FIRST INTERNATIONAL
CONFERENCE ON
VACCINES
AGAINST VIRAL AND
RICKETTSIAL DISEASES OF MAN
PAN AMERICAN HEALTH ORGANIZATION
Pan
American Sanitary Bureau, Regional Office of the
WORLD HEALTH ORGANIZATION
FIRST INTERNATIONAL CONFERENCE
ON
VACCINES AGAINST
VIRAL
AND
RICKETTSIAL DISEASES OF MAN
Papers Presented and Discussions Held
Washington, D.C., 7-11 November 1966
r-éRIA
Scientific Publication No. 147
PAN AMERICAN HEALTH ORGANIZATION Pan American Sanitary Bureau, Regional Office oí the
WORLD HEALTHORGANIZATION 525 Twenty-third Street, N.W. Washington, D.C. 20037, U.S.A.
Preface
A survey of opinion among members of the scientific community,
con-ducted late in 1965, made clear the desirability of convening an international
conference to summarize present knowledge and future needs in the field
of vaccines against viral and rickettsial diseases of man, and to consider
chemotherapeutic and other approaches in the control of these diseases.
The Conference was organized by the Pan American Health Organization
and the World Health Organization and was held at the PAHO Headquarters
in Washington, D.C., from 7 to 11 November 1966. It was structured to
serve as a forum for the presentation of individual papers by acknowledged
authorities from all parts of the world, and for prepared discussions and
open debate on those presentations.
The current and new information on practically all important aspects of
the subject, presented by nearly 300 distinguished scientists from 27 nations,
is recorded in the following pages.
We wish to express our gratitude to all those who contributed their efforts
to ensure the success of the Conference and the early publication of the
proceedings. We are particularly indebted to the Program Committee for
the excellent content of the agenda, and to the participants for the high
quality of their presentations, which have made a contribution of inestimable
value to the body of knowledge in this field.
ABRAHAM HORWITZ
Editorial Note
TABLE OF CONTENTS
Page
List of Participants ... ... ... xi
Opening Statement-Dr. Abraham Horwitz ... xxi
Keynote Address: Present Accomplishments and Future Needs for Viral and Rickettsial Vaccines-Dr. C. H. Stuart-Harris ... ... . xxv
SESSION I. CONTROL OF ACUTE RESPIRATORY DISEASES Section A. Influenza Virus Vaccines Present Status of Inactivated Influenza Virus Vaccines-Fred M. Davenport ... 3
Present Status of Live Influenza Virus Vaccines-V. M. Zhdanov ... 9
Animal Reservoirs of Influenza-L. Syruiek ... ... 16
Problems of Antigenic Variations and Broadening of Immunologic Response-Werner Henle 20 Discussants Dr. H. G. Pereira ... ... 24
Dr. Roslyn Q. Robinson ... ... ... 24
Dr. Anatoli A. Smorodintsev ... 25
Sir Christopher Andrewes ... ... 26
Colonel Edward L. Buescher ... 27
Dr. R. Sohier ... 28
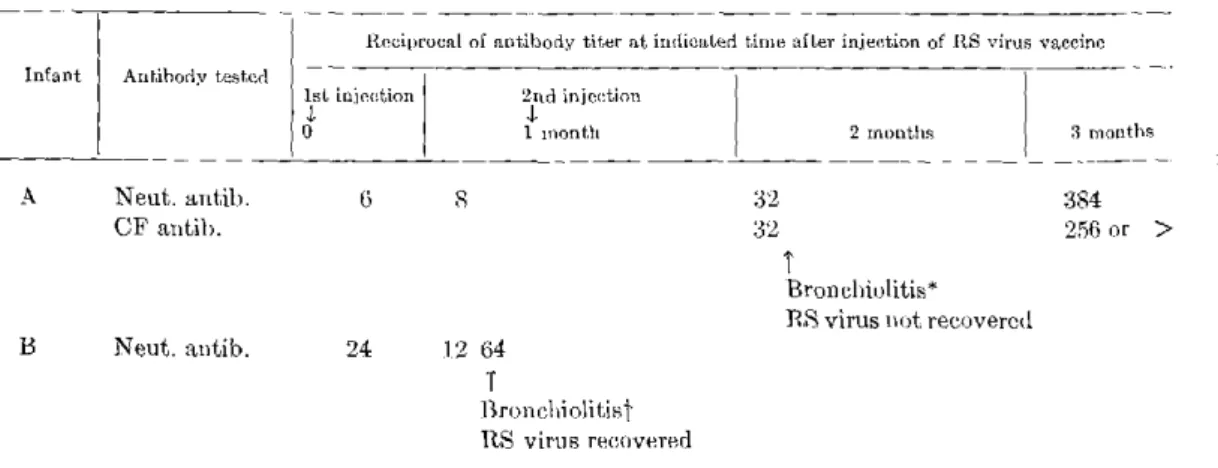
Section B. Respiratory Syncytial and Parainfluenza Virus Vaccines Experience with Inactivated Respiratory Syncytial and Parainfluenza Virus Vaccines in Infants-Robert H. Parrott, Hyun Wha Kim, Julita O. Arrobio, José G. Canchola, Carl D. Brandt, Joseph L. DeMeio, Keith E. Jensen, and Robert M. Chanock ... 35
Parainfluenza Type 3 Vaccine in Cattle-Z. Dinter ... 42
Discussants Dr. S. B. Mohanty ... 50
Dr. Robert M. Chanock ... 53
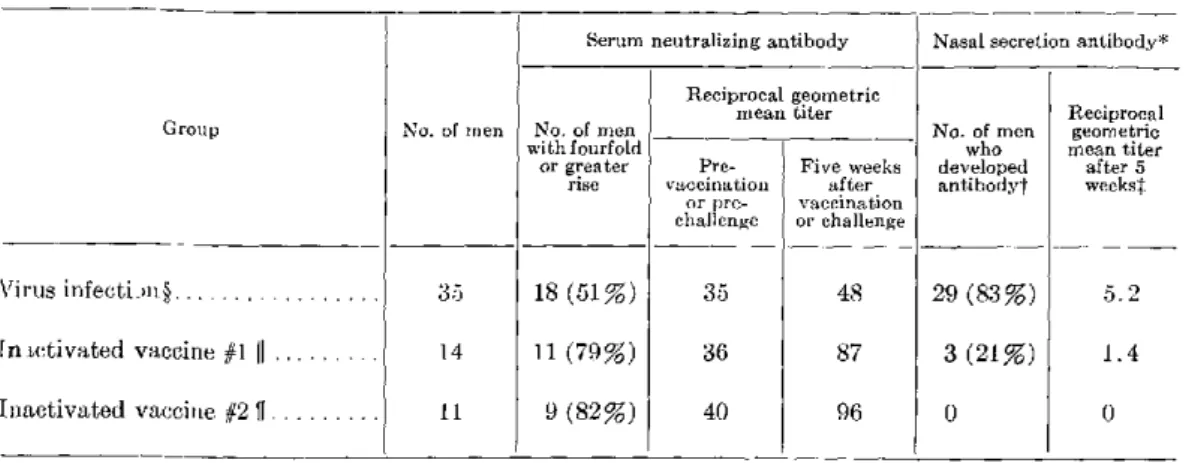
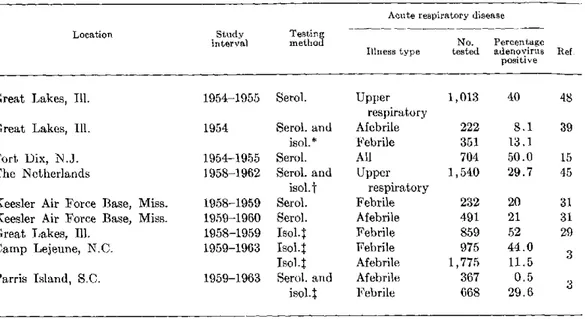
SESSION II. CONTROL OF ACUTE RESPIRATORY DISEASES (cont.) Section C. Adenoviruses Efficacy of Killed and Live Adenovirus Vaccines-Maurice A. Mufson ... 65
The Problem of Oncogenicity of Adenoviruses-Robert J. Huebner ... 73
Discussants Dr. Julius A. Kasel ... ... 81
Dr. John J. Trentin ... ... 82
Dr. H. G. Pereira ... 83
Dr. A. A. Selivanov ... 83
Page Section D. Rhinoviruses
Classification of Rhinoviruses-Herbert A. Wenner ... 9... 94
Tests of Rhinovirus Vaccines in Man-D. A. J. Tyrrell ... 102
Problems and Approaches to Control of Rhinovirus Infections-William S. Jordan, Jr ... 107
Discussants Dr. William J. Mogabgab ... 113
Dr. Dorothy M. Hamre ... 117
Section E. Mycoplasma Vaccines Classification of Mycoplasma of Man-D. G. ff Edward ... 120
Field Evaluation of Killed M. Pneumoniae Vaccine-William J. Mogabgab ... 125
Mycoplasma Pneumoniae Infection-Prospects for Live and Inactivated Vaccines-R. M. Chanock, C. B. Smith, W. T. Friedewald, R. Gutekunst, P. Steinberg, S. Fuld, K. E. Jensen, L. B. Senterfit, and B. Prescott ... . 132
Section F. Combined Respiratory Virus Vaccines Development and Field Evaluation of Combined Polyvalent Respiratory Virus Vaccines-M. R. Hilleman, R. E. Weibel, A. F. Woodhour, J. Stokes, Jr., C. C. Mascoli, Vaccines-M. B. Leagus, A. A. Tytell, and P. P. Vella ... 141
Discussants (Sections E. and F.) Dr. L. Hayflick ... ... ... 155
Dr. B. P. Marmion ... ... 156
Dr. Keith E. Jensen ... 157
SESSION III. ENTEROVIRUSES Section A. Poliomyelitis [nactivated Poliomyelitis Vaccine-Present and Future-Sven Gard ... 161
Poliomyelitis: Accomplishments of Live Virus Vaccine-Albert B. Sabin ... 171
Duration of Immunity Following Live Poliovirus Vaccine-Herald R. Cox ... 179
Poliovaccines Prepared in Human Diploid Cells-D. Ikic ... 185
The Present Status of Poliovirus Immunization in the USSR-M. P. Chumakov and M. K. Voroshilova ... 190
The State of Poliomyelitis in the World-S. G. Drozdov and W. Chas. Cockburn ... 198
Discussants Dr. George W. A. Dick ... 210
Dr. Andrew J. Rhodes ... ... 211
Dr. Manuel Ramos Alvarez ... 213
Dr. Jacobus D. Verlinde ... 218
Section B. Coxsackie and Echoviruses Importance and Prospects for Control of Coxsackievirus and Echovirus Infections-Joseph L. Melnick ... 223
Discussants Dr. M arina K. Voroshilova ... ... 233
Dr. Norman R. Grist ... 234
Dr. Dorothy Horstmann ... 234
Page
SESSION IV. ARBOVIRUSES AND HERPESVIRUS GROUP VIRUSES
Section A. Arboviruses
Classification of Arboviruses and Delineation of Clinically Important Types-Jordi Casals 243
Present and Future of Killed and Live Arbovirus Vaccines-William McD. Hammon .... 252
Possibilities for Control of Hemorrhagic Fevers in Latin America-R. B. MacKenzie,
M.
L. Kuns, and P. A. Webb ... ... ... 260Section B. Herpesvirus Group Viruses Vaccination Against Herpesvirus Infections-R. N. Hull and F. B. Peck, Jr. ... 266
Prospects for Immunization against Varicella and Cytomegalovirus Infections-Thomas H. Weller ... ... . 276
Discussants (Sections A. and B.) Colonel Edward L. Buescher ... ... ... 283
Dr. Karl M. Johnson ... . ... ... ... 283
Dr. Charles L. Wisseman, Jr. ... ... ... 284
Dr. Donald R. E. MacLeod ... ... ... ... .. ... 285
Colonel William D. Tigertt ... ... 286
Dr. T. F. McNair Scott ... 287
SESSION V. EXANTHEMS AND MUMPS Section A. Rubeola Present Status of Live Rubeola Vaccines in the United States-John F. Enders and Samuel L. Katz ... 295
Present Status of Killed Measles Vaccine-Erling C. Norrby ... . 301
Mass Rubeola Immunization in Africa-René Labusquiere ... 312
Mass Measles Immunization in South America-C. Ristori, J. M. Borgoñio, R. Greiber, and O. Avendaño ... 318
Immunization Against Measles in the USSR-V. M. Bolotovsky ... ... 325
Rubeola Immunization in the United Kingdom-Frank T. Perkins ... 330
Combined Measles-Smallpox and other Vaccines-H. M. Meyer, Jr., H. E. Hopps, B. C. Bernheim, and R. D. Douglas ... ... 336
Discussants Dr. W. Chas. Cockburn ... ... ... 343
Dr. Samuel L. Katz ... .... . ... .. ... ... ... 343
Dr. M. V. Milovanovic ... ... ... 345
Dr. Minoru Matumoto ... ... .... ... 346
Dr. Francois Kalabus ... ... ... 347
Dr. Saul Krugman ... ... 353
Dr. Anton J. Schwarz ... ... ... 361
Section B. Rubella Epidemiology of Rubella-John L. Sever .... ... 366
Gamma Globulin Prophylaxis of Rubella-J. C. McDonald ... ... 371
SESSION VI. EXANTHEMS AND MUMPS (cont.) Section B. Rubella (cont.) Laboratory Studies with an Attenuated Rubella Virus-Paul D. Parkman, H. M. Meyer, Jr., H. E. Hopps, and R. L. Kirschstein ... ... 381
Page Clinical Studies with an Attenuated Rubella Virus-Harry M. Meyer, Jr., P. D. Parkman,
T. C. Panos, G. L. Stewart, T. E. Hobbins, and F. A. Ennis ... 390
Prospects for Vaccination against Rubella-Saul Krugman ... 399
Discussants D r. Joseph Stokes, Jr .. .... ... ... 402
Dr. Stanley A. Plotkin ... ... 405
Col. Edward L. Buescher ... 408
Dr. Thomas H. Weller ... ... 408
Dr. Frederick C. Robbins ... ... 4... 409
Section C. Mumps Epidemiology and Clinical Importance of Mumps Infection in Man-Frederick C. Robbins 412 Present Knowledge of Killed Mumps Vaccine-Victor J. Cabasso ... 417
Experience with Live Mumps Vaccine in the USSR-A. A. Smorodintsev, N. S. Klyachko, M. N. Nasibov, and E. S. Schickina ... ... 422
Evaluation of Live Attenuated Mumps Virus Vaccine, Strain Jeryl Lynn-R. E. Weibel, E. B. Buynak, J. Stokes, Jr., J. E. Whitman, Jr., and M. R. Hilleman ... 430
Discussants Dr. Friedrich W. Deinhardt ... ... 438
Dr. E. B. Buynak ... ... 440
Dr. Werner Henle ... 442
SESSION VII. SMALLPOX, RABIES, AND HEPATITIS Section A. Smallpox Problems with Smallpox Vaccines and Possible Solutions-M. F. Polak ... 449
Problems of Mass Vaccination Programs-J. D. Millar ... 460
Smallpox Vaccines: Attributes of Egg and Calf Lymph Vaccines-J. A. Espmark ... 466
Discussants Dr. A. W. Downie ... ... 475
Dr. C. Henry Kempe ... ... 476
Dr. O. G. Andzaparidze ... ... 477
Dr. K. Raska ... 478
Section B. Rabies Present Concepts of the Epidemiology of Rabies-J. Frederick Bell ... . 481
Vaccines against Rabies: Present and Future-H. Koprowski ... 488
Discussants Dr. Paul Fenje ... ... 494
Dr. Martin Kaplan ... 495
Dr. Harald N. Johnson ... 496
Section C. Hepatitis Present Knowledge of the Etiology of Hepatitis-Robert W. McCollum . ... 500
Discussants Dr. I. William McLean, Jr. ... ... 505
Dr. Saul Krugman ... .... 506
Dr. Robert G. Ward .... ... ... 509
Dr. Friedrich W. Deinhardt .. ... 510
Dr. Werner Henle ... 511
Dr. Frederick O. MacCallum ... ... 512
Page
SESSION VIII. RICKETTSIAE, BEDSONIAE, AND ADJUVANTS
Section A. Rickettsiae
New Concepts on the Epidemiology of Typhus Fever--Cornelius B. Philip and Imam Z.
E. Imam ... 517
The Present and Future Immunization against the Typhus Fevers-Charles L. Wisseman, Jr. 523 Vaccination against Q Fever-Paul Fiset ... ... 528
Discussants Dr. Imam Z. E. Imam ... ... 532
Dr. John P. Fox ... ... ... 533
Dr. Herald R. Cox ... 534
Dr. B. P. Marmion ... ... 534
Section B. Bedsoniae Evaluation of Chemotherapy of Trachoma-Edward S. Murray and Roger L. Nichols .... 537
Immunization against Trachoma-J. Thomas Grayston ... 546
Section C. Adjuvants Problems and Future of Immunologic Adjuvants-Geoffrey Edsall ... 560
Discussants (Sections B. and C.) Dr. Roderick Murray ... 565
Dr. Frank T. Perkins ... 566
Dr. A. F. Woodhour ... ... 566
Dr. Richard Haas ... 569
SESSION IX. VACCINATION PROBLEMS AND OTHER APPROACHES TO CONTROL OF VIRAL DISEASES Section A. Problems of Vaccine Development Contemporary Problems in Regulating the Potency and Safety of Viral Vaccines-Roderick Murray ... ... 577
The Primary Problem with Virus Vaccines-Leonard Hayflick ... 581
Section B. Chemotherapy Chemoprophylactic Approach to the Control of Smallpox-D. J. Bauer ... 588
Chemoprophylaxis of Viral Respiratory Diseases-George Gee Jackson, Edith D. Stanley, and Robert Lee Muldoon ... 595
Chemotherapy of Herpesvirus Infections-Herbert E. Kaufman ... . 604
Discussants (Sections A. and B.) Dr. Frank T. Perkins ... ... 609
Dr. Frederick P. Nagler ... ... ... ... . 610
Dr. C. Henry Kempe ... 610
Dr. Kenneth W. Cochran ... 611
Section C. Interferon The Interferons: Some Unsolved Problems of Action and Biosynthesis-Robert R. Wagner and Thomas J. Smith ... . ... 616
The Role of Interferon in Human Viral Infections-E. Frederick Wheelock ... 623
Prospects for Applying Interferon to Man-Monto Ho and Bosko Postic . ... ... 632
Discussants
D r. A . B illiau . ... ...
Dr. Julius S. Youngner ... ... ... Dr. John H. Dingle ... ... .... ... Dr. D. Blalkovié . ... . . ... ... Dr. Samuel Baron ... . .... ... Dr. V. D. Soloviev ...
SESSION X. GENERAL DISCUSSION Summary of the Conference-Dr. Sven Gard ..
General Discussion ...
Closing Remarks-Dr. Abraham Horwitz ...
.. .. 663 ... 668 681 I n d e x . . . 683 Page
PARTICIPANTS
DR. FRANCIS R. ABINANTI
National Institutes of Health Bethesda, Maryland, USA
SIR CHRISTOPHER H. ANDREWES
Overchalke, Coombe Bisset Salisbury, Wilts.
England
DR. O. G. ANDZAPARIDZE
Research Institute of Virus Preparations Moscow, USSR
DR. M. S. ARTENSTEIN
Walter Reed Army Institute of Research Washington, D.C., USA
DR. KARL BAKOS
National Veterinary Institute Stockholm, Sweden
DR. JAMES BANTA
National Institutes of Health Bethesda, Maryland, USA
DR. SAMUEL BARON
National Institutes of Health Bethesda, Maryland, USA
DR. O. V. BAROYAN
Gamaleya Institute of Epidemiology and Microbiology
Moscow, USSR
DR. JOHN D. BAUER
The Wellcome Laboratories of Tropical Medicine Beckenham, Kent, England
DR. P. L. BAZELEY
The Salk Institute for Biological Studies La Jolla, California, USA
DR. ALAN J. BEALE
Virus Vaccine Department Glaxo Laboratories, Ltd. Buckinghamshire, England
DR. EARL S. BECK
National Institutes of Health Bethesda, Maryland, USA
DR. WALTER B. BECKER
National Institutes of Health Bethesda, Maryland, USA
DR. J. FREDERICK BELL
Rocky Mountain Laboratory Hamilton, Montana, USA
xi
DR. JOSEPH A. BELL
National Institutes of Health Bethesda, Maryland, USA
DR. ABRAM S. BENENSON
Jefferson Medical College Philadelphia, Pennsylvania, USA
DR. CHRISTEL M. BENITZ
Medical Research Cyanamid International Pearl River, New York, USA
DR. SANFORD BERMAN
Walter Reed Army Institute of Research Washington, D.C., USA
DR. BARBARA C. BERNHEIM
National Institutes of Health Bethesda, Maryland, USA
DR. A. J. D. A. BILLIAU
Rega Institute University of Louvain Louvain, Belgium
DR. LEONARD N. BINN
Walter Reed Army Institute of Research Washington, D.C., USA
DR. JAMES L. BITTLE
Infectious Disease Research The Dow Chemical Company Zionsville, Indiana, USA
DR. DIONYZ BLASKOVIC
Institute of Virology Bratislava, Czechoslovakia
DR. HENRY H. BLOOM
Microbiological Associates, Inc. Bethesda, Maryland, USA
DR. VLADIMIR M. BOLOTOVSKY
Central Institute of Epidemiology Moscow, USSR
DR. OTTO BONIN
Paul Ehrlich Institute Frankfurt am Main, Germany DR. F. L. BOWLING
DR. F. MARILYN BOZEMAN
Walter Reed Army Institute of Research Washington, D.C., USA
DR. ROBERT G. BRACKETT
Virus Research
Parke, Davis & Company Detroit, Michigan, USA
DR. JACOB A. BRODY
National Institutes of Health Bethesda, Maryland, USA
COL. EDWARD L. BUESCHER
Walter Reed Army Institute of Research Washington, D.C., USA
DR. EUGENE B. BUYNAK
Merck Institute for Therapeutic Research West Point, Pennsylvania, USA
DR. VICTOR J. CABASSO
Virus Immunological Research Lederle Laboratories
Pearl River, New York, USA
DR. J. CAMPBELL
Walter Reed Army Institute of Research Washington, D.C., USA
DR. EDWARD A. CARBREY
National Animal Disease Laboratory Ames, Iowa, USA
DR. JORDI CASALS
Yale University and Rockefeller Foundation New Haven, Connecticut, USA
DR. GABRIEL CASTELLANO
Microbiological Associates, Inc. Bethesda, Maryland, USA
DR. ROBERT M. CHANOCK
National Institutes of Health Bethesda, Maryland, USA
DR. PROCTOR CHILD
Armed Forces Institute of Pathology Washington, D.C., USA
DR. MIKHAIL P. CHUMAKOV
Institute of Poliomyelitis and Viral Encephalitides
Moscow, USSR
DR. W. CHAS. COCKBURN
World Health Organization Geneva, Switzerland
DR. KENNETH W. COCHRAN
The University of Michigan Ann Arbor, Michigan, USA
DR. HANS COHEN
Rijks Instituut voor de Volksgezondheid Utrecht, The Netherlands
DR. LEWIS L. CORIELL
South Jersey Medical Research Foundation Camden, New Jersey, USA
DR. HERALD R. Cox
Viral and Rickettsial Research Lederle Laboratories
Pearl River, New York, USA
DR. E. J. CUNNINGHAM
Medical Division VISTA
Washington, D.C., USA
DR. EDWARD C. CURNEN
Columbia University New York, New York, USA
DR. ARTHUR C. CURTIS
Public Health Division
U.S. Agency for International Development Washington, D.C., USA
DR. ROBERT T. CUTTING
U.S. Army Medical Research and Development Command
Washington, D.C., USA
DR. PETER DANS
National Institutes of Health Bethesda, Maryland, USA
DR. HARRY E. DASCOMB
Louisiana State University New Orleans, Louisiana, USA
DR. FRED M. DAVENPORT
The University of Michigan Ann Arbor, Michigan, USA
DR. DORLAND J. DAVIS
National Institutes of Health Bethesda, Maryland, USA
DR. ALBERT DAWKINS
The Johns Hopkins University Baltimore, Maryland, USA
DR. F. W. DEINHARDT
Presbyterian-St. Luke's Hospital Chicago, Illinois, USA
DR. P. DE SOMER*
Rega Institute University of Louvain Louvain, Belgium
DR. GEORGE W. A. DICK
Bland-Sutton Institute London, England
DR. JOHN H. DINGLE
Western Reserve University Cleveland, Ohio, USA
DR. ZVONIMIR DINTER
University of Uppsala Uppsala, Sweden DR. A. W. DOWNIE
The University of Liverpool Liverpool, England
DR. CHRISTOPHER C. DRAPER
The Wellcome Research Laboratories Beckenham, Kent, England
DR. HAROLD A. DRAYTON
University of Guyana Georgetown, Guyana
DR. T. D. DUBLIN
U.S. Agency for International Development Washington, D.C., USA
DR. JOHN A. DUDGEON
The Hospital for Sick Children London, England
DR. JAMES T. DUFF
National Institutes of Health Bethesda, Maryland, USA DR. H. BRUCE DULL
Communicable Disease Center Atlanta, Georgia, USA
DR. GEOFFREY EDSALL
Massachusetts Institute of Laboratories Boston, Massachusetts, USA
DR. D. G. FF EDWARD
The Wellcome Research Laboratories Beckenham, Kent, England
DR. BENNETT L. ELISBERG
Walter Reed Army Institute of Research Washington, D.C., USA
DR. JUAN E. EMBIL
Dalhousie University Halifax, N.S., Canada
DR. JOHN F. ENDERS
The Children's Hospital Medical Center Boston, Massachusetts, USA
DR. J. A ESPMARK
M.D. Anderson Hospital and Tumor Institute Houston, Texas, USA
DR. ALFRED S. EVANS
Yale University
New Haven, Connecticut, USA
DR. JACQUELINE FABIA
International Children's Center Paris, France
DR. AKINYELE FABIYI
National Institutes of Health Bethesda, Maryland, USA
DR. RAYMOND FAGAN
Virology Research Wyeth Laboratories Radnor, Pennsylvania, USA
DR. EGISTO FALCHETTI
Istituto Sieroterapico e Vaccinogeno Toscano Sclavo
Siena, Italy
DR. HARRY A. FELDMAN
State University of New York Syracuse, New York, USA
DR. PAUL FENJE
Connaught Medical Research Laboratories Toronto, Ontario, Canada
DR. MARY FINK
National Institutes of Health Bethesda, Maryland, USA
DR. PAUL FISET
University of Maryland Baltimore, Maryland, USA
DR. ALLAN L. FORBES
Life Sciences Division
Department of the Army
Arlington, Virginia, USA
DR. JOHN P. Fox
University of Washington
Seattle, Washington, USA
DR. THOMAS FRANCIS, JR. The University of Michigan Ann Arbor, Michigan, USA
DR. HARALD FREDERIKSEN
Office of Technical Cooperation and Research
U.S. Agency for International Development Washington, D.C., USA
DR. DAVID A. FUCILLO
National Institutes of Health Bethesda, Maryland, USA
DR. GEORGE J. GALASSO
The University of Virginia
Cliarlottesville, Virginia, USA
DR. SVEN GARD
Karolinska Institute
Stockholm, Sweden
DR. Ross L. GAULD
Walter Reed Army Institute of Research
Washington, D.C., USA
DR. A. GIOVANARDI
Universitá di Milano Milan, Italy
DR. GARY L. GITNICK National Institutes of Health
Bethesda, Maryland, USA
DR. PAULO DE GÓES Instituto de Microbiologia
Universidade do Brasil
Rio de Janeiro, Brasil
DR. FRANCIS B. GORDON
Department of Microbiology
Naval Medical Research Institute Bethesda, Maryland, USA
DR. ARTHUR N. GORELICK
Virus and Rickettsial Department
U.S. Army Biological Center
Frederick, Maryland, USA
DR. ALAN GRAY
Bacterial and Viral Vaccines
Merck, Sharp & Dohme
West Point, Pennsylvania, USA
DR. J. THOMAS GRAYSTON
University of Washington
Seattle, Washington, USA
DR. HERSCHEL E. GRIFFIN
Preventive Medicine Division
Office of the Surgeon General of the Army
Washington, D.C., USA
DR. NORMAN R. GRIST
University of Glasgow
Glasgow, Scotland
DR. RICHARD GRUNERT
Pharmaceutical Research
E. 1. du Pont de Nemours & Co.
Wilmington, Delaware, USA
DR. RICHARD HAAS
University of Freiburg
Freiburg, Germany
DR. WILLIAM McD. HAMMON
University of Pittsburgh
Pittsburgh, Pennsylvania, USA
DR. DOROTHY M. HAMRE
University of Chicago
Chicago, Illinois, USA
DR. V. R. HARRISON
Walter Reed Army Institute of Research
Washington, D.C., USA
DR. LEONARD HAYFLICK
The Wistar Institute
Philadelphia, Pennsylvania, USA
DR. R. HEBERLING
National Institutes of Health
Bethesda, Maryland, USA
DR. ALFRED HELLMAN
Oak Ridge National Laboratory
Oak Ridge, Tennessee, USA
DR. WERNER HENLE
The Children's Hospital of Philadelphia
Philadelphia, Pennsylvania, USA
DR. WALTER A. HENNESSEN
Behringwerke, AG.
Marburg/Lahn, Germany
DR. CASPAR W. HIATT
National Institutes of Health
Bethesda, Maryland, USA
DR. MAURICE R. HILLEMAN
Merck Institute for Therapeutic Research
WesI Point, Pennsylvania, USA
DR. MONTO Ho
University of Pittsburgh Pittsburgh, Pennsylvania, USA
DR. HORACE L. HODES The Mount Sinai Hospital New York, New York, USA
DR. ARIEL C. HOLLINSHEAD The George Washington University Washington, D.C., USA
DR. JACOB C. HOLPER
Infectious Diseases Research Division
Abbott Laboratories Chicago, Illinois, USA Djt. H. E. Hopps
National Institutes of Health Bethesda, Maryland, USA
DR. RICHARD HORNICK The University of Maryland
Baltimore, Maryland, USA
DR. DOROTHY HORSTMANN Yale University
New Haven, Connecticut, USA
DR. ABRAHAM HORWITZ
Pan American Health Organization
Washington, D.C., USA
DR. ROBERT J. HUEBNER National Institutes of Health Bethesda, Maryland, USA
DR. ROBERT N. HULL
The Lilly Research Laboratories Indianapolis, Indiana, USA
DR. DAVID L. HUXSOLL
Walter Reed Army Institute of Research
Washington, D.C., USA
DR. HOWARD J. IGEL National Institutes of Health
Bethesda, Maryland, USA
DR. DRAGO IKIC Institute of Immunology Zagreb, Yugoslavia
DR. IMAM Z. E. IMAM Virus Research Center Production Laboratories Agouza, Cairo, UAR
DR. GEORGE G. JACKSON The University of Illinois Chicago, Illinois, USA
DR. LEON JACOBS
National Institutes of Health Bethesda, Maryland, USA
DR. KEITH E. JENSEN
Medical Research Laboratories Chas. Pfizer and Company, Inc.
Groton, Connecticut, USA
DR. ERIK JOHANSSON
Hem Research Incorporated Bethesda, Maryland, USA
DR. DONALD W. JOHNSON
Animal Health Division U.S. Department of Agriculture Hyattsville, Maryland, USA
DR. HARALD N. JOHNSON
Arthropod-Borne Virus Studies The Rockefeller Foundation Berkeley, California, USA DR. KARL McK. JOHNSON Middle America Research Unit Balboa Heights, Canal Zone
DR. WILLIAM S. JORDAN, JR.
The University of Virginia Charlottesville, Virginia, USA
DR. J. MEHSEN JOSEPH
Maryland State Department of Health Baltimore, Maryland, USA
DR. FRANCOIS A. KALABUS
Ministere de la Santé Quebec, Canada
DR. ALBERT Z. KAPIKIAN
National Institutes of Health Bethesda, Maryland, USA
DR. MARTIN M. KAPLAN
World Health Organization Geneva, Switzerland
DR. JULIUS A. KASEL
National Institutes of Health Bethesda, Maryland, USA
DR. SAMUEL L. KATZ
The Children's Hospital Medical Center Boston, Massachusetts, USA
DR. HERBERT E. KAUFMAN
The University of Florida Gainesville, Florida, USA
DR. C. HENRY KEMPE
The University of Colorado Medical Center Denver, Colorado, USA
DR. RUTH L. KIRSCHSTEIN
National Institutes of Health Bethesda, Maryland, USA
DR. MORTON KLEIN
Temple University
Philadelphia, Pennsylvania, USA
DR. HILARY KOPROWSKI
The Wistar Institute
Philadelphia, Pennsylvania, USA
DR. SAUL KRUGMAN New York University New York, New York, USA
DR. RENÉ LABUSQUIERE
Organization for Coordination and Cooperation in the Control of Major Endemic Diseases for the Countries of Central Africa
Yaoundé, Republic of Cameroon
DR. NICHOLAS M. LARIN
Biologicals Research Department The Pfizer Group
Kent, England
DR. EDWIN H. LENNETTE
Viral and Rickettsial Disease Laboratory State of California Department of Public Health Berkeley, California, USA
DR. PIERRE R. LEPINE
Institut Pasteur Paris, France
DR. STANLEY LEVIN
Kaplan Hospital Rehovot, Israel
DR. L. JAMES LEWIS
Sterling-Winthrop Research Institute Biological Division
Rensselaer, New York, USA
DR. FLORENCE LIEF
University of Pennsylvania Philadelphia, Pennsylvania, USA
DR. ROYCE Z. LOCKART, JR. Central Research Department E. I. du Pont de Nemours & Co. Wilmington, Delaware, USA
DR. WILLIAM T. LONDON
National Institutes of Health Bethesda, Maryland, USA
DR. CLAYTON G. LOOSLI
University of Southern California Los Angeles, California, USA
DR. JOSEPH P. LOWENTHAL
Walter Reed Army Institute of Research Washington, D.C., USA
DR. LAURI LUOTO
National Institutes of Health Bethesda, Maryland, USA
DR. RONALD B. MACKENZIE
Rockefeller Foundation Bogotá, Colombia
DR. FREDERICK O. MACCALLUM
Virus Laboratory The Radcliffe Infirmary Oxford, England
DR. DONALD R. E. MAcLEOD
Connaught Medical Research Laboratories University of Toronto
Toronto, Ontario, Canada
DR. HERDIS VON MAGNUS
Statens Seruminstitut Copenhagen, Denmark
DR. BARRIE P. MARMION
Monash University Melbourne, Australia
DR. SALVADOR MARTIN
Instituto de Vacunas y Biológicos México, D.F., México
DR. MAURICIO MARTINS DA SILVA
Pan American Health Organization Washington, D.C., USA
DR. MINORU MATUMOTO
The University of Tokyo Tokyo, Japan
DR. GORDON MEIKLEJOHN
University of Colorado Medical Center Denver, Colorado, USA
DR. JOSEPH L. MELNICK
Baylor University Houston, Texas, USA
DR. HARRY M. MEYER, JR.
National Institutes of Health Bethesda, Maryland, USA
DR. JACK W. MILLAR
Bureau of Medicine and Surgery Washington, D.C., USA
DR. JOHN D. MILLAR
Communicable Disease Center Atlanta, Georgia, USA
DR. WILLIAM S. MILLER
U.S. Army Biological Center Frederick, Maryland, USA
DR. MILAN V. MILOVANOVIC
World Health Organization Rio de Janeiro, Brazil
DR. WILLIAM J. MOGABGAB
Tulane University
New Orleans, Louisiana, USA
DR. S. B. MOHANTY
University of Maryland College Park, Maryland, USA
DR. J. ANTHONY MORRIS
National Institutes of Health Bethesda, Maryland, USA
DR. MAURICE A. MUFSON
University of Illinois Chicago, Illinois, USA
DR. DANIEL MULLALLY
National Institutes of Health Bethesda, Maryland, USA
DR. EDWARD S. MURRAY
Harvard University
Boston, Massachusetts, USA
DR. RODERICK MURRAY
National Institutes of Health Bethesda, Maryland, USA
DR. SAMUEL L. MUSSER
Research Department Philips Roxane, Inc. St. Joseph, Missouri, USA
DR. ROBERT W. MCCOLLUM
Yale University
New Haven, Connecticut, USA
DR. STEWART J. MCCONNEL
U.S. Army Medical Research Unit Frederick, Maryland, USA DR. J. CORBETT McDoNALD
McGill University Montreal, Canada
LT. COL. ROBERT W. McKINNEY
U.S. Army Medical Research Unit Frederick, Maryland, USA DR. I. WILLIAM McLEAN, JR.
Microbiological Research Parke, Davis & Company Detroit, Michigan, USA DR. FREDERICK P. NAGLER
Virus Laboratories
Department of National Health and Welfare Ottawa, Ontario, Canada
DR. NEAL NATHANSON
The Johns Hopkins University Baltimore, Maryland, USA
DR. CHARLES F. NEEDY
Walter Reed Army Institute of Research Washington, D.C., USA
DR. THIEU L. NGHIEM
Rubella Vaccine Program Microbiological Associates Bethesda, Maryland, USA
DR. ERLING C. NORRBY
Karolinska Institute Stockholm, Sweden
DR. FERNANDO C. OTTATI
Medical Research and Development Cyanamid International
Pearl River, New York, USA
DR. JOHN R. OVERMAN
National Institutes of Health Bethesda, Maryland, USA
DR. PAUL D. PARKMAN National Institutes of Health Bethesda, Maryland, USA
DR. ARMANDO S. PARODI Universidad de Buenos Aires Buenos Aires, Argentina
DR. ROBERT H. PARROTT
Children's Hospital of the. District of Columbia and Georgetown University
Washington, D.C., USA
DR. VYTAUTAS PAVILANIS
University of Montreal Montreal, Quebec, Canada
DR. ANTHONY M.-M. PAYNE
World Health Organization Geneva, Switzerland
DR. GERALD V. PEACOCK
U.S. Department of Agriculture Hyattsville, Maryland, USA DR. F. BRUCE PECK
The Lilly Clinical Research Laboratories Indianapolis, Indiana, USA
DR. R. 0. PECKINPAUGH Naval Medical Research Unit Great Lakes, Illinois, USA
DR. H. G. PEREIRA
National Institute for Medical Research London, England
DR. FRANK T. PERKINS
National Institute for Medical Research London, England
DR. JOHN C. PERKINS
National Institutes of Health Bethesda, Maryland, USA
DR. CORNELIUS B. PHILIP
Rocky Mountain Laboratory Hamilton, Montana, USA
DR. CHARLES E. PHILLIPS
National Animal Disease Laboratory Ames, Iowa, USA
DR. MARGARET PITTMAN
National Institutes of Health Bethesda, Maryland, USA
DR. STANLEY A. PLOTKIN
The Wistar Institute
Philadelphia, Pennsylvania, USA
DR. MARIUS F. POLAK
Rijks Instituut voor de Volksgezondheid Utretch, The Netherlands
DR. BERNARD PORTNOY
University of Southern California Los Angeles, California, USA DR. BosKO POSTIC
University of Pittsburgh Pittsburgh, Pennsylvania, USA
DR. ABEL L. PRINZIE
Rega Institute University of Louvain Louvain, Belgium
DR. ROBERT H. pURCELL
National Institutes of Health Bethesda, Maryland, USA
DR. MANUEL RAMOS ALVAREZ
Hospital Infantil de México México, D.F., México
DR. KAREL RASKA
World Health Organization Geneva, Switzerland
DR. A. F. RASMUSSEN
University of California Los Angeles, California, USA
DR. FRANK RAUSCHER
National Institutes of Health Bethesda, Maryland, USA
DR. ROBERT C. REINSINGER
Animal Health Division U. S. Department of Agriculture Hyattsville, Maryland, USA DR. ANDREW J. RHODES University of Toronto Toronto, Ontario, Canada
DR. LIONEL E. RHULAND
Department of Virus Research The Upjohn Company Kalamazoo, Michigan, USA
DR. WILTON A. RIGHTSEL
Virus Research Department Parke, Davis & Company Detroit, Michigan, USA
DR. HARRIS D. RILEY
The University of Oklahoma Medical Center Oklahoma City, Oklahoma, USA
DR. CONRADO RISTORI
Servicio Nacional de Salud Santiago, Chile
DR. FREDERICK ROBBINS
Western Reserve University Cleveland, Ohio, USA DR. ROSLYN Q. ROBINSON Communicable Disease Center Atlanta, Georgia, USA
DR. EUGENE I. ROSANOFF
Virology Research Department Wyeth Laboratories
Philadelphia, Pennsylvania, USA
DR. MAX J. ROSEMBAUM
Department of the Navy Medical Research Unit Great Lakes, Illinois, USA
DR. C. E. VAN ROOYEN
Dalhousie University Halifax, N.S., Canada
DR. ALBERT B. SABIN
DR. SAMUEL SASLAW
The Ohio State University Columbus, Ohio, USA
DR. MORRIS SCHAEFFER
Bureau of Laboratories
The New York City Department of Health New York, New York, USA
DR. ANTON J. SCHWARZ
Pittman-Moore Research Center Zionsville, Indiana, USA
DR. THOMAS MCNAIR SCOTT
The Children's Hospital of Philadelphia Philadelphia, Pennsylvania, USA
DR. JOHN R. SEAL
National Institutes of Health Bethesda, Maryland, USA
DR. EDWARD S. SELIGMANN
National Institutes of Health Bethesda, Maryland, USA
DR. A. A. SELIVANOV
Institute of Experimental Medicine Leningrad, USSR
DR. JOHN L. SEVER
National Institutes of Health Bethesda, Maryland, USA DR. E. D. SHAW
Armed Forces Institute of Pathology Washington, D.C., USA
Da. ALEXIS SHELOKOV
National Institutes of Health Bethesda, Maryland, USA
DR. RALPH C. SINGER
Communicable Diseases Branch
Office of the Surgeon General of the Army Washington, D.C., USA
DR. THOMAS J. SMITH
Walter Reed Army Institute of Research Washington, D.C., USA
DR. A. A. SMORODINTSEV
Institute of Experimental Medicine Leningrad, USSR
DR. R. M. SOMIER
Faculté de Medecine Lyon, France
DR. V. D. SOLOVIEV
Gamaleya Institute of Epidemiology and Microbiology
Moscow, USSR
DR. FRED L. SOPER
Pan American Health Organization Washington, D.C., USA
DR. JAMES V. SORRENTINO
Walter Reed Army Institute of Research Washington, D.C., USA
DR. LESLIE P. SPENCE
Trinidad Regional Virus Laboratory Port-of-Spain, Trinidad
DR. EDITH STANLEY
University of Illinois
Chicago, Illinois, USA
DR. ALEX J. STEIGMAN
Mount Sinai Medical College and Hospital New York, New York, USA
DR. LJUBINKO STOJKOVIC
Serbian Institute of Public Health Belgrade, Yugoslavia
DR. JOSEPH STOKES
The Henry Phipps Institute Philadelphia, Pennsylvania, USA
DR. JURAJ STRAUSS
National Institutes of Health Bethesda, Maryland, USA
DR. CHARLES H. STUART-HARRIS
The University of Sheffield Sheffield, England
DR. EUGENIO SUÁREZ
Pan American Health Organization Washington, D.C., USA
DR. BENJAMIN H. SWEET
Gulf South Research Institute New Orleans, Louisiana, USA
DR. LUBOS SYR ClEK
Institute of Epidemiology and Microbiology
Prague, Czechoslovakia
DR. K. J. TAYLOR
Microbiological Screening Service Cutter Laboratories
Berkeley, California, USA
DR. HELEN L. TEPPER
National Institutes of Health Bethesda, Maryland, USA
DR. WILLIAM J. THOMAS Virology Research
National Drug Company
Swiftwater, Pennsylvania, USA DR. WILLIAM D. TIGERTT
Walter Reed Army Institute of Research Washington, D.C., USA
DR. HOWARD TINT
Biological and Chemical Development Wyeth Laboratories
Philadelphia, Pennsylvania, USA DR. Y. Toco
University of Maryland Baltimore, Maryland, USA
DR. DAVID A. TYRRELL
National Institute for Medical Research Salisbury, Wilts, England
DR. JOSEPH UNGAR
Swiss Serum and Vaccine Institute Berne, Switzerland
DR. JACOBUS D. VERLINDE
University of Leiden Leiden, The Netherlands
DR. MARINA VOROSHILOVA
Institute of Poliomyelitis and Viral Encephalitides
Moscow, USSR
DR. JOHN C. WAGNER
National Institutes of Health Bethesda, Maryland, USA
DR. R. R. WAGNER
The Johns Hopkins University Baltimore, Maryland, USA
DR. ROBERT WARD
University of Southern California Los Angeles, California, USA
DR. THOMAS G. WARD
Microbiological Associates, Inc. Bethesda, Maryland, USA
DR. JOEL WARREN
Biologics Research Department Chas. Pfizer and Company Terre Haute, Indiana, USA
DR. ROBERT E. WEIBEL
University of Pennsylvania Philadelphia, Pennsylvania, USA DR. THOMAS H. WELLER
Harvard University
Boston, Massachusetts, USA
DR. HERBERT A. WENNER
University of Kansas School of Medicine Kansas City, Kansas, USA
DR. EARLE F. WHEELOCK
Western Reserve University Cleveland, Ohio, USA
DR. CARRIE E. WHITMIRE
Biological Development and Production Winthrop Laboratories
Rensselaer, New York, USA
DR. T. J. WIKTOR
The Wistar Institute
Philadelphia, Pennsylvania, USA
DR. ROBERT J. WILSON
Connaught Medical Research Laboratories Toronto, Ontario, Canada
DR. CHARLES L. WISSEMAN, JR.
University of Maryland Baltimore, Maryland, USA
DR. RUTH G. WITTLER
Walter Reed Army Institute of Research Washington, D.C., USA
DR. ALBERT E. WOELTJEN
Clinical Research Department Pfizer International, Inc.
New York, New York, USA
DR. ALLEN F. WOODHOUR
Merck Institute for Therapeutic Research West Point, Pennsylvania, USA
DR. THEODORE WOODWARD
University of Maryland Baltimore, Maryland, USA
DR. ROBERT L. WOOLRIDGE
National Institutes of Health Bethesda, Maryland, USA
DR. CHARLES J. YORK
Institute for Comparative Biology San Diego, California, USA
DR. JULUS S. YOUNGNER
University of Pittsburgh Pittsburgh, Pennsylvania, USA
DR. KAREL ZACEK
Institute of Epidemiology and Microbiology Prague, Czechoslovakia
DR. VICTOR M. ZHDANOV
Institute of Virology Moscow, USSR
Program Committee
DR. W. CHAS. COCKBURN World Health Organization Geneva, Switzerland
DR. SVEN GARD Karolinska Institute Stockholm, Sweden
DR. WERNER HENLE
The Children's Hospital of Philadelphia Philadelphia, Pennsylvania, USA
DR. M. R. HILLEMAN (Consultant)
Merck Institute for Therapeutic Research West Point, Pennsylvania, USA
DR. M. MARTINS DA SILVA (Conference Secretary)
Office of Research Coordination Pan American Health Organization Washington, D.C., USA
INTRODUCTORY REMARKS
ABRAHAM HORWITZ
Director, Pan American Sanitary Bureau,
Regional Office of
the World Health Organization
for the Americas
DR. HORWITZ: 1 wish to express, in the name
of the Pan American Health Organization and the World Health Organization, our deep grati-tude for your presence in this house during this week. For you are going to benefit us-and through us, millions of human beings all over the world-with your knowledge and experience in a field of deep significance for science and for the well-being of man.
We consider this Conference to be a direct continuation of the dialogue started a few years ago, under our sponsorship, on live poliovirus vaccines. The presence of many of you today who also attended the two conferences on live poliovirus vaccines is in the tradition of Aristotle when he stated that a community is viable when everyone knows the face of his neighbor. We, of this Organization, are anxious to create a true intellectual community of the sciences and arts of health as the most essential force in fostering progress in the well-being of people.
A few years ago, in my introductory remarks to the First PAHO/WHO Conference on Live Poliovirus Vaccines, I declared that "in the evo-lution of ideas in search for those truths which bear on the lives of many people it is indispensa-ble, from time to time, to pause and to analyze what is known, what still remains to be learned, and to determine the course which must be fol-lowed to reach the original objectives." Thus we are assembled here to analyze the past and the present and to project the future of vaccines against viral and rickettsial diseases. The pro-gram for the week ahead is ambitious, but we feel certain that the eminence and high compe-tence of the speakers and participants are full
guarantee that the Conference's objectives will he attained.
If there is a characteristic common to all or most of the subjects to be discussed, it is their complexity-complexity in the sense that nature still keeps many secrets to be unravelled. The more we know, the greater the stimulus for those especially endowed with an inquisitive mind and great imagination to penetrate to the very es-sence of each process in order to interpret those forces revealing the existence of new relation-ships which require new principles and laws. Ortega y Gasset wisely observed that facts "cover" reality and that the role of science is precisely to "uncover" it. He symbolized science as "construction" and judged it, as it related to matters corporeal or spiritual, as much a prod-uct of imagination as of observation, the latter not being possible without the former. He added: "This characteristic, in part at least, the imagina-tive element of science, makes of her a sister of poetry."*
In keeping with the best of their postulates, both the Pan American Health Organization and the World Health Organization are eager to learn from your experience what knowledge is ready to he applied to prevent or cure disease, as well as to learn of those concepts that will stimulate further investigation. The history of the recent past is rich in accomplishments and justifies these new explorations. We need only to mention vaccines against yellow fever, small-pox, and poliomyelitis. Where the vaccines have
beea applied in a systematic way, these diseases * Ortega y Gasset, José. En torno a Galileo-Esquema de las crisis, page 17, Espasa-Calpe, S.A., Madrid, 1965.
xxii Introductor y Remarks * Horwitz
have been brought to comparative inconsequence and we look forward to similar success in the most recent initiative of Governments in their agreement to undertake a world-wide effort to eradicate smallpox, coordinated by the World
Health Organization.
In view of such background and purpose, it seems fitting that this international scientific conference is being held in our new house and is devoted to viral and rickettsial diseases and to measures that have been or are being developed
for their prevention. We would hope to be able in the future to continue this dialogue so as to consolidate some of the experiences, as well as to explore others, such as those related to chronic and degenerative diseases, neoplasia, autoim-mune and other maladies of long-term occur-rence, which only now are beginning to demon-strate or to suggest a viral etiology.
Since the days of Jenner and Pasteur, who used intact animals, to our days in which cell-culture procedures are commonly employed, great progress has occurred in virus vaccine production. It is to be regretted that the same accomplishment has not been registered in their application for human betterment, even in the case of those vaccines whose preventive capacity is solid and long-proven and whose cost, through industrial production, is relatively small. As a consequence, millions of human beings living in the so-called underdeveloped world are deprived of the benefits of immunization and specific dis-eases still show high incidence and mortality. Under the circumstances, the question has been raised as to whether these countries should be concerned with advanced research and its ef-fects, or whether they should concentrate on the traditional procedures for the solution of basic health problems.
Colm has stated that development derives from "growth and change" involving the economy, the organization and administration of services, and social welfare. All of them are preconditions to the application of modern technology. In this complex interplay of forces, health care is one of the essential components because, jointly with education, it contributes to the development of each personality and, through this process, to productivity and production. Progress is mainly the result of the spirit of creation, inventiveness and ingenuity, as well as a recognition of
re-sponsibility and of the need for decision on the part of those endowed with a sense of national purpose. All other elements, capital investment included, are complementary. Development will not be realized unless increasing social opportuni-ties are offered within each society, so that each human being "will be more, knowing more."
In the field of health, people do not accept discrimination with regard to the quality of cura-tive and prevencura-tive medicine to which they as-pire. They want immediately for themselves and for their children that which is modern as soon as it becomes practical and is known. Govern-ments must satisfy these aspirations, as a moral imperative and as a fundamental activity for the future of the country. For this very important purpose, scientists and the other elements of the intellectual community should keep in active as-sociation with Governments, regardless of cur-rents of political opinion, because it is the well-being of the people that is at stake. International organizations, intergovernmental in nature and in objective, such as the World Health Organiza-tion and the Pan American Health OrganizaOrganiza-tion, should contribute their efforts to spread modern knowledge, to assist in its application, and to create the appropriate atmosphere for the best minds in each discipline to coordinate and to systematize their thoughts and experiences. In this way, old concepts and techniques are mod-ernized; new ideas are explored. As in any at-tempt to explain all phenomena of the real
world, the process is never ending.
Introductory Rernarks * Horwitz
understood properly as a form of culture, is a means of eventually providing the whole com-munity with an objective awareness of the proper context of man; it gives a holistic view of the universe, in keeping with man's intellectual na-ture; it will eventually provide a basis for mutual understanding; and it is in any case a proper basis on which to build education." *
· Report to the Director. First Meeting, PAHO Advisory Committee on Medical Research, 18-22 June 1962, Washington, D. C.
xxiii
The opening address for this Conference will he presented by Dr. Stuart-Harris, who is Pro-fessor of Medicine at the University of Sheffield, England. Dr. Stuart-Harris has had many years
KEYNOTE ADDRESS:
PRESENT ACCOMPLISHMENTS AND FUTURE NEEDS
C. H. STUART-HARRIS
Departinent of Medicine, University of Sheffield
Sheffield, England
DR. STUART-HARRIS: The infections that will be
considered by this Conference attack the popu-lations of all countries throughout the world to a
greater or lesser degree. Their control by means that will be both safe and effective is the dream of those charged with responsibility for the health of the people. Yet the very success of some of the weapons forged in the laboratory and applied in the field has served but to high-light the failures in other directions. Sometimes the failure has been attributed to the weapons themselves or to the characteristics of the in-fectious agent; sometimes it has been in the method of application of the prophylactic to the population. Nevertheless, as it is my task to ring up the curtain on this Conference, I prefer to deal first with success rather than failure, in the hope that the principles that have led to success may also guide us in the consideration of future needs.
The Achievements
It is now a little over 200 years since Edward Jenner embarked on the career that made him the pioneer in the control of disease by artificial immunization. From the time of the first vac-cination in a boy with lymph from the vesicle of a milkmaid's cowpox, immunization against smallpox has had a chequered career. When Jenner died in 1823, his work was only partly recognized by his countrymen, and it was bitterly criticized after his death. Variolation with ma-terial from cases of variola continued to be prac-ticed in Britain until 1840 in spite of protests and a firm recommendation for Jennerian vac-cination by the Royal College of Physicians of
London. As vaccination gradually came to be adopted during the latter half of the 19th cen-tury, so did variola decline in Europe and North America.
Today, however, the situation is different. There remain foci of virulent smallpox in Asia and elsewhere that threaten the rest of the world where variola major has ceased to occur. In Britain the rate of acceptance for vaccinial im-munization in babies and infants, is now only about 30 per cent unless a special effort is made. Thus every importation of variola is a cause of public alarm and perhaps mass vaccination cam-paigns, with their attendant risk of severe re-actions and hazards for eczematous babies. Only recently has the World Health Assembly decided to devote large resources in an effort to vanquish smallpox at its source. Truly, the achievement so far obtained could have been infinitely greater had the necessary effort been made.
But Jenner's torch lit the path for Pasteur, and it was Pasteur who showed the way to at-tenuate microbial virulence artificially. The prin-ciples enunciated by Pasteur in his work on fowl cholera, anthrax, and rabies have stood the test of time and are still in use today. They were the inspiration for Theiler and his colleagues of the Rockefeller Foundation in their successful cultivation of the 17D strain of yellow fever virus. They led also to the growth and develop-ment of immunology, which today has become a key scientific discipline in its own right.
The control of disease by artificial immuniza-tion based on Pasteurian principles is of course most strikingly instanced in our time by polio-myelitis. The fact that in this vast country of
Keynote Address · Stuart-Harris the United States paralytic poliomyelitis has
be-come reduced from a disease numbered in thou-sands of cases annually to a mere 61 in 1965 is an astonishing and solid achievement directly attributable to immunization. The fact that this has been brought about by the deployment of two entirely different potent vaccines-those of Salk and of Sabin-is also remarkable. Had these weapons not been employed in dramatic mass campaigns in both the United States and the USSR, the course of history might well have been different. The lesson that a vaccine must be not only safe and effective but also ac-ceptable to the people should by now have been well and truly learned.
It is not for me to adjudicate upon the respec-tive merits of different forms of poliovirus vac-cines, and I know that all those present would prefer that I honor the one to whom the modern work on poliomyelitis can be traced-John En-ders. Not only did he and his co-workers at the Boston Children's Hospital provide the tissue-culture tools requisite for the preparation of virus vaccines in bulk, but he himself has since tamed the measles virus and provided the world with yet another potent weapon. Nor does the story end here, for already a tissue-culture line of rubella virus has been established by Park-man and others at the National Institutes of Health, which may provide another safe and ef-fective prophylactic against a disease that is trivial for the child but hazardous indeed for the fetus. The application of both measles and rubella vaccines for the attempted control of their respective diseases is an unfinished story, but no one can fail to be amazed at the pace of development in the past ten years.
Present Challenges
It is time now to turn to the challenges pre-sented to us by the unconquered infections of our several populations in order to assess the present needs. The commonest, if not the most deadly, infections in all parts of the world are still the acute infections of the respiratory tract. The fact that it is more than 30 years since the recovery of the influenza virus in the laboratory, and that influenza is still an undefeated disease, is surely a sobering thought. Is it because our weapons are too blunt or inadequately applied, or are we defeated by the changing virus whose
antigenic variation is so sharp a contrast to the stability of the viruses of smallpox, yellow fever, poliomyelitis and measles? Probably all three reasons are valid but the debate about to begin may serve to assess the matter.
Then there are the hordes of cases of common colds, of pharyngitis, and of the acute lower respiratory tract infections that press upon peo-ple of all ages and whose rule is one of etiolo-gical diversity. The seroloetiolo-gical complexity of certain species such as the rhinoviruses make the immunological problem enormous. The pos-sibility of formulating polyvalent vaccines made up of several species is to be discussed. If only some common product of the virus particles existed that would immunize against different serotypes, the task might seem less formidable. Moreover, the need to define the respiratory vi-rus problems at different ages and in different groups of the population is still outstanding. It is perhaps peculiarly my prerogative in this Conference to declare that immunization must begin by defining its objective. Unless there is a clinical problem whose etiology has been estab-lished, then there is no basis for formulating and using a vaccine.
Let us turn to the still relatively poorly under-stood group of enterovirus infections. Among these one would select aseptic meningitis as the most serious clinical condition, but the diversity of viruses concerned makes illness a challenging problem indeed. There are those who believe in the possible harmful effects of Coxsackie and echoviruses in obscure conditions of the nervous system. Others point to the cardiac effects of certain enteroviruses or to their role in gastro-intestinal disease. The sum total of clinical hazards of this ubiquitous group of viruses is hard to fathom. How much these remarks may apply also to the complex arbovirus field, others more competent than I must judge. But the challenge of virus encephalitis is one that must be felt in many widely separated countries throughout the world.
The conquest of disorders in which there is an acarine or insect vector raises the possibility of control measures other than immunization. The rickettsial infections illustrate this problem as well as, if not better than virus diseases. Those of us who witnessed the historical success of insecticides in the control of louse-borne
epi-.
xxvii
demic typhus in Naples in 1944 are not likely to forget the value of vector destruction. Im-munization against epidemic typhus is now feasi-ble by more than one method, yet other rickett-siae such as those of scrub typhus and Q fever are more recalcitrant. The clinical need for con-trol measures has to be established before the worthwhileness of immunization can be assessed. Wartime epidemics revealed the existence of Rickettsiae orientalis, but the endemic import-tance of this infection to populations of South-east Asia and elsewhere has still to be assessed. Much the same can be said of tick-borne and Q fever rickettsiae that flourish in the animal kingdom in many countries all over the world. Action against the numerous animal species con-cerned seems difficult to effect. The existence of satisfactory chemotherapeutic agents against the rickettsial infections has taken away much of the urgency of prophylaxis. Does the experi-ence of the fight against the rickettsiae have a wider message? The tantalizing prospect of chemotherapy against the true viruses is now doubly attractive in view of the problems just outlined. Chemoprophylaxis which has a better theoretical chance of success is already being explored against smallpox. The future possi-bility of extending this method, particularly for epidemic control, must clearly now be taken into account.
Present Needs
There is at the present time much talk of the possible eradication of disease. Coming as I do from an island that has successfully eradicated at least one virus disease-rabies-by the simple expedient of quarantine of potentially infected animals, it might be thought that I would speak strongly in favor of this concept. On the con-trary, the word "eradication" seems to me to foster an illusion. It is perfectly possible to eradicate a particular infection from one par-ticular country or area, but the reality is that the world is an epidemiological unit and that other areas may continue to foster the infection long after eradication has been achieved else-where. The existence of animal reservoirs of infection in any case makes eradication an im-possibility for certain of the virus diseases now
to be discussed.
I should like to propose that the primary aim
of preventive immunization may be stated quite simply as the artificial induction of resistance to infection so that children may be born healthy and attain adult life without the risk of repeated illnesses to which they are now subject. Where the infectious agent is ubiquitous-as in the case of the polioviruses, measles, and rubella-it is necessary to immunize each generation anew. Where the agent persists in localized areas, as for instance, in the case of yellow fever or vari-ola, the induction of resistance by immunization campaigns in these areas may be successful in quenching the fire of infection at its source. This is essentially the technique of epidemic control. We need at the present time to take stock of the diseases caused by the major groups of the virus infections of man. We need to define our objectives anew. This may demand further detailed clinical and epidemiological studies com-bined with an assessment of the virus flora. Only then can it be stated whether the problem is best met by basic immunization of children or by some form of epidemic control.
Second, there is a great need for work on the immunizing materials themselves with a view to improving their purity and antigenic effective-ness. As was pointed out by the WHO Scientific Group on Human Viral and Rickettsial Vaccines* some virus vaccines honored by tradition fail to conform to the standards set for the more re-cently developed vaccines. Smallpox and rabies vaccines are examples of this, and even yellow fever and live influenza vaccines need to be cleansed of accompaniments such as the fowl leukosis complex. The existence of known ex-traneous agents in living tissue and the possi-bility of further as yet unknown contaminants frightens many workers and greatly complicates the problems of safety and control of live at-tenuated vaccines. The preparation of such vac-cines in cell strains that can be subjected to defined criteria and experimental tests is an attractive method that is already being explored as an alternative to cultures from animal tissues. Even where inactivated vaccines furnish a good alternative to living vaccines, from the standpoint of protection there is much to be learned in regard to the methods of administra-tion, precise composiadministra-tion, and number of doses. Take, for example, the question of influenza.
xxviii
Inactivated saline vaccines exert so transient an effect that they are only protective for relatively short periods of time and injections must be practiced annually. The oily adjuvants hitherto used afford an excellent chance of prolonging the antibody response, yet give troublesome local reactions in a small number of recipients. The recent introduction of purified hemagglutinin, which avoids the pyrogenic effect of whole virus vaccine, may therefore be the best approach to the influenza problem. But will this afford merely basic immunization for certain groups of the population, and ought reliance for epidemic con-trol be placed on an attenuated living vaccine? Many in my own country feel unable to accept the latter mode of immunization until a labora-tory method of test for attenuation of influenza virus is formulated which will provide the equiv-alent for influenza to the monkey nervous system for poliomyelitis virus.
Third, there is need to develop new vaccines from etiological agents shown to be responsible for a significant amount of illness. The respira-tory virus field is one that cries out for explora-tion and that can only be approached on the basis of a vaccine containing many constituents. Will the number of doses required for an inac-tivated polyvalent respiratory vaccine be then too great for acceptability, or will needle-less methods of administration soothe away the sting and fear of injections?
Fourth, we need to obtain a far greater de-gree of cooperation from the population in the future. The extension of immunization demands this, unless we are merely to substitute one vac-cine for another. In my country, where drugs are not only tolerated but requested, vaccination still conjures up an unpleasant image of a
pro-cedure bringing with it the hazard of reactions and only the negative benefit of freedom from a disease which might never happen. Fear is, however, not the basic reason for the low rates of acceptance of vaccination. A recent example may be given of the effect of improving the ad-ministration of immunization by the installation of a computer system for supervising the call of parents to the clinic at the appropriate time. In one area in England where the rate of immuniza-tion of children against smallpox was formerly 30 per cent, computer control raised the figure to more than 80 per cent.
Perhaps this Conference will also give some attention to the basic need for supervision or surveillance of the population so that a watch can be kept not only on the state of immunity but on all possibly harmful reactions or ill-nesses attributable to immunization. This will extend the present form of control exerted pri-marily on the manufacture and test of the vac-cines themselves.
Conclusion
The famous exhortation sent to Jenner by John Hunter-"Why think? Why not try the experi-ment?"-was written about Jenner's work on hedgehogs. It has been taken out of its context and used repeatedly to uphold the experimental method. This Conference is not concerned with the hibernation of hedgehogs but with man's conflict with his environment. Even so, it would do well to take heed of Hunter's comments in the unfinished business of the prophylactic im-munization against infectious disease. May 1 paraphrase this by saying: "We need to think. We need also to try the experiment."
Keynote Address o StuLart-Harris
SESSION I
CONTROL OF ACUTE RESPIRATORY DISEASES
Monday, 7 November 1966, 8:30 a.m.
CHAIRMAN
DR. THOMAS FRANCIS, JR.
RAPPORTEUR
DR. EDWIN H. LENNETTESection A.
Influenza Virus Vaccines
Presentation of Papers by:
Dr. Fred M. Davenport
Dr. Victor M. Zhdanov
Dr. Lubomir Syrúiek
Dr. Werner Henle
Discussants:
Dr. H. G. Pereira
Dr. Roslyn Q. Robinson
Dr. Anatoli A. Smorodintsev
Sir Christopher H. Andrewes
Col. Edward L. Buescher
Dr. R. Sohier
Section B.
Respiratory Syncytial and Parainfluenza Virus Vaccines
Presentation of Papers by:
Dr. Robert H. Parrott
Dr. Z. Dinter
Discussants:
1
1
1