www.bjorl.org
Brazilian
Journal
of
OTORHINOLARYNGOLOGY
ORIGINAL
ARTICLE
The
impact
of
laronidase
treatment
in
otolaryngological
manifestations
of
patients
with
mucopolysaccharidosis
夽
Ana
Paula
Fiuza
Funicello
Dualibi
a,∗,
Ana
Maria
Martins
b,
Gustavo
Antônio
Moreira
b,
Marisa
Frasson
de
Azevedo
a,
Reginaldo
Raimundo
Fujita
a,
Shirley
Shizue
Nagata
Pignatari
aaUniversidadeFederaldeSãoPaulo(EPM---UNIFESP),EscolaPaulistadeMedicina,DepartamentodeOtorrinolaringologiae
CirurgiadeCabec¸aePescoc¸o,SãoPaulo,SP,Brazil
bUniversidadeFederaldeSãoPaulo(EPM---UNIFESP),EscolaPaulistadeMedicina,DepartamentodePediatria,SãoPaulo,SP,Brazil
Received24July2015;accepted3September2015 Availableonline17December2015
KEYWORDS
MucopolysaccharidosisI; Laronidase;
Enzymatic
replacementtherapy; URTinfections; Sleepapnea; Hearingloss
Abstract
Introduction:Mucopolysaccharidosis(MPS)isalysosomalstoragediseasecausedbydeficiency
of␣-l-iduronidase.Theotolaryngologicalfindingsincludehearingloss,otorrhea,recurrent
oti-tis,hypertrophyoftonsilsandadenoid,recurrentrhinosinusitis,speechdisorders,snoring,oral
breathingandnasalobstruction.
Objective:To evaluate the impact of enzymatic replacement therapy with laronidase
(Aldurazyme®)inpatients with mucopolysaccharidosis (MPSI), regarding sleepand hearing
disorders,andclinicalmanifestationsintheupperrespiratorytract(URT).
Methods:NinepatientswithMPSI(8Hurler-Scheie,and1Scheiephenotypes)ofbothsexes,
agesranging between3and20 years,wereincludedinthisstudy.Patientswere evaluated
betweensevenand11monthsbeforethetreatmentandbetween16and22monthsafterthe
onsetoftheenzymaticreplacement.Theywereallsubmittedtoaclinicalandotolaryngological
evaluation,includingnasofibroscopical,polysomnographicandaudiologicexams.
Results:Theresults’datashoweddecreasingofthefrequencyofear,noseandthroat
infec-tions, with improvementof the rhinorrheaand respiratory quality. No remarkable changes
wereobserved regardingmacroglossiaandtonsilandadenoidhypertrophy. Audiometricand
polysomnographicevaluationsdidnotshowstatisticalsignificance.
夽 Pleasecitethisarticleas:DualibiAP,MartinsAM,MoreiraGA,deAzevedoMF,FujitaRR,PignatariSS.Theimpactoflaronidasetreatment inotolaryngologicalmanifestationsofpatientswithmucopolysaccharidosis.BrazJOtorhinolaryngol.2016;82:522---8.
∗Correspondingauthor.
E-mail:jpedualibi@uol.com.br(A.P.Dualibi).
http://dx.doi.org/10.1016/j.bjorl.2015.09.006
Conclusion: EnzymaticreplacementtherapyinpatientswithmucopolysaccharidosisIprovides
controlofrecurrentURTinfections,rhinorrheaandrespiratoryquality,howeveritisdoesnot
seemtoimproveaudiologicandpolisomnographicparameters,withnoeffectonadenoidand
tonsilshypertrophyandmacroglossia.
© 2015 Associac¸˜ao Brasileira de Otorrinolaringologia e Cirurgia C´ervico-Facial. Published
by Elsevier Editora Ltda. This is an open access article under the CC BY license (http://
creativecommons.org/licenses/by/4.0/).
PALAVRAS-CHAVE
MucopolissacaridoseI; Laronidase;
Terapiadereposic¸ão enzimática;
Infecc¸õesdoTRS; Apneiadosono; Perdaauditiva
Impactodotratamentocomlaronidasenasmanifestac¸õesotorrinolaringológicas depacientescommucopolissacaridose
Resumo
Introduc¸ão: Mucopolissacaridose(MPS) éuma doenc¸a de depósitolisossômico causada pela
deficiênciade␣-l-iduronidase.Osachadosotorrinolaringológicosincluemperdaauditiva,
otor-reia,otitesderepetic¸ão,hipertrofiaadenotonsilar,rinossinusiterecorrente,distúrbiosdafala,
roncos,respirac¸ãobucaleobstruc¸ãonasal.
Objetivo: Avaliaroimpactodaterapiadereposic¸ãoenzimáticacomlaronidase(Aldurazyme®)
em pacientescommucopolissacaridose I(MPSI) em relac¸ãoao sono, distúrbios auditivose
manifestac¸õesclínicasdotratorespiratóriosuperior(TRS).
Método: NovepacientescomMPSI(oitocomfenótipoHurler-ScheieeumcomfenótipoScheie),
deambosossexos,comidadesvariandoentre3e20anos,foramincluídosnesteestudo.Os
pacientes foramavaliadosentre7 e11 mesesantes dotratamentoe entre16 e 22 meses
após o início da reposic¸ão enzimática. Todos foram submetidos a uma avaliac¸ão clínica e
otorrinolaringológica,incluindonasofibroscopia,polissonografiaeexamesradiológicos.
Resultados: Os dados dos resultados mostraram diminuic¸ão da frequência de infecc¸ões de
orelha, nariz e garganta, commelhora da rinorreia e da qualidade respiratória. Mudanc¸as
significativasnão foramobservadasemrelac¸ãoàmacroglossiaeàhipertrofiaadenotonsilar.
Avaliac¸õesaudiométricasepolissonográficasnãoapresentaramsignificânciaestatística.
Conclusão:Aterapiadereposic¸ãoenzimáticaempacientescommucopolissacaridoseIfornece
controledeinfecc¸õesrecorrentesdoTRS,rinorreiaequalidaderespiratória,porém,nãoparece
melhorarosparâmetrosaudiológicosepolissonográficos,ouexercerefeitosobreahipertrofia
adenotonsilaremacroglossia.
© 2015 Associac¸˜ao Brasileira de Otorrinolaringologia e Cirurgia C´ervico-Facial. Publicado
por Elsevier Editora Ltda. Este ´e um artigo Open Access sob uma licenc¸a CC BY (http://
creativecommons.org/licenses/by/4.0/).
Introduction
Mucopolysaccharidosis(MPS)isalysosomalstoragedisease
caused bydeficiency ofan enzyme involved inthe
degra-dation of glycosaminoglycans (GAGs). They are classified
accordingtotheinvolvedenzymeandGAGinseventypes:
I (Hurler, Hurler Scheie and Scheie), II (Hunter), III
(San-fillipo), IV (Morquio), VI (Maroteaux Lamy), VII (Sly) e IX
(Natowicz).1---3
MPSIis causedby deficiencyof ␣-l-iduronidase,which
leads tointralysosomal depositsofdermatan andheparan
sulfate. Itis an autosomicrecessive geneticdisease,with
estimatedincidencevaryingfrom1:100,000forseverecases
to1:800,000forcaseswithmildmanifestations.4
Clinical manifestations of MPS I are extremely
het-erogeneous, with symptoms that evolve in many ways,
fromverymild manifestationsof latedevelopment,
with-out cognitive disorders, and high life time expectation
(Scheie),toverysevere casesof early onset,rapidly
pro-gressive,withneuraldegenerationandlimitedcapabilities
in life, usually manifested by the first decade (Hurler),
and passing through an intermediary level of severity
(Hurler---Scheie).1,2,5
The disease may involve nervous, skeleton, digestive,
cardiac,superiorandinferiorrespiratorysystemspresenting
different levels of severity in an independent manner.
Regardingtheotolaryngologicalfindings,themostfrequent
symptomsincludehearing loss,otorrhea, recurrentotitis,
hypertrophyof tonsilsand adenoid,recurrent
rhinosinusi-tis, speech disorders, snoring, oral breathing and nasal
obstruction.1,2,6
Obstructivesleepapneaandhypopneasyndrome(OSAHS)
isfrequentlydiagnosed inMPSIpatients.Obstructiveand
restrictivefactorssuchasreductionofthethoracicvolume
(musculoskeletal alterations), restriction of the
diaphrag-maticmovement due tohepatosplenomegaly,presence of
atelectasis secondary to the reduction of the lung
vol-ume,depositofGAGsintothepulmonaryinterstitialtissue,
trachealstenosis,vocal cordthickening,adenoidand
positioned epiglottis, presence of abundant thick nasal
mucous,andlimitedmouthopeningaremainlyresponsible
for therespiratory disorders.6---11 Hearing lossis also
com-moninpatientswithMPSI.Althoughthenatureofhearing
lossmaybeconductive,sensorineuralormixed,conductive
hearinglossismorefrequentandcanbeexplainedby
sev-eralfactors,suchasthethickeningofthemiddleearmucus
andeardrumproducedbydepositsofGAGs,Eustachiantube
obstruction, ossicular chain malformation,
hypopneumati-zationofthetemporalboneandpresenceofthickcopious
mucus.Mechanismstoexplainthesensorineuralhearingloss
areunclear.Itisbelievedthataprogressivehyperplasiaof
thearachnoidmembranecancompressthecochlearnerve,
and that the storage of GAGs inside neurovascular
struc-turesof theinner ear,along withalterations of thehairy
cellsconsequenttometabolicdisordersusuallypresent in
patientswithMPS,maybecontributoryfactors.2,9,12---17
Duetothemultisystem involvement,treatment is
usu-allymultidisciplinary.Untilthe1970s,treatmentconsisted
in palliative methods toimprove the qualityof life.
Cur-rently, the two principal therapeutic tools of MPS I are
basedonenzymaticreplacement(ERT)andtransplantation
of hematopoietic cells (HCT). The enzymes produced by
stemcells frombone marrowor umbilicalcordrestore to
thepatienttheabilityofdegradation.Since1981,morethan
200childrenhavebeensubmittedtothistypeoftreatment.
TheHCTproducesresolutionorimprovementofOSAHSand
conductivehearingloss.Themajorproblemrelatedtothis
treatmentisthehighrateofmorbidityandmortality(40%),
andthedifficultytofindcompatiblebonemarrow.Because
ofthe high risk relatedtothis therapy,it is restricted to
children with severe disease, preferable before the 18th
month of life, when the CNS alterations usually begin to
occur.18---21
Laronidase(Aldurazyme®)istheenzyme usedinMPSI,
and it is produced by recombinant DNA technique.
Clini-caltrialswithERThaveshownimprovingoftherespiratory
status. It was approved by commercial use in 2003, and
sincethenmorethan330patients havebeentreated.22---26
Althoughimprovementoftherespiratoryconditionsis
fre-quently mentioned in the majority of the reports, few
studieshaveaddressedthehearingproblemsandother
oto-laryngologicalfindingsthatremainunclear.27,28
Theindividualsincludedinthisstudyarepartofthefirst
clinicaltrialoftreatmentofMPSIwithlaronidaseinBrazil.
The objective wastoevaluate the impactof ERT with
laronidaseinrespiratoryandaudiologicalmanifestationsof
patientswithMPSIandclinicalmanifestationsintheupper
respiratorytract(URT).
Method
NinepatientswithMPSI(no-Hurlerphenotype)were
evalu-atedafterapprovaloftheCommitteeofEthicsinResearch
(protocol0337/05).
Each patient received 52 infusions of laronidase,
0.54mg/kg/dose.
Theywereallsubmittedtoatleasttwootolaryngological
evaluations, including nasofibroscopic, polysomnographic
andaudiologicalexamination,betweensevenand11months
beforeandbetween16and22monthsafterthebeginning
ofthetreatment.
Tonsils3 and4 accordingtoBrodsky classification,and
adenoids occupying more than 70% of the choanae were
consideredhypertrophic.29
Thedata obtainedfrompolysomnographic examination
(PSG) wereanalyzed accordingtothe parametersdefined
bytheAmericanThoracicSociety(indexofapneahipopnea
>1event/hinchildrenunder14yearsofageand>5events/h
inolderchildren).30,31
Audiological evaluation was accomplished by
conven-tionalaudiometricexamaloneorwithvisualreinforcement.
AnalysisofthedatawasbasedonSRT(‘‘speechreception
threshold’’). It was considered normal when the SRTwas
< or equal 20(dB).Imitanciometric curvewas interpreted
accordingtoJergerclassification(1970).32,33
StatisticalevaluationwasanalyzedbytheFriedmantest
(non-parametric).The valueofrejection forhypothesis of
nullitywasfixedforvalues<orequal0.05.Significancewas
markedas(*).Non-significant(NS).
Results
Fromtheninepatientsinitiallyevaluated,twodidnotreturn
for the second visit afterthe beginning of treatment and
werenotincludedinthestatisticalanalysis.Fourpatients
werefemaleandfivemale,andagesrangedbetween3and
20years(medianof8years).
One patientdid not have a pre-treatment
polysomno-graphicexamandwasnotincludedinthefinal
polysomno-graphicevaluation.
Three patients had undergone ENT surgery before the
startofthisstudy:inadditiontomyringotomywith
ventilat-ingtubeplacement,onepatientunderwentadenoidectomy
at the ageof 7, onepatientwasreferred to
adenotonsil-lectomy attheageof4 andanotherpatientheldelective
tracheotomyandadenoidectomyat4yearsofage.
Fig. 1 shows that nasal obstruction, snoring, oral
breathing,apnea,rhinorrhea,andrecurrentURTinfections
improvedwithERTinpracticallyallpatients.
Otolaryngologicalexaminationshowedthatmacroglossia
andappearanceofthetympanicmembrane(retraction)did
notchangeafterERT.Inthethreepatientswithouttympanic
membraneretraction, twohadpatentventilatingtubesin
place. All patients experienced reduction of nasal mucus
afterERT(Fig.2).
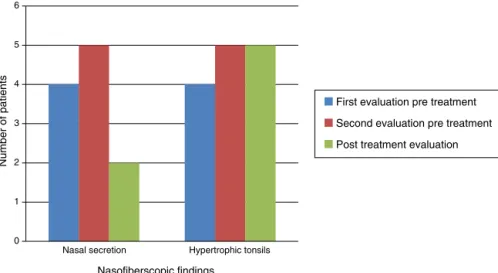
Naso-fiberscopic examination alsoshowed reduction of
the nasalsecretion,however, reductionof tonsil sizewas
notobserved(Fig.3).
The results obtained by audiometric evaluation are
shown in Table1. Statistical analysisdidnot demonstrate
significanceofthedifferencesbetweenpre-andpost-ERT.
Tympanometrywasperformedinonlyfivepatients,since
twohad bilateralpatent ventilatingtubesin placeat the
moment of the evaluation. In the first evaluation four of
thempresentedB-typecurveandonepatientpresented
A-typecurve,remainingunchangedafterERT.
Resultsobtainedwithpolysomnographicexamsareshown
in Table 2. Statistical analysis did not show significance
0 1 2 3 4 5 6 7
Number of patients
URT symptoms
First evaluation pre treatment
Second evaluation pre treatment
Post treatment evaluation
Nasal obstr uction
Snor ing
Oral breathing apnea
Rhinorrea
Recurrent UR T inf
ections
Hear ing loss
Figure1 Otolaryngologicalsymptomsobtainedfromdirectedanamnesis.
0 1 2 3 4 5 6 7 8
Macroglossia Nasal secretion Tympanic membrane retraction
Number of patients
Otolaryngological findings
First evaluation pre treatment
Second evaluation pre treatment
Post treatment evaluation
Figure2 Otolaryngologicalfindings.
Discussion
Sincethefirstdescription ofMPS, at thebeginningof the
20thcentury,theknowledgeofthisprogressivediseaseof
largephenotypicdiversity,aswellassignificant limitation
ofqualityandexpectancyoflifehaveexpanded
consider-ably.However,despitetheremarkableimprovementofthe
understanding of its natural history and treatment, many
questionsremainunsolved.There is alack of information
regarding the evolution of non-treated patients, and the
0 1 2 3 4 5 6
Nasal secretion Hypertrophic tonsils
Number of patients
Nasofiberscopic findings
First evaluation pre treatment
Second evaluation pre treatment
Post treatment evaluation
Table1 ResultsofSTR(dB).
Patients 1stevaluation
pretreatment
2ndevaluation
pretreatment
3rdevaluation
posttreatment
RE LE RE LE RE LE
1 30 35 15 20 20 15
2 20 20 25 25 20 30
3 50 60 50 60 65 55
4 55 50 60 35 55 35
5 80 60 90 95 75 80
6 45 50 75 70 80 70
7 75 75 60 60 55 70
Friedman’stest:2calc=0.240;N.S.,p=0.887.
rarity and large phenotypicheterogenicity of MPSI make thedevelopmentofastudy ofclinical relevancedifficult, particularlydue tothe diversity of clinical presentations. The absenceof specific scores and biomarkers represents anadditionalproblemtomonitortheefficacyofanykindof therapeuticprotocol.4
MostofthestudiesenrollingpatientswithMPSIandERT
haveaddressed moreadvancedstagesof thedisease.The
presentstudyisprospective,observationallongitudinal,and
the patients were already presenting established lesions.
Thismayhavecontributedtothelowexpressiveresults.
Otolaryngological complaints (oral breathing, snoring,
hearingloss, nasalobstruction, rhinorrhea, recurrentURT
infections)andclinicalfindings(macroglossia,hypertrophy
oftonsilsandadenoid,nasalsecretionandretractionof
tym-panic membrane) are frequently reported in the medical
literature.8---12
Inourstudy,weobservedadecreaseinfrequencyof
prac-ticallyallotolaryngologiccomplainsafterERT,withmarked
reductionin rhinorrhea and URT recurrent infections. We
believethatthereductionoftheGAGdepositsinthemiddle
earandURT,andconsequentimprovementofthenasalflow
andEustachiantube could have been thereasonfor such
improvement.Interestingly,hypoacusiafrequencyincreased
afterERT.
Most of our results are similar to those reported in
the literature. In phase I/II clinical trials, authors have
reported90%ofthepatientspresentingwithrecurrentURT
infections, and improvement after ERT.23 Sardón et al.
describedtwopatientsunderERT,withsignificantreduction
ofthetracheotomysecretionsinoneofthepatientsafter
40 weeks of laronidase, although no improvement of the
hypoacusia could be observed.28 Tokic et al., in 2007,
reported two cases of MPS I submitted to ERT, with
remarkable improvement of the URT recurrent infections
inbothpatients,andalsoimprovementoftheaudiological
parameters.29
Alongthetime,naso-fiberopticexaminationshowed
con-tinuousincreaseofhypertrophyoftonsilsandadenoids,as
muchasnasalsecretions,inevaluationsbeforeERT.After
the beginning of ERT, nasal secretion showed important
reduction, but no changes regarding tonsils’ hypertrophy
were observed. There areno references in the literature
regarding the volume of the tonsils and adenoids in MPS
I patients submitted to ERT. However, some authors
per-formed tonsillectomy and adenoidectomy in at least five
patients undergoing ERT, and this fact may reflect the
absenceofimprovementofthisclinicalparameter.27
Regardingthehearingloss,thereisa consensusamong
thereportsthatMPSIpatientspresentwithhypoacusia,and
althoughsomeauthorsreportimprovementafterHCT,few
haveevaluatedtheevolutionofdysacusiaafterERT.Sardón
et al., in 2005, evaluated two patients performing brain
evokedresponseaudiometry(BERA),detecting conductive
hearing lossin one.The patientdid notpresent
improve-mentafterERT.23 Tokicetal.,in2007,alsoevaluatedtwo
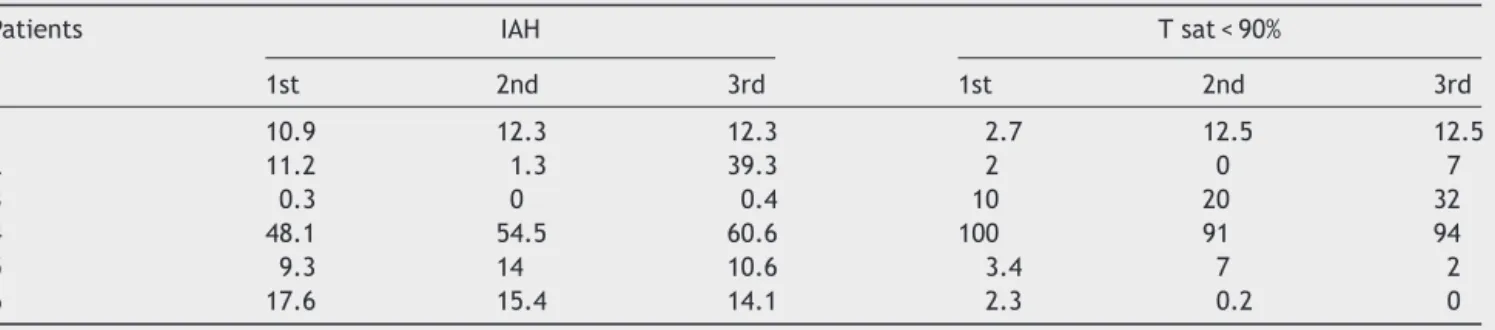
Table2 Apnea---hypopneaindexes(IAH)andtimeofsleepwithoxygensaturationinferiorto90%.
Patients IAH Tsat<90%
1st 2nd 3rd 1st 2nd 3rd
1 10.9 12.3 12.3 2.7 12.5 12.5
2 11.2 1.3 39.3 2 0 7
3 0.3 0 0.4 10 20 32
4 48.1 54.5 60.6 100 91 94
5 9.3 14 10.6 3.4 7 2
6 17.6 15.4 14.1 2.3 0.2 0
Friedman’stest.
IAH,2calc=1.826;N.S.,p=0.401.
patients hearingby using traditionalaudiometry.A mixed conductive-sensorineuralhearinglosswasobservedin one patient,withnochangesafterERT,andconductivehearing loss wasobservedin theother, which presented improve-mentofthethresholdsfrom30dBto10dBinoneearand 90dBto60dBintheotherearafterERT.29
Onehundredpercentofourpatientspresentedhearing
loss of varying levels. Two patients had ventilation tubes
inplaceandwerenotsubmittedtotympanometry.Type-B
curvewasobserved in80% oftheremaining fivepatients.
Therewasnostatisticalsignificanceofthevaluesbetween
justbeforeandafterERT,however,accordingtothe
medi-ans,itwaspossibletoverifythatthehearinglosswasgetting
worsebeforeERT,andhadaslightincreaseaftertreatment.
AlongwiththeimprovementoftheURTinfectionsand
rhin-orrhea,abetterresultregardingthehearingthresholdswas
alsoexpected,but possiblyother factorssuchasossicular
chainmalformation,thickeningofthemiddleearmucosa,
andEustachian tube dysfunctionwere responsible for the
maintenanceoftheaudiologicalstatus.Ontheotherhand,
sensorineuralhearinglossesareusuallyprogressiveandtend
toworsentheaudiologicthresholdofMPSIpatients,even
duringERT,sincetheenzymedoesnotcrossthe
hematoen-cephalicbarrier.17
Whenfirstsubmittedtopolysomnographicexamination,
allpatientspresented OSAHS,exceptonepatientwhohad
hadtracheostomybeforethebeginningofthestudy,andno
OSAHSwasregisteredinanyoccasion. Overall,theresults
did not show statistical differencebetween the pre- and
post-ERTmeasurements,however,basedonthemedians,we
observedthatthepolysomnographicparameterswere
dete-riorating beforetherapy and presented stabilization after
theERT.Thisphenomenonwasalsoobservedforthemedians
ofthetimeofoxyhemoglobinunder90%.
Accordingtotheliteraturemostofthepatientsimproved
their respiratory status after ERT. Phases I/II clinical
tri-als have shown that all patients with OSAHS presented
reduction of the IAH after 52 weeks of ERT (mean of
2.1---1event/h); two patients had reductionof sleeptime
withoxygensaturationunder90%;onepatienthad
improve-mentinsleeptime.Aftersixyearsoffollow-up,fiveoutof
sixpatientswerere-evaluated,fourpresentedimprovement
or stabilization of therespiratory status, andone patient
wasworse.26
Phase III study also showed reduction of the IAH in
patientstreatedwithenzymewhencomparedtoaplacebo
group(reductionof6events/hinthetreatedgroup,increase
of0.3event/hintheplacebogroup).Inastudyaddressing
childrenundertheageof5years,reductionof8.5%ofthe
IAH was observed. However,amongsix patients who
pre-sentednormalIAHbeforetreatment,fourremainednormal
andtwogotworseaftertherapy.25Tokicetal.alsoreported
normalization to the IAH in two patients after 12 weeks
of treatment, along with improvement of the respiratory
pattern.29
Few isolatedreports,however,present contraryresults
regardingtherespiratoryquality.Sardónetal.hadapatient
with tracheotomy whodid not show improvement of the
respiratory quality after ERT.23 Thomas et al. described
a severely diseased patient treated with ERT for three
yearswithnochangesontheprogressionoftheobstructive
picture.34
DuetotheprogressivepatternofMPSI,stabilizationor
reductionoflesionsandspeedofthediseaseprogressionare
consideredasbenefitsfromthetherapy.2,26
Whenaquestionnaireiscompletedbythepatients,
stud-ieshaveshownimprovementindailyactivitiesperformance.
Ourpatients have alsodescribed ageneral improvement,
evenregardingthehypoacusia,sleepandrespiratory
qual-ity, which we could not demonstrate through the results
oftheobjectiveexams(audiometricandpolysomnographic
evaluations).
CurrentchallengesforERT,besidesthedevelopmentof
anearlydiagnosticprotocol(neonatalscreeningtest),
con-sistofpredictionoftheseverityofthedisease,inorderto
makeanadequatechoiceoftreatment,todevelopan
ade-quatetreatmentfortheneuropathies,andtofindwaysfor
aneffectivemonitoring,withspecificinstrumentsto
quan-tifytheimprovementoflifequalityaswellasefficacyand
effectivenessof the employed therapy.Longer follow-ups
canalsocontributetotheknowledgeofthesideeffectsof
theERTasmuchastheevaluationoftheevolutionofeach
systeminordertoplanassociatedtreatmentprograms.2,35,36
Although thiswasa study project ofa smallsampling,
overalltherewasaremarkableimprovementofthequality
of life of patients and a high level of satisfactionby the
relativesandhealthcareprofessionalsproducedbytheERT.
Conclusion
According to the results obtained from this study, we
observed that MPS I patients submitted to ERT with
laronidase(Aldurazyme®)presentimprovementoftheURT
recurrent infections, rhinorrhea, and general respiratory
status,without howeverpresenting expressiveand
signifi-cantimprovementofthehearingloss,tympanometriccurve
pattern,sleepdisorders,macroglossiaandtonsilsand
ade-noidhypertrophy.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.NeufeldEF,MuenzerJ.Themucopolysaccharidoses.In:Scriver CR,BlaudetAL,SlyWS,ValleD,editors.Themetabolicbases of inherited disease. 8th ed. New York: McGraw Hill; 2001. p.3421---52.
2.Wraith JE. The first 5 years of clinical experience with laronidaseenzymereplacementtherapyfor mucopolysaccha-ridosisI.ExpertOpinPharmacother.2005;6:489---506.
3.MartinsAM.Mucopolissacaridoses:ManualdeOrientac¸ões;2003 http://www.unifesp.br/centros/creim/downloads/gz-mps-apostila-2003.pdf
4.PastoresGM,Arn P,Beck M,ClarkeJTR,GuffonN,KaplanP, et al. The MPS I Registry: design, methodology and early findings of a global disease registry for monitoring patients with mucopolysaccharidosis type I. Mol Genet Metabol. 2007;91:37---47.
6.NayakDR,BalakrishnanR,AdolphS.Endoscopicadenoidectomy inacaseofScheiesyndrome(MPSIH).IntJPediatr Otorhino-laryngol.1998;44:177---81.
7.Brady RO.Enzymereplacementfor lysosomaldiseases. Annu RevMed.2006;57:283---96.
8.Leighton SEJ, Papsin B, Vellodi A, Dinwiddie R, Lane R. Disorderedbreathingduringsleepinpatientswith mucopolysac-charidoses.IntJPediatrOtorhinolaryngol.2001;58:127---38. 9.Ruckenstein MJ, MacDonald RE, Clarke JTR, Forte V. The
managementofotolaryngologicalproblemsinthe mucopolysac-charidoses: a retrospective review. J Otolaryngol. 1991;20: 177---83.
10.Semenza GL, Pyeritz RE. Respiratory complications of mucopolysaccharide storage disorders. Medicine. 1988;67: 209---19.
11.ShapiroJ,StromeM,CrockerAC.Airwayobstructionandsleep apneainHurlerandHuntersyndromes.AnnOtolRhinol Laryn-gol.1985;94:458---61.
12.GocerC,LinthicumFH.Hurlerdisease.OtolNeurotol.2004;25: 81---2.
13.Bredenkamp JK, Smith ME, Dudley JP, Williams JC, Crum-ley RL, Crockett DM. Otolaryngologic manifestations of the mucopolysaccharidoses. Ann Otol Rhinol Laryngol. 1992;101: 472---8.
14.FriedmannI,SpellacyE,CrowJ,WattsRWE.Histopathological studiesofthetemporalbonesinHurler’sdisease.JLaryngol Otol.1985;99:29---41.
15.Hayes E, Babin R, Platz C. The otologic manifestations of mucopolysaccharidosis.AmJOtol.1980;2:65---9.
16.Komura Y, Kaga K, OgawaY,YamaguchiY, TsuzukuT, Suzuki JI. ABRandtemporalbonepathology inHurler’sdisease. Int JPediatrOtorhinolaryngol.1998;43:179---88.
17.SchachernPA,SheaDA,PapparellaMM.Mucopolysaccharidosis IH(Hurler’sSyndrome)andhumantemporalbone histopatho-logy.AnnOtolRhinolLaryngol.1984;93:65---9.
18.Guffon N, Souillet G, MaireI, StraczekJ,Guibaud P.Follow upofninepatientswithHurlersyndromeafterbonemarrow transplantation.JPediatr.1998;33:119---25.
19.MaloneBN,WhitleyCB, DuvallAJ,BelaniK,SibleyRK, Ram-sayNKC,etal.ResolutionofobstructivesleepapneainHurler syndrome after bone marrow transplantation. Int J Pediatr Otorhinolaryngol.1988;15:23---31.
20.Papsin BC, Vellodi A, Bailey CM, Ratcliffe PC, Leighton SEJ. Otologic and laryngologicmanifestationsof mucopolysaccha-ridoses afterbonemarrowtransplantation.OtolaryngolHead NeckSurg.1998;118:30---6.
21.Peters C, Steward CG. Hematopoietic cell transplantation for inherited metabolic diseases: an overview of outcomes and practice guidelines. Bone Marrow Transplant. 2003;31: 229---39.
22.KakkisED,MuenzerJ,TillerGE,WaberL,BelmontJ,PassageM, etal.EnzymereplacementtherapyinmucopolysaccharidosisI. NEnglJMed.2001;344:182---8.
23.ClarkeLA,WraithJE,BeckM,KolodnyEH,PastoresGM, Muen-zerJ. Aldurazyme® enzyme replacementtherapy for MPS I: 48-weekextensiondata.AmJHumGenet.2003;73:623. 24.WraithJE,ClarkeLA,BeckM,KolodnyEH,PastoresGM,Muenzer
J, et al. Enzyme replacement therapy for mucopolysaccha-ridosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human a-l-iduronidase (laronidase).JPediatr.2004;144:581---8.
25.SifuentesM,DoroshowR,HoftR,MasonG,WalotI,DiamentM, etal.Afollow-upstudyofMPSIpatientstreatedwithlaronidase enzymereplacementtherapyfor6years.MolGenetMetabol. 2007;90:171---80.
26.Wraith JE, Beck M, Lane R, Ploeg A, Shapiro E, Xue Y, et al. Enzyme replacement therapy in patients who have mucopolysaccharidosisIandareyoungerthan5years:results of a multinational study of recombinant human alpha-l -iduronidase(laronidase).Pediatrics.2007;120:e37---46. 27.Sardón O, Pardos CG, Mintegui J, Ruiz EP, Coll MJ, Chabás
A,et al.Evolucióndedospacientes conSíndromedeHurler en tratamiento con enzima recombinante humana alpha-l -iduronidasa.AnPediatr(Barc).2005;63:61---7.
28.TokicV,BarisicI,HuzjakN,PetkovicG, FumicK,PaschkeE. Enzymereplacementtherapyintwopatientswithanadvanced severe(Hurler) phenotype ofmucopolysaccharidosis I. EurJ Pediatr.2007;166:727---32.
29.BrodskyL.Modernassessmentoftonsilsandadenoids.Pediatr ClinNorthAm.1989;36:1551---71.
30.American Thoracic Society. Indications and standards for cardiopulmonary sleep studies. Am Rev Respir Dis. 1989;153:866---78.
31.AmericanThoracicSociety.Indicationsandstandardsfor car-diopulmonarysleepstudiesinchildren.AmJRespirCritCare Med.1996;139:559---68.
32.Pereira LD, Ziliotto KN. Logoaudiometria. In: Campos CAH, Costa HOO, editors. Tratado de Otorrinolaringologia da SociedadeBrasileiradeORL.1sted.SãoPaulo:EditoraRoca; 2002.p.490---9.
33.JergerJ.Clinicalexperiencewithimpedanceaudiometry.Arch Otolaryngol.1970;92:311---24.
34.ThomasJA,JacobsS,KiersteinJ,VanHoveJ.Outcomeafter three years of laronidase enzyme replacementtherapy in a patientwithHurlersyndrome.JInheritMetabDis.2006;29:762. 35.MillingtonDS.Newbornscreeningforlysosomalstorage
disor-ders.ClinChem.2005;51:808---9.