystudiesreportingdecreasedanthelmintic effective-nesshavebeenpublished.
Anthelminticsderivedfromplantscanbean alterna-tivefor the treatmentof parasitic infections (Akhtaret al.,2000).Researchin thefield of medicinalplantsis a goodsourceofknowledgeregardingthepotentialaction ofplantextractsoncertaindiseasesandpests.Asaresult, thisareaofstudyhaswitnessedimpressivedevelopment relatedtohumanandanimalhealth.Therearereports indi-catingantiparasiticeffectsofsomeplantspecies,suchas Pipertuberculatum,Lippia sidoides,Mentha piperita,Hura crepitansandCarapaguianensis.Themaincharacteristics oftheseplantsaredescribedinthefollowingparagraphs.
ThecommonnameofP.tuberculatum (Piperaceae)in Brazilis “pimentalonga” or“pimento-d’arta”. ThePiper genusisdistributedinbothhemispheresintropicaland subtropicalregions(JaramilloandManos,2001).Ithasbeen thesubjectofstudiesthathavenoteditsinsecticidal, fungi-cidal,andtrypanocidalactions(Mirandaetal.,2002;Scott etal.,2008; Freire-de-Limaet al.,2008).The toxicityof PiperaduncumtothecattletickRhipicephalus(Boophilus) microplushasalsobeenreported(Silvaetal.,2009).
NativeofnortheasternBrazil,L.sidoides(Verbenaceae) ispopularlyknownas“alecrim-pimenta”,“estrepa-cavalo” and“alecrim-bravo”. Scientific studieshave revealedits effectsagainstsomebacteria(Aguiaretal.,1984;Baraand Vanetti,1998),Leishmania(Oliveiraetal.,2009a)andAedes aegyptilarvae(Carvalhoetal.,2003).
Inturn,M.piperita(Lamiaceae),whichcomesfromthe Mediterraneanregionandisknownaspeppermint,is culti-vatedasahybridofMenthaaquaticaL.andMenthaspicataL. acrosstheworld.Itexhibitsantiseptic,antibacterial, fungi-cidal,antispasmodicandstimulantactions(Mimica-Duki ´c etal.,2003;McKayandBlumberg,2006).
H. crepitans (Euphorbiaceae) is naturally distributed throughoutCentralandSouthAmerica,fromCostaRicato theAmazon.InBrazil,itispopularlyknownas“assacu” or“ac¸ac¸u”(Brondani,2006).Itsseedsandsapwere for-merlyusedasapurgativeandalsoasapopularmedicine totreat elephantiasis,leprosy,rheumaticfever,swelling and intestinal parasites (Francis, 1990). The latex has beenobservedtohaveaneffectonlarvaeoftheticksR. (Boophilus)microplusandR.sanguineus(Brondani,2006).
Finally,C.guianensis(Meliaceae)isatreethatis gener-allyfoundbothinCentralandinSouthAmerica,popularly knownas“andiroba”.Theessentialoilextractedfromthis plant is used industrially in theproduction of candles, shampoos,soapsandrepellents(PastoreJuniorandBorges,
1998,1999).Somestudieshavereportedvarious effects producedbythisplant,suchasanti-allergicandanalgesic effects(Penidoetal.,2006a),acaricidalaction(Fariasetal., 2009),anti-inflammatoryeffect(Penidoetal.,2006b)and insectrepellentaction(Miotetal.,2004;Mendonc¸aetal., 2005).Furthermore,theteapreparedwithC.guianensis’s barkandflowersisusedbothasananthelminticand heal-ingagentinhumans(Boufleuer,2004).
Considering the advances made in this research in recent years, this study aimed to evaluate the in vitro antiparasitic action of five plants (P. tuberculatum, L. sidoides,M.piperita,H.crepitansandC.guianensis)against H.contortus,and theinvivo actionagainstStrongyloides venezuelensisinrats.
2. Materialsandmethods
2.1. Plantmaterial
Theextractsfromthefiveplantspecieswereproduced inthelaboratoriesofseveralinstitutions.TheInstituteof ChemistryofPaulistaStateUniversity,SãoPaulostate, pro-videdthecrudeextractpreparedfrom18kgofleavesofP. tuberculatumcultivatedingreenhouseatAraraquara,São Paulo.The green leavesweredried in a stovewithhot aircirculationandthermostatizedat40◦Cduring4days. Aftergrindingthem,theresultingmaterialwassubjected toextractionwithethylacetateandethanol(3:1),using 9L ofthismixtureforeachextraction. Thisprocesswas repeatedfour timeswithan interval of7daysbetween extractions.Thetotalweightoftheextractobtained cor-respondedto5.5%offreshplantmass(Cotinguibaetal., 2009).
TheessentialoilsfromL.sidoidesandM.piperitawere obtainedattheEmbrapaWesternAmazonResearch Sta-tion fromplants cultivated in Manaus, Amazonasstate, Brazil.TheleavesofL.sidoidesandM.piperitawerecutat groundlevelandplacedinafreezeruntilextraction.After separation oftheleaves,two samplesof20gwereused todeterminemoisturebydryingan ovenat65◦Cfor 3 days.Twoothersamplesof100geachwereusedtoextract theessentialoilbyhydrodistillationinaClevengertype apparatusfor3h.
H.crepitanslatexwascollectedinthetreeslocatedin thecityofPortoVelho,Rondôniastatebyemployeesof EmbrapaRondônia.TheseedoilofC.guianensiswas pro-ducedandacquiredinthelocalmarketofPortoVelho.
2.2. Chemicalanalyzes
TheactivesubstancesfromP.tuberculatumusedinthe presenttrialwerepreviouslydescribedbyCotinguibaetal. (2009).
anHP-5MScapillarycolumn(5%diphenyl,95% dimethyl-silicone,30m×0.25mm;filmthickness0.25m).Helium was used as the carrier gas (1.0mLmin−1), with injec-tion of 1.0mL of a 1% solution of the essential oil in dichloromethaneinaninjectorheatedto250◦C, operat-ing in split mode(splitratio 1:100). Oventemperature wasvariedfrom60to240◦Catarateof3◦Cmin−1.The massdetectorwasoperatedinelectronionization(70eV) withthemassanalyzermaintainedat150◦C,the ioniza-tionsourceat220◦Candtransferlineat260◦C.Toobtain thequantification, theessential oilswere alsoanalyzed in an Agilent 7890A chromatograph (Agilent Technolo-gies, Delaware, USA) equipped with a flame ionization detector (FID) kept at 280◦C and fitted with an HP-5 capillary column(5%diphenyl–95%-dimethyl silicone; 30m×0.32mm;filmthickness0.25m).Thesame injec-tionandchromatographicconditionsabovewereapplied, buthydrogenwasusedasthecarriergas,at1.5mLmin−1. Theresultswereindicatedthroughrelativearea(%area). Linearretentionindiceswerecalculatedbyinjectionofa seriesofn-alkanes(C7–C26)inthesamecolumnand con-ditions stated for GC-FID analyses.Identification of the essentialoils’componentswasdonebycomparisonofboth massspectraandretentionindiceswiththeWiley6th edi-tionspectraldatabaseandliteraturedata.
FortheH.crepitanslatexanalysis,protein
electrophore-sis in polyacrylamide gel was performed, using the
PROTEAN II xi Cell system from BIORAD, according to themethodologyproposedbyLaemmli(1970).The elec-trophoresis was performed over a period of 7h and a voltageof 100V.Proteins fromgelsremainedovernight in astainingsolutionofacetic acid10%(v/v),methanol 40%(v/v)andCoomassiebrilliantblueR2501%(v/v).The gelwasdestainedinasolutioncontaining10%aceticacid (v/v)and40%methanol(v/v),withsolutionrenewedevery 30minuntilobtainingaclearrevelation.Thecalculationof molecularweightproteinfractionswasperformedbythe constructionofstandardcurves,withmolecularmarkers againsttheirrespectivedistancesinthegel.
2.3. Invitroassays
2.3.1. Egghatchtest(EHT)
EggsofH.contortuswereobtainedfromfeces,directly collectedfromtherectumoftwolambs(Ovisaries) exper-imentallyinfected withthestrain H.contortus Pratânia, which hasshown anthelminticresistance to all classes ofdrugsavailablecommerciallyinBrazil(Almeidaetal., 2010).Theeggsweretakenfromfecesaccordingtothe methodologydescribed byBizimenyeraetal.(2006),an adaptationoftheoriginalmethodproposedbyColesetal. (1992). Feces were mixed with distilled water and fil-teredthrough100,56,30and25maperturesieves.In thelast sieve,theeggswereretainedand washedwith distilled water and centrifuged in Falcon tubes (50mL) at 3000rpm/5min filled with water. Then the super-natant wasremovedand aNaCl saturatedsolutionwas added. This solution was once again centrifuged under
the same conditions and the supernatant was washed
through a 25m sieve. The eggs collected were put inside a wine glass toallow sedimentation for 30min.
Theeggcountwasperformedin fivealiquots,andthen 100eggsinsuspensionwereadded perwellto24-well plates. The plantextracts were dilutedin a solutionof Tween 80 (3%) to obtainthe concentrations, and incu-bated in wells (0.3mL per well) for 48h at 25◦C. The concentrations were determined following the ratio of 2 in at least six replicates for each concentration. The highestandthelowestconcentrationsevaluatedforeach plant extract were as follows: 0.313–0.0195mgmL−1 for P. tuberculatum, 1.25–0.039mgmL−1 for L. sidoides, 0.156–0.0098mgmL−1 forM.piperita,2.5–0.156mgmL−1 for H.crepitans and 10–0.625mgmL−1 for C. guianensis. Afterincubation,Lugol’siodinesolutionwasaddedinthe wellstostopthehatchingprocess.ThenumberofL1larvae andeggsperwellwasthencountedusingareverse micro-scopeunder100×magnification(Olympus,modelCK-2, Japan).Thenegativecontrolcontained3%Tween80and thepositivecontrolwaspreparedwith0.025mgmL−1of albendazole,inbothcasesinsixreplicates.
2.3.2. Larvaldevelopmenttest(LDT)
Eggs were recovered according the above
proce-duresandtheLDTwasperformedfollowingthemethod described byHubert and Kerboeuf (1992).In summary, 0.2mLofeggsuspension,containingapproximately100 eggs, wasadded perwell,in 24-well plates.They were incubatedfor24hat27◦CtoobtainL1.Afteregg hatch-ing,90Lofculturemedium(containingEscherichiacoli and yeast extract) was added to each well, followed by the test extract. The concentrations were deter-mined following the ratio of 2 from 5gmL−1 to 0.0003gmL−1 (i.e.,5, 2.5, 1.25gmL−1 and soon) in at leastsix replicatesfor each concentration.The high-est and the lowest concentrations evaluated for each plantextractwereasfollows:0.313–0.0195mgmL−1for
P. tuberculatum, 0.0195–0.0003mgmL−1 for L. sidoides, 0.156–0.0098mgmL−1forM.piperita,2.5–0.0078mgmL−1 forH.crepitansand5–0.039mgmL−1forC.guianensis.The plateswereincubated for6 daysat25◦C, andthen the differentialcountfromL3,L2andL1wasperformed.A solu-tionof0.5%DMSOwaspreparedasnegativecontroland 0.64mgL−1ofivermectinaspositivecontrol,insix repli-cates.
In both in vitro tests, the lowest concentration was determined when thehatching and larval development weresimilartothecontrol.Thehighestconcentrationwas basedonthesolubilityorontheturbiditythatlimitsits readability.
2.4. Invivoassay
Table1
LC50andLC90obtainedintheegghatchtest(EHT)forPiper tubercula-tum,Lippiasidoides,Menthapiperita,HuracrepitansandCarapaguianensis extractsonHaemonchuscontortus.
Species LC50(mgmL−1) LC90(mgmL−1)
P.tuberculatum 0.031(0.029–0.033) 0.09(0.08–0.10)
L.sidoides 0.04(0.038–0.053) 0.13(0.12–0.14)
M.piperita 0.037(0.035–0.039) 0.10(0.09–0.11)
H.crepitans 2.16(1.60–3.40) 17.13(8.61–57.21)
C.guianensis 2.03×10−6(3.78 ×
10−17–4.86 ×10−4)
1.22×10−12(2.53 ×
10−34–7.66 ×10−8)
Thenumbersinparenthesesrefertovaluesofupperandinferior confi-dencelimits95%.
G5– L. sidoides essential oil(150mgkg−1) and G6 – L. sidoidesessentialoil(250mgkg−1).Infectionintensitywas determinedbycountingthenumberofeggspergramof feces(EPG)ondays1,2,3,4and6afterthefirstdayof treatmentandbycountingthenumberofparthenogenetic femalewormsfoundinthefirst-thirdportionofthesmall intestine.Toobtainadultworms,theupperthird ofthe smallintestine wasremovedfromtherats13days after infection,cutlongitudinallyandincubatedinsaline solu-tion(0.9%NaCl)for4hat39◦C(NakaiandAmarante,2001). Theaimofthistestwastoevaluateinratstheextracts thatshowedgreatest activityinthein vitrotests.Thus, itwouldbepossibletoobtainanindicationofthemost active extract in an in vivo model for future testing in sheep.AlthoughtheessentialoilofM.piperitashowedgood resultsintheinvitrotests,itwasnotpossibletoperform theinvivotestwithitduetothesmallamountofextract thatwasprovidedbythepartnerinstitution.
2.5. Statisticalanalysis
IntheEHTandLDT,theefficacyofeachtreatmentwas determinedbasedonhatchingordevelopmentpercentage accordingtothefollowingequation: Inhibition(%)=100 (Ptest/Ptotal),where,Ptestreferstothenumberofeggsinthe EHTornumberoflarvaethatdidnotdevelopuntilL3inthe LDTandPtotalcorrespondstothenumberofeggs+L1(EHT) ornumberofL1+L2+L3(LDT).
The50%lethalconcentration(LC50),i.e.,effective con-centrationtokill50%oftheeggsorlarvae,wasdetermined byProbitanalysis(SASInstitute,2003).
Fortheinvivotests,thevalueswerelogtransformed [log(x+1)]andsubjectedtoanalysisofvariance.The aver-ageswere compared bythe Tukeytest at 5%using the Minitab®statisticalsoftware.
3. Results
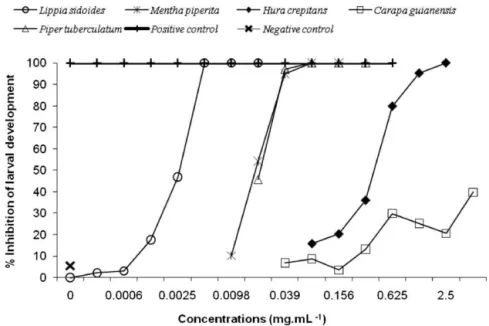
Threeofthefiveextractstested–M.piperita,L.sidoides andP.tuberculatum–exhibitedsatisfactoryresultsbythe EHT(Fig.1),withlowLC50values(Table1).Thepositive controlwas100%effectiveininhibitingegghatchingand thenegativecontrolhadeffectivenessof3.5%.
AccordingtotheLDT,allextractsprovidedsatisfactory resultswiththeexceptionoftheextractofC.guianensis, whichdidnotprovideeffectiveinhibition(Fig.2).Thedata ontheLC50 are shown in Table 2.The positive control
Table2
LC50andLC90obtainedinthelarvaldevelopmenttest(LDT)forPiper tuberculatum,Lippiasidoides,Menthapiperita,HuracrepitansandCarapa guianensisextractsonHaemonchuscontortus.
Species LC50(mgmL−1) LC90(mgmL−1)
P.tuberculatum 0.020(0.0197–0.0206) 0.031(0.029–0.033)
L.sidoides 0.002(0.0016–0.0025) 0.004(0.003–0.007)
M.piperita 0.018(0.0166–0.0186) 0.03(0.03–0.04)
H.crepitans 0.36(0.33–0.40) 0.91(0.80–1.05)
C.guianensis 17.65(7.39–93.01) 1890(257–105,990)
Thenumbersinparenthesesrefertovaluesofupperandinferior confi-dencelimits95%.
presented100%inhibitionoflarvaldevelopmentandthe negativecontrol5.43%.
H.crepitansshowedbetterresultsintheLDT,100% inhi-bitionataconcentrationof2.5mgmL−1,whileatthissame concentrationtheinhibitionintheEHTwasonly16.84%. Incontrast,C.guianensisdidnotshowinhibitoryeffecton thedevelopmentofeggsandlarvae.Atthehighest con-centrationevaluated(10mgmL−1),only8.52%inhibition wasobservedintheEHT,whileintheLDT(5mgmL−1)the inhibitionwas39.74%.
Cotinguibaetal.(2009)performedqualitative identifi-cationofthemainsubstancesintheP.tuberculatumextract and indicated thepresenceofpiperamides, suchas(Z )-piplartine,(E)-piplartine,8,9-dihydropiplartine,piperine, 10,11-dihydropiperine, 5,6-dihydropiperlongumine and pellitorine.TheessentialoilsofL.sidoidesandM.piperita wereanalyzedbygaschromatography-massspectrometry andpresentedastheirmaincomponentsthymol(76.6%) and menthol(27.5%),respectively.TheoilofC. guianen-sis wasevaluated and presented oleic acid(46.8%) and palmiticacid(39.0%)asitsmajorconstituents(Table3).
IntheevaluationoftheextractofH.crepitans,the pres-enceoftwobandswasobserved,a stronglycoloredone correspondingtothepolypeptidechainofmassbetween 36.5and 49.5kDaandweaklystainedpolypeptidechain correspondingtoamassbetween36.5and28.8kDa.From themassof28.8kDa,therewasdiffusestainingwith spe-cificstainingfortheproteinthatdiffusedthroughtheend ofthegel.Thepresenceofproteinmaterialwithmolecular massabove200kDawasobservedontopofthegel.
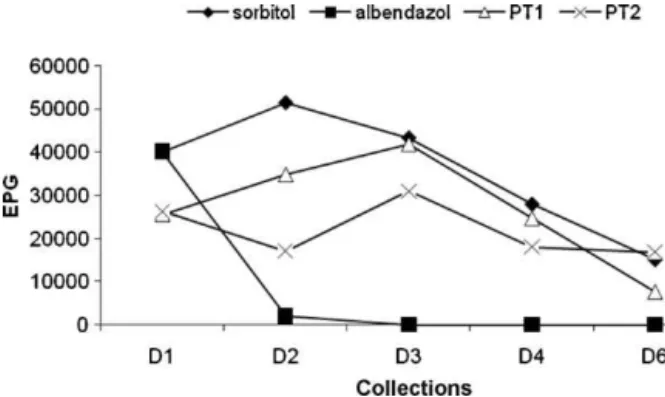
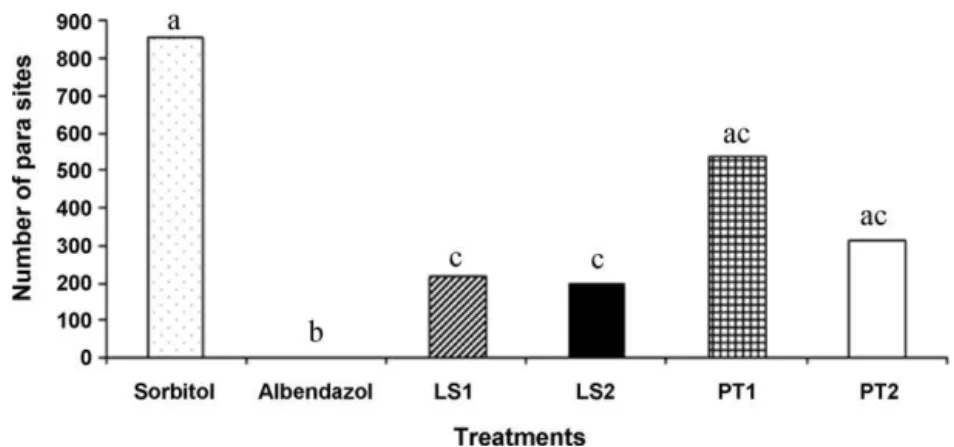
TheinvivoassaywasperformedwiththeextractsofP. tuberculatumandL.sidoides.Theinvivoassayconducted withtheextractofP.tuberculatumshowedthattherewas nosignificantreductionin theEPG(Fig.3)andinadult parasiteaverages incomparison withthecontrolgroup (P>0.05)(Fig.4).ConcerningtheextractofL.sidoides,there wassedativeactiononratsthatreceivedbothdoses.Asa result,theadministrationofthethreeconsecutivedosesof thisextractwasnotpossible,sofecalexamswerealsonot performed.Consideringadultparasites,therewere statisti-caldifferencesbetweenthetreatmentgroups(G5showed areductionof74.4%andG6of76.8%)andsorbitolcontrol group(Fig.4).
4. Discussion
a-Fig.1.MeanpercentageofinhibitionofHaemonchuscontortusintheegghatchtest(EHT)performedwithextractsofPipertuberculatum,Lippiasidoides, Menthapiperita,HuracrepitansandCarapaguianensis.
Vasconcelosetal.(2007)evaluatedinvitrotheanthelmintic potentialoftheessentialoilofthesameplantagainsteggs andlarvaeofH.contortusandobtainedmeaningfulresults: attheconcentrationof0.62mgmL−1therewas94.88%egg hatchinginhibition.TheLC50was0.40mgmL−1intheEHT and2.97mgmL−1intheLDT.Weobserved100%inhibition ofegghatchingataconcentrationof0.625mgmL−1 and theLC50was0.04mgmL−1 intheEHTand0.02mgmL−1 intheLDT.ThesedifferencesbetweentheLC50ofthetwo studiesispossiblyduetothecompositionoftheessential oils.Inthepresentstudy,thymolaccountedfor76.6%of theL.sidoidesessentialoilwhilethissubstanceonly rep-resented59.65%oftheoiltestedbyCamurc¸a-Vasconcelos etal.(2007).Themainelementof theessential oilofL.
sidoidesisthymolandtherearereportsofitsantimicrobial activity(Helanderetal.,1998;Nostroetal.,2007), mol-luscicidalactivity(Singhetal.,1999)andlarvicidalactivity (Carvalhoetal.,2003).Camurc¸a-Vasconcelosetal.(2007) conductedtestswiththymolandobtainedresultsvery sim-ilartothatfoundwiththeessentialoil,whichsuggeststhat thiscompoundmayhaveovicidalandlarvicidalaction.
TheessentialoilofM.piperitaalsoprovidedinteresting resultsin vitro.The action of menthol, oneof the con-stituentsofthisoil(includedintheterpenoidsclass)has alsobeenreportedinsomestudies.Júnior(2003)reported theinsecticidalactivityofsometerpenoids,whichmightbe involvedintheinhibitionorretardationofgrowth, matu-rationdamage,reducedreproductivecapacityorappetite
Table3
PercentagecompositionofLippiasidoides,MenthapiperitaandCarapaguianensisessentialoilsobtainedbygaschromatography/massspectrometry.
L.sidoides % M.piperita % C.guianensis %
␣-Tujene 0.3 ␣-Tujene t Oleicacid 46.8
␣-Pinene 0.1 ␣-Pineno 0.8 Palmiticacid 39
Mircene 1.1 3-Methyl-cyclohexanone 0.1 ␣-Copaene 2.3
␣-Terpinene 0.7 Sabinene 0.4 Stearicacid 1.7
o-Cimene 6.3 -Pinene 1.3 ␣-Cubebene 0.5
Limonene 0.4 Mircene 0.6 n.i. 8.7
1.8-Cineol 0.7 3-Octanol 0.1
␥-Terpinene 2.0 o-Cimene 0.1
Ipsdienol 0.6 Limonene 3.5
Umbelulone 0.2 1,8-Cineole 2.1
4-Terpinenol 1.0 cis--Ocimene 0.1
␣-Terpineol 0.2 ␣-Terpinene 0.1
Timil-methyl-ether 1.0 cis-Sabinenehydrate 0.2
Thymol 76.6 Terpinolene 0.1
␣-Copaene 0.4 Linalool 0.1
-Caryophylene 5.0 neo-Isopulegol 0.1 Aromadendrene 0.4 p-Ment-3-en-8-ol 0.1
␣-Humulene 0.3 Menthone 11.0
Ledene 0.3 Menthofuran 22.5
␦-Cadinene 0.3 Menthol 27.5
Caryophylleneoxide 0.7 4-Terpineol 1.0
iso-Menthol 0.2
␣-Terpineol 0.3
Pulegone 12.8
Piperitone 0.6
neo-Menthylacetate 0.7 Menthytlacetate 12.5
trans--Caryophyllene 0.5 Mintlactone 0.1
t,traces;n.i.,notidentified.
suppression,allofwhichcancauseinsectmortality.The activityoftheessentialoilofM.piperitaoneggsandlarvae ofH.contortusislikelyrelatedtoallorsomeoftheactivities describedabovefortheterpenoids.
RegardingtheoilextractedfromseedsofC.guianensis, therewasnoovicidalorlarvicidalactivityatthe concentra-tionstested.Onedifficultyfacedinhandlingthisoilinthe
invitrotestswithgastrointestinalnematodeswasits solu-bilization.Itwouldbenecessarytoincreasetheamountof solvent,buttheparasitesareverysensitive,whichexplains thelowconcentrationsusedinthisstudy.Itwasnecessary toincreasetheconcentrationsofC.guianensisandH. crepi-tansconsiderablytoobtainbetterresultsintheEHTand LDTandtoreachtheLC50.Sothestandarddeviationfor thesetwospecies,andespeciallyforC.guianensisinthe LDT,presentedlargevariability.
The latex extracted from H. crepitans did not show anyovicidalactivity,butwaseffectiveintheLDT.Inthe EHT, it was not possible to assess this latex in higher concentrationsbecausetheextract possesseddarkcolor
and contained many particles, making the subsequent
visualization and countingof larvae and eggs impossi-ble.Brondani(2006)analyzedtheactivityofthelatexof this plant oninfective larvae of ticks(R. microplus and R. sanguineus) and observed mortality rates above 95% atallconcentrations.Someconstituentsofthelatexare lectin,creptin(bothglycoproteins)andhurin(proteolytic enzyme)(Brondani,2006).Therearestudiesthatindicate theharmful action of proteases onthe cuticle of some
nematodes(Stepeketal.,2004).Forinstance,Jaffé(1943) comparedtheactionofhurin(fromthelatexofH.crepitans) withpapain(comingfromthesapofFicus)onAscaris lum-bricoidesandearthworms.Theresultsshowedthatpapain digestedbothspecies, whilehurindigestedearthworms but notA.lumbricoides,althoughitcaused deathof this species. Lectin, which is alsopresent in thelatex of H. crepitans,actsbybindingspecificallytocarbohydratesand otherresiduesofglycoconjugatesonthecellsurface.Its potentialhasbeenreportedasaninsecticideagainstsome
Fig.4.MeannumberofStrongyloidesvenezuelensisadultwormsrecoveredfromtheinitialthirdofintestineofratstreatedwithLippiasidoidesessential oil(LS1– 150mgkg−1andLS2– 250mgkg−1)andPipertuberculatumextract(PT1– 150mgkg−1andPT2–250mgkg−1).Meanswithdifferentlettersare significantlydifferentbytheTukeytest(P<0.05).
species,bybindingtotheperitrophicmembrane(the acel-lularchitinstructurethatlinesthedigestivetractofsome ColeopteraandLepidoptera),interferinginthefeeding pro-cess(Fitchesetal.,2001).Takingintoaccounttheactionof lectin,weassumedthatitmaybeconsumedbytheL1 lar-vae,leadingtoinhibitionoftheirdevelopmentuntiltheL3 stage.Therewasanincreaseininhibitionofdevelopment withincreasingextractconcentration.
InrelationtotheextractofP.tuberculatum,satisfactory resultswerefoundinboththeEHTandtheLDT.
The Piperaceae contains several plants with insecti-cidal effects, especially thePiper genus,which contains species withsecondarymetabolitessuchaslignans and amides,usedintheirdefenseagainstherbivores. Piplar-tine,identifiedbyDuhandWu(1990)asoneofthetoxic componentsofP.arborescens,hasdemonstratedcytotoxic activityoncells.Bezerraetal.(2005)comparedthemitotic activityofpiperineand piplartineagainst differentcells andobservedamorepotenteffectofpiplartine.Piperine, inturn,hasantiparasiticactivity,asobservedbyRibeiro etal.(2004)againstepimastigotesandamastigotesof Try-panosoma cruzi.Also, Freire-de-Lima etal.(2008) noted thatpiperinecausedadelayincellcycleofthatparasite.
Thebestresultsfromthisstudywerewiththeextracts of P. tuberculatum (EHT with LC90=0.09mgmL−1 and LDT withLC90=0.03mgmL−1)and L.sidoides(LDTwith LC90=0.03mgmL−1).Theseresultsweresignificantwhen comparedtothoseobservedfortheotherplantextracts evaluatedagainstH.contortus.Spigeliaanthelmiashowed 100%inhibitionintheEHTand81.2%intheLDTata con-centrationof50mgmL−1(Assisetal.,2003).Macieletal.
(2006) observed better efficacy of ethanol seed extract (100%inhibitionat1.56mgmL−1)andleafextract(98.24% at12.5mgmL−1)ofMeliaazedarachoneggsofH.contortus andtheethanolextractofleaves(91.64%at50mgmL−1) onthelarvaeofthesamespecies.Macedoetal.(2010),in turn,analyzedtheeffectofEucalyptusstaigeriana essen-tialoilandobservedinhibitoryeffectoneggs(99.27%at 1.35mgmL−1)andlarvae(99.20%at5.4mgmL−1).Cocos
nuciferashowed 100%of inhibition at 5mgmL−1 in the EHTand99.77%at80mgmL−1intheLDT(Oliveiraetal.,
2009b).The potential larvicidal activity of the aqueous extract (97.3% inhibition at 150mgmL−1) and ethanol extract (99.6% inhibition at 60mgmL−1)of Anacardium
humileleavesongastrointestinalnematodesinsheepwas reportedbyNeryetal.(2010).
Some studies have analyzed the antiparasitic action ofplantextractsonparasites ofratsormiceandsheep. Amongthese,BorbaandAmorim(2004)studiedtheaction ofextractsofChenopodiumambrosioidesL.ontheoxyurids SyphaciaobvelataandAspiculuristetraptera,obtaining neg-ativeresultsforallconcentrationstested.Thissamespecies wasevaluatedinratsagainstStrongyloidesvenezuelensis and showed efficacy in reducing the EPG(75.89%) and the number of adult parasites (86.31%) at 400mgkg−1 (Bernardes, 2006). In field trials with sheep, Camurc¸ a-Vasconcelosetal.(2008)administeredtheessentialoilof L.sidoidesatconcentrationsof230and283mgkg−1 and observedareductionintheEPGcountintheevaluations conducted7and14daysafterthetreatment:38%and30% and45%and54%,respectively.
Inourevaluationwithrats,asignificantreductionwas observedinthenumberofadultparasites atbothdoses tested (150 and 250mgkg−1) compared to the control group,treatedwithsorbitol.Thehighestmeannumberof EPGwasrecorded7or8daysafterinfectionofratswith S.venezuelensis,afterwhicheggoutputshowed progres-sivereduction.Therefore,thetrendinEPGvaluesobserved in thesorbitolgroupreflects a naturalreduction of the eggelimination.Duetoitssedativeeffect,observedafter thefirstadministrationof theoil, wechosenotto per-formthefollowingtwotreatmentstopreventtheanimals’ death.Invivotestsareneededtoevaluatetheeffectofplant extractswithsignificantresultsinvitroonparasites. How-ever,thepossibletoxiceffectsonthetargethostsshould beperformedearlier.
actioninrats.Nevertheless,futurestudieswithP. tuber-culatumextractsandM.piperitaandL.sidoidesoilswillbe necessarytounderstandtheirabsorptionandmetabolism inratsandsheep.
Acknowledgments
Wegratefullyacknowledgethetechnicalassistanceof AndrineM.C.Navarro,CésarC.BassettoandLetícia Bos-chini.ThisworkreceivedfinancialsupportfromFAPESP (SãoPauloStateResearchFoundation).CamilaO.Carvalho hasagrantfromFAPESP.
References
Aguiar,M.L.B.A.,Matos,F.J.A.,Moura,V.R.,1984.Aatividadeantibióticade plantasdafloranordestina.Ciênc.Cult.36,547.
Akhtar,M.S.,Iqbal,Z.,Khan,M.N.,Lateef,M.,2000.Anthelminticactivity ofmedicinalplantswithparticularreferencetotheiruseinanimals inIndo-Pakistansubcontinent.SmallRumin.Res.38,99–107. Almeida,F.A.,Garcia,K.C.O.D.,Torgerson,P.R.,Amarante,A.F.T.,2010.
MultipleresistancetoanthelminticsbyHaemonchuscontortusand TrichostrongyluscolubriformisinsheepinBrazil. Parasitol.Int.59, 622–625.
Assis,L.M.,Bevilaqua,C.M.,Morais,S.M.,Vieira,L.S.,Costa,C.T.,Souza,J.A., 2003.OvicidalandlarvicidalactivityinvitroofSpigeliaanthelmia Linn.extractsonHaemonchuscontortus.Vet.Parasitol.117,43–49. Bara,M.T.F.,Vanetti,M.D.,1998.Estudodaatividadeantibacterianade
plantasmedicinais,aromáticasecorantesnaturais.Rev.Bras. Farma-cogn.7–8,22–34.
Bezerra,D.P.,Pessoa,C.,Moraes,M.O.,Silveira,E.R.,Lima,M.A.,Elmiro,F.J., Costa-Lotufo,L.V.,2005.Antiproliferativeeffectsoftwoamides, piper-ineandpiplartine,fromPiperspecies.Z.Naturforsch.60,539–543. Bernardes, H.M.D., 2006. Estudo doefeito anti-helmínticodoextrato
hidroalcoólico e frac¸ões de Chenopodium ambrosioides L. sobre Strongyloidesvenezuelensis(BRUMPT,1934)98f.Tese(Doutorado emParasitologia)—ProgramadePós-graduac¸ãoemParasitologia. Uni-versidadeEstadualdeCampinas,Campinas.
Bizimenyera,E.S.,Githiori,J.B.,Eloff,J.N.,Swan,G.E.,2006.Invitroactivity ofPeltophorumafricanumSond(Fabaceae)extractsontheegg hatch-ingandlarvaldevelopmentoftheparasiticnematodeTrichostrongylus colubriformis.Vet.Parasitol.142,336–343.
Borba,H.R., Amorim,A., 2004. Ac¸ãoanti-helmíntica de plantas XIV Avaliac¸ãodaatividadedeextratosaquososdeChenopodium ambro-sioidesL. (Erva-de-Santa-Maria) em camundongos naturalmente infectadoscomSyphaciaobvelataeAspiculuristetraptera.Rev.Bras. Parasitol.Vet.13,133–136.
Boufleuer,N.T.,2004.Aspectosecológicosdaandiroba(Carapa guianen-sisAubletMelicaceae):subsídiosparaomanejo.90f.Dissertac¸ão (MestradoemEcologiaeManejodeRecursosNaturais).Universidade FederaldoAcre,RioBranco.
Brondani,F.M.M.,2006.AtividadecarrapaticidadolátexdaplantaHura crepitans L. e do extrato etanólico da planta Rinorea pubiflora (Benth.)Sprague&SandwithemlarvasdeBoophilusmicropluse Rhipicephalussanguineus72f.Dissertac¸ão(Mestrado em Biolo-giaExperimental)—ProgramadeBiologiaExperimental.Universidade FederaldeRondônia,Rondônia.
Camurc¸a-Vasconcelos,A.L.F.,Bevilaqua,C.M.L.,Morais,S.M.,Maciel,M.V., Costa,C.T.C.,Macedo,I.T.F.,Oliveira,L.M.B.,Braga,R.R.,Silva,R.A., Vieira,L.S.,2007.AnthelminticactivityofCrotonzehntneriandLippia sidoidesessentialoils.Vet.Parasitol.148,288–294.
Camurc¸a-Vasconcelos,A.L.F.,Bevilaqua,C.M.L.,Morais,S.M.,Maciel,M.V., Costa,C.T.C.,Macedo,I.T.F.,Oliveira,L.M.B.,Braga,R.R.,Silva,R.A., Vieira,L.S., Navarro,A.M.C.,2008. Anthelminticactivityof Lippia sidoidesessentialoilonsheepgastrointestinalnematodes.Vet. Par-asitol.154,167–170.
Carvalho,A.F.U.,Melo,V.M.M.,Craveiro,A.A.,Machado,M.I.L.,Bantim, M.B.,Rabelo,E.F.,2003.Larvicidalactivityoftheessentialoilfrom Lip-piasidoidesCham.againstAedesaegyptiL.Mem.Inst.OswaldoCruz 98,569–571.
Coles,G.C.,Bauer,C.,Borgsteede,F.H.M.,Geerts,S.,Klei,T.R.,Taylor, M.A.,Waller,P.J.,1992.WorldAssociationfortheAdvancementof VeterinaryParasitology(W.A.A.V.P.)methodsforthedetectionof anthelminticresistanceinnematodesofveterinaryimportance.Vet. Parasitol.44,35–44.
Cotinguiba,F.,Regasini,L.O.,Bolzani,V.S.,Debonsi,H.M.,Passerini,G.D., Cicarelli,R.M.B.,Kato,M.J.,Furlan,M.,2009.Piperamidesandtheir derivativesaspotentialanti-trypanosomalagents.Med.Chem.Res. 18,703–711.
Duh,C.,Wu,Y.,1990.CytotoxicpyridonealkaloidsfromtheleavesofPiper aborescens.J.Nat.Prod.53,1575–1577.
Drudge,J.H.,Szanto,J.,Wyant,Z.N.,Elam,G.,1964.Fieldstudiesonparasite controlinsheep:comparisonofthiabendazole,ruelene,and phenoth-iazine.Am.J.Vet.Res.25,1512–1518.
Farias,M.P.O.,Sousa,D.P.,Arruda,A.C.,Wanderley,A.G.,Teixeira,W.C., Alves,L.C.,Faustino,M.A.G.,2009. Potencialacaricidadoóleo de andirobaCarapaguianensisAubl.sobrefêmeasadultasingurgitadas deAnocentornitensNeumann, 1897eRhipicephalussanguineus Latreille,1806.Arq.Bras.Med.Vet.Zootec.61,877–882.
Fox,M.T.,1993.PathophysiologyofinfectionwithOstertagiaostertagiin cattle.Vet.Parasitol.46,143–158.
Francis,J.K.,1990.HuracrepitansL.Sandbox,Molinillo,Jabillo. Euphor-biaceae.Spurgefamily.USDA.For.Serv.So.Res,5p.
Freire-de-Lima,L.,Ribeiro,T.S.,Rocha,G.M.,Brandão,B.A.,Romeiro,A., Mendonc¸a-Previato,L.,Previato,J.O.,Lima,M.E.D.,Carvalho,T.M.U., Heise,N.,2008.ThetoxiceffectsofpiperineagainstTrypanosomacruzi: ultrastructuralalterationsandreversibleblockageofcytokinesisin epimastigoteforms.Parasitol.Res.102,1059–1067.
Fitches,E.,Woodhouse,S.D.,Edwards,J.P.,Gatehouse,J.A.,2001.Invitro andinvivobindingofsnowdrop(Galanthusnivalisagglutinin;GNA) andjackbean(Canavalia ensiformis;ConA)lectinswithintomato moth(Lacanobiaoleracea)larvae;mechanismsofinsecticidalaction. J.Insect.Physiol.47,777–787.
Helander,I.M.,Alakomi,H.L.,Latva-Kala,K.,Mattila-Sandholm,T.,Pol, I.,Smid,E.J.,Gorris,L.G.M.,Wright,A.,1998.Characterizationofthe actionofselectedessentialoilcomponentsonGram-negative bacte-ria.J.Agric.FoodChem.46,3590–3595.
Hubert,J.,Kerboeuf,D.,1992.Amicrolarvaldevelopmentassayforthe detectionofanthelminticresistanceinsheepnematodes.Vet.Rec. 130,442–446.
Jaffé,W.G.,1943.Hurain,anewplantproteasefromHuracrepitans.J.Biol. Chem.149,1–7.
Jaramillo,M.A.,Manos,P.S.,2001.Phylogenuandpatternsoffloral diver-sityinthegenusPiper(Piperaceae).Am.J.Bot.88,706–716. Júnior,V.C.,2003.Terpenoscomatividadeinseticida:umaalternativapara
ocontrolequímicodeinsetos.Quim.Nova26,390–400.
Laemmli,U.K.,1970.Cleavageofstructuralproteinsduringtheassembly oftheheadbacteriophageT4.Nature227,680–685.
Macedo,I.T.,Bevilaqua,C.M.,Oliveira,L.M.,Camurc¸a-Vasconcelos,A.L., Vieira,L.S.,Oliveira,F.R.,Queiroz-Junior,E.M.,Tomé,A.R.,Nascimento, N.R.,2010.AnthelminticeffectofEucalyptusstaigerianaessentialoil againstgoatgastrointestinalnematodes.Vet.Parasitol.173,93–98. Maciel,M.V.,Morais,S.M.,Bevilaqua,C.M.L.,Camurc¸a-Vasconcelos,A.L.F.,
Costa,C.T.C.,Castro,C.M.S.,2006.Ovicidalandlarvicidalactivityof MeliaazedarachextractonHaemonchuscontortus.Vet.Parasitol.140, 98–104.
Mendonc¸a,F.A.C.,Silva,K.F.S.,Santos,K.K.,Ribeiro-Júnior,K.A.L.,Sant’Ana, A.E.G,2005.ActivitiesofsomeBrazilianplantsagainstlarvaeofthe mosquitoAedesaegypti.Fitoterapia76,629–636.
Miot,H.A.,Batistella,R.F.,Batista,K.A.,Volpato,D.E.C.,Augusto,L.S.T., Madeira,N.G.,Junior,V.H.,Miot,L.D.B.,2004.Comparativestudyof thetopicaleffectivenessoftheandirobaoil(Carapaguianensis)and DEET50%asrepellentforAedessp.Rev.Inst.Med.Trop.SaoPaulo46, 253–256.
Mimica-Duki ´c,N.,Bozin,B.,Sokovi ´c,M.,Mihajlovi ´c,B.,Matavulj,M., 2003.AntimicrobialandantioxidantactivitiesofthreeMenthaspecies essentialoils.PlantaMed.69,413–419.
Miranda, J.E.,Oliveira, J.E.M., Rocha, K.C.G., Bortoli, S.A.,Navickiene, H.M.D.,Kato,M.J.,Furlan,M.,2002.Potencialinseticidadoextratode Pipertuberculatum(Piperaceae)sobreAlabamaargillacea(Huebner, 1818)(Lepidpoptera:Noctuidae).Rev.Bras.Ol.Fibrs.6,557–563. McKay,D.L.,Blumberg,J.B.,2006.Areviewofthebioactivityandpotential
healthbenefitsofpepperminttea(MenthapiperitaL.).Phytother.Res. 20,619–633.
Nakai,E.S.,Amarante,A.F.T.,2001.Experimentalinfectioninmice(Mus musculus)and(Rattusnorvegicus)byStrongyloidesvenezuelensis.Braz. J.Vet.Parasitol.10,1–6.
Nery,P.S.,Nogueira,F.A.,Martins,E.R.,Duarte,E.R.,2010.Effectsof Anac-ardiumhumileleafextractsonthedevelopmentofgastrointestinal nematodelarvaeofsheep.Vet.Parasitol.171,361–364.
Oliveira,L.M.B.,Bevilaqua,C.M.,Costa,C.T.,Macedo,I.T.,Barros,R.S., Rodrigues,A.C.,Camurc¸a-Vasconcelos,A.L.,Morais,S.M.,Lima,Y.C., Vieira, L.S., Navarro, A.M., 2009a. Anthelmintic activity of Cocos nuciferaL.againstsheepgastrointestinalnematodes.Vet.Parasitol. 159,55–59.
Oliveira,V.C.,Moura,D.M.,Lopes,J.A.,deAndrade,P.P.,daSilva,N.H., Figueiredo,R.C.,2009b.EffectsofessentialoilsfromCymbopogon cit-ratus(DC)Stapf.,LippiasidoidesCham.,andOcimumgratissimumL. ongrowthandultrastructureofLeishmaniachagasipromastigotes. Parasitol.Res.104,1053–1059.
PastoreJunior,F.,Borges,V.,1998.Produtosflorestaisnão-madeireiros: processamentoecomercializac¸ão.In:IITO/Funatura/UnB,Ibama,p. 54pp.
PastoreJunior,F.,Borges,V.,1999.Extrac¸ãoflorestalnão-madeireirana Amazônia:armazenamentoecomercializac¸ão.In:IITO/Funatura/UnB, Ibama,p.73pp.
Penido, C., Costa, K.A., Pennaforte, R.J., Costa, M.F.S., Pereira,J.F.G., Siani,A.C.,Henriques,M.G.M.O.,2006a.Anti-allergiceffectsof natu-raltetranortriterpenoidsisolatedfromCarapaguianensisAubleton allergen-inducedvascularpermeabilityandhyperalgesia.Inflamm. Res.54,295–303.
Penido,C.,Conte,F.P.,Chagas, M.S.S.,Rodrigues,C.A.B.,Pereira,J.F.G., Henriques,M.G.M.O., 2006b. Antiinflammatory effects ofnatural tetranortriterpenoids isolated from Carapa guianensis Aublet on zymosan-inducedarthritisinmice.Inflamm.Res.55,457–464. Ribeiro,T.S.,Freire-de-Lima,L.,Previato,J.O.,Mendonc¸a,L.,Heise,N.,Lima,
M.E.F.,2004.Toxiceffectsofnaturalpiperineanditsderivativeson epimastigotesandamastigotesofTrypanosomacruzi.Bioorg.Med. Chem.Lett.14,3555–3558.
Scott,I.M.,Jensen,H.R.,Philogène,B.J.R.,Arnason,J.T.,2008.Areviewof Piperspp(Piperaceae)phytochemistry,insecticidalactivityandmode ofaction.Phytochem.Rev.7,65–75.
Singh,V.K.,Singh,S.,Singh,D.K.,1999.Effectofactivemolluscicidal com-ponentofspicesondifferentenzymeactivitiesandbiogenicamine levelsinthenervoustissueofLymnaeaacuminata.Phytother.Res.13, 649–654.
Silva,W.C.,Martins,J.R.S.,Souza,H.E.M.,Heinzen,H.,Cesio,M.V.,Mato,M., Albrecht,F.,Azevedo,J.L.,Barros,N.M.,2009.ToxicityofPiperaduncum L(Piperaceae)fromtheAmazonforestforthecattletickRhipicephalus (Boophilus)microplus(Acari:Ixodidae).Vet.Parasitol.164,267–274. Stepek,G.,Behnke,J.M.,Buttle,D.J.,Duce,I.R.,2004.Naturalplantcysteine