1
Methimazole (1-methyl-2-mercaptoimidazole, tapazole) is an orally taken drug used in the therapy of hyperthyroidism (overactivity of the thyroid gland). Its action is to slow iodide integration into tyrosine and thus inhibit the production of thyroid hormones [1]. Methimazole is used as a drug to manage hyperthy-roidism associated with Grave’s disease, but it has such a side effect as possible decrease of white blood cells count [2]. Methimazole may also cause such side effects as nephritis, liver cirrhosis, irritation of the skin, allergies and pharyngitis with fever [3]. Thus, the development of methods for the determination of methimazole is obviously of significance.
To date, a number of methods to determine methi-mazole have been developed, such as thin layer chro-matography [4], high performance liquid chromatog-raphy-mass spectrometry [5, 6], HPLC with ultravio-let detection [7], resonance light scattering spectroscopy [8], pharmacopeia method [9], flow-injection with ultraviolet detection [10], and electro-chemistry method [11, 12]. However, these methods have their own different disadvantages with respect to practical application to the determination of methim-azole.
In this paper, a spectrophotometric method for the determination of methimazole is presented. This
1The article is published in the original.
method is based on the reaction of the system methim-azole–FeCl3–K3[Fe(CN)6]. The results show that Fe(III) is reduced to Fe(II) by the sulfhydryl group containing in methimazole at pH 4.0. In situ formed Fe(II) reacts with potassium ferricyanide to form the soluble Prussian Blue (KFeIII[FeII(CN)
6]) [13a]. The
absorbance of soluble Prussian Blue is measured at 735 nm, which is a 483 nm shift against the λmax of
methimazole itself (252 nm). According to the absor-bance of the product, the amount of methimazole can be obtained. The linear range is 0.02–6.00μg/mL and the apparent molar absorption coefficient of the deter-mination of methimazole is 5.7 × 104L/mol cm. This
method has been applied to the determination of methimazole in the pharmaceutical, serum and urine samples. Analytical results obtained with this novel method are satisfactory.
EXPERIMENTAL
Reagents and solutions. Unless specially stated, all reagents used were of analytical grade and all solutions were prepared with distilled water. The main solutions were prepared as follows. A standard methimazole stock solution (3.00 mg/mL) was prepared by dissolv-ing 0.30 g of methimazole (A.R., Shanghai Huanghai Pharmaceutical Plant, Shanghai, China) in 100 mL of distilled water and stored at 4°C in dark place. Stocks
Spectrophotometric Determination of Methimazole
in Pharmaceutical, Serum and Urine Samples by Reaction
with Potassium Ferricyanide-Fe(III)
1Chunhong Donga, c, Yan Zhangb, Li Guoa, and Quan-min Lia
a College of Chemistry and Environmental Science, Henan Normal University Xinxiang, Henan, 453007 P.R. China b College of Chemistry and Chemical Engineering, Henan Institute of Science and Technology Xinxiang,
Henan, 453003 P.R. China
c Department of Chemistry and Engineering, Jiaozuo University Jiaozuo, Henan, 454003 P.R. China Received March 19, 2009; in final form December 9, 2009
Abstract—This paper describes a novel method to determine methimazole by spectrophotometry using a potassium ferricyanide-Fe(III) reaction. The study indicates that at pH 4.0 Fe(III) is reduced to Fe(II) by methimazole and in situ formed Fe(II) reacts with potassium ferricyanide to give soluble Prussian Blue which is characterized by means of XRD analysis. The absorbance of Prussian Blue is measured at the absorption maximum of 735 nm, and the amount of methimazole can be determined based on this absorbance. Beer’s law is obeyed in the range of methimazole concentrations of 0.02–6.00 µg/mL. The equation of the linear regression is A = –0.0058 + 0.49988c (µg/mL), with a correlation coefficient of 0.9998 and RSD of 0.80%. The detection limit (3σ/k) is 0.015 µg/mL, and the apparent molar absorption coefficient of indirect deter-mination of methimazole is 5.7 ± 104 L/mol cm. This method has been successfully applied to the determi-nation of methimazole in pharmaceutical, serum and urine samples, and average recoveries are in the range of 98.6–102.4%. Analytical results obtained with this novel method are satisfactory.
DOI: 10.1134/S1061934810070099
of standard solutions of 1.5 × 10–2 M potassium
ferri-cyanide (A.R., Peking Chemical Plant, Peking, China) and 1.5 × 10–2 M ferric chloride (A.R., Tianjin
Chemical Plant, Tianjin, China) were obtained by dis-solving 1.2347 g of potassium ferricyanide and 1.0137 g of ferric chloride in 250 mL standard flask with distilled water, respectively. A 0.1% (v/v) solution of Tween-20 (Qingdao Shijixing Chemical Reagent Company, Qingdao, China) was prepared by diluting 0.10 mL of Tween-20 in 100 mL of distilled water. A 0.1% (m/v) solution of sodium dodecyl sulfate (SDS) (Tianjin Kemiou Chemical Reagent Company, Tian-jin, China) was prepared by dissolving 0.10 g in 100 mL distilled water. A 0.1% (m/v) solution of cetyl-tri methyl ammonium bromide (CTMAB) (Beijing Chemical Reagent Plant, Beijing, China) was pre-pared in the same way.
Apparatus. Bruker D8 Advance X-ray powder dif-fractometer (Germany) was employed for XRD mea-surements. A model T6 UV/VIS spectrophotometer (Peking purkinje general instrument Co., Peking, China) was used for scanning the absorption spectrum and photometric measurements. A model CS-501 super thermostat instrument (Chongqing Experiment Equipment Plant, Chongqing, China) was utilized for temperature control.
Sample treatment. Prior to analysis, four methima-zole tablets (5.00 mg each, Shanghai Huanghai Phar-maceutical Plant, Shanghai, China) were well ground, 10.00 mg of the powder was accurately weighed using a BS 110s electro-analytical balance (Beijing Sartorius Balance Ltd, Beijing, China) and then dissolved in distilled water. The solution was filtered and the filtrate was taken into a 100-mL volumetric flask, diluted to the mark, mixed well and preserved without light at 4°C.
Procedure. 1.00 mL of 30 μg/mL methimazole, 1.00 mL of 1.5 × 10–2 M ferric chloride and 1.00 mL of
1.5 × 10–2 M potassium ferricyanide were transferred
into a 25-mL color comparison tube, the solution was diluted to the mark with distilled water and mixed well (pH of this solution is 4.0). Then it was stored for 40 min at room temperature and the absorbance was measured at 735 nm against a reagent blank prepared in the same way, but with no methimazole.
RESULTS AND DISCUSSION
Absorption spectrum. The absorption spectrum of soluble Prussian Blue formed from the reaction of methimazole, Fe(III) and potassium ferricyanide and the corresponding reagent blank are shown in Fig. 1. It can be seen that the product of soluble Prussian Blue has an absorption peak at 735 nm (a). In comparison, the absorbance of the reagent blank (b) and methima-zole (c) are almost zero in the range of 500–900 nm. In order to obtain the highest sensitivity, all the follow-ing measurements were carried out at 735 nm.
Effects of ferric chloride and potassium ferricyanide. The effect of ferric chloride on absorbance can be seen in Fig. 2, the amount of ferric chloride ranging from 0.00 to 1.40 mL was studied. The absorbance reaches its maximum when the amount of ferric chloride is 0.80 mL, and it does not change any further with the increasing amount of ferric chloride. This clearly indi-cates that all methimazole is oxidized by Fe(III) and the amounts of Fe(II) and the formed Prussian Blue reach their maximum.
The effect of potassium ferricyanide on absorbance has been studied, and the result can seen in Fig. 3. The solution absorbance reaches its maximum when the amount of potassium ferricyanide is 0.80 mL and keeps constant when this amount is above 0.80 mL. 0.6
0.4
0.2
0
900 800
700 600
500
λ, nm a
b c
A
Fig. 1. Absorption spectra. a: Product against the reagent blank; b: mixed solution of FeCl3 + K3[Fe(CN)6] against
water; c: Methimazole against water. Methimazole (30 μg/mL): 1.00 mL; FeCl3 (1.5 × 10–2 M): 1.00 mL; K3[Fe(CN)6] (1.5 × 10–2 M): 1.00 mL.
0.60
0.45
0.30
0.15
0 0.2 0.4 0.6 0.8 1.0 1.2 V, mL A
Fig. 2. Effect of FeCl3 concentration. Methimazole
(30 μg/mL): 1.00 mL; FeCl3: 1.5 × 10–2 M; K3[Fe(CN)6]
This means that the amount of the formed soluble Prussian Blue reaches its maximum.
So, 1.00-mL aliquots of 1.5 × 10–2 M of both ferric
chloride and potassium ferricyanide have been selected.
Effects of temperature and reaction time. Keeping other conditions constant, the effect of temperature on absorbance was studied. The absorbance of the solution is maximum at room temperature. In order to make the determination of methimazole both sensitive and simple, room temperature has been chosen as optimum.
Absorbance has been also determined at various times after reaction start at room temperature. The results show that the reaction methimazole–FeCl3–
K3[Fe(CN)6] occurs very fast but the absorbance
reaches its maximum at 40 min, remaining stable for at least 30 min. Therefore, 40 min time and room tem-perature have been selected for further experiments.
Effect of different acids. Keeping other conditions unaltered, the influence of different acids (2.00 M) on the determination of methimazole was studied.
The results show that the absorbance declines to a different degree with the increasing amount of acids. This may be attributed to the fact that the oxidation potential of sulfliydryl-containing compounds increase with pH [13b], which is supposed to decrease the reducing ability of methimazole. From Fig. 4 we can see that H3PO4 has a great influence on the absor-bance. With the amount of H3PO4 the absorbance declines from 0.594 (0.00 mL) to 0.403 (0.30 mL). Due to the formation of a colourless and stable com-plex ( ) between H3PO4 and Fe(III) [14], the oxidation potentials of Fe(III) shifts negatively and the absorbance decreases accordingly. The influence of H3BO3 on the absorbance is less than the influence
Fe HRO( 4)2–
of HCl. It is likely that H3BO3 is a weaker acid
com-pared to HCl.
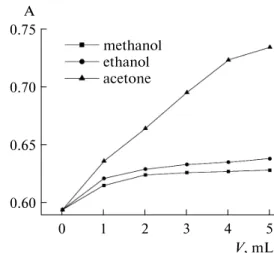
Effect of organic solvents. Figure 5 shows the effect of different organic solvents on absorbance. In con-trast to CH3OH and CH3CH2OH, the effect of
CH3COCH3 is more remarkable: absorbance increases
by 23% with 5 mL of added CH3COCH3. This result is
due to low polarity of CH3COCH3 compared to that of
CH3OH and CH3CH2OH. Meanwhile, the stability constant of soluble Prussian Blue is maximum in the solution containing CH3COCH3 because the stability
constant of the complex increases along with the decrease of the organic solvent polarity [15]. In other words, the concentration of both soluble Prussian Blue and absorbance are at the maximum in a solution con-taining CH3COCH3.
0.6
0.4
0.3
0.1
0 0.2 0.4 0.6 0.8 1.0 1.2 V, mL A
0.5
0.2
Fig. 3. Effect of K3[Fe(CN)6] concentration. Methima-zole (30 μg/mL): 1.00 mL; FeCl3 (1.5 × 10–2 M): 1.00 mL; K3[Fe(CN)6]: 1.5 × 10–2 M; reaction time: 40 min; total volume: 25 mL.
0.6
0.5
0.4
0.3
1.5 1.2 0.9 0.6 0.3 0
V, mL A
0.7 HCl
H3PO4
H3BO3
Fig. 4. Effect of acids. Methimazole (30 μg/mL): 1.00 mL; FeCl3 (1.5 × 10–2 M): 1.00 mL; K3[Fe(CN)6] (1.5 × 10–2 M): 1.00 mL; HCl, H3BO3 and H3PO4: 2.00 M; reaction time:
40 min; total volume: 25 mL.
0.75
0.70
0.65
0.60
5 4 3 2 1 0
V, mL A
methanol ethanol acetone
Effect of surfactants. From Fig. 6 we can see the influence of various surfactants on the determination of methimazole. When the cationic surfactant CTMAB (0.1%) and non-ionic surfactant Tween-20 (0.1%) are added in the range of 0.00–2.50 mL, the absorbance changes slightly. However, the anionic sur-factant SDS (0.1%) decreases the absorbance, mainly because it can react with Fe(III) [16] and some floccu-lation is produced, which is consistent with experi-mental observations. In the same way, the surfactant affects the reaction between methimazole and Fe(III), namely the amount of formed Prussian Blue reduces and the absorbance decreases accordingly.
Interference of coexisting components. A systematic study of the influence of excipients, aminoacids, car-bohydrate and minerals on the determination of methimazole was carried out. The tolerance levels are defined with an error of the determination less than ±5%. It has been found that 2.0 mg/mL starch; 4.0 mg/mL dextrose; 0.5 mg/mL serine, proline, leu-cine, arginine and glutamic acid; 50 μg/mL Bi3+,
Mn2+, Co2+, Cd2+, Cr3+, Ni2+, Pb2+, Ca2+, Zn2+, and
Al3+; large amounts of K+, Na+, Cl–, and
Br– do not affect the determination. The measured
concentrations of excipients, aminoacids, carbohy-drate and minerals are higher than the content within humans, as reported by the World Health Organiza-tion in 2002 [17].
Calibration curve. According to the procedure, a linear relationship between the absorbance (A) of product and the concentration (C) of methimazole is obtained in the range of 0.02–6.00 μg/mL. The linear regression equation is A = –0.0058 + 0.49988 C (μg/mL) with a correlation coefficient of 0.9998. Also, the molar absorption coefficient in the determination of methimazole is 5.7×104 L/mol cm. The absorbance
has been determined according to the procedures, for a total of times, which gave a relative standard devia-tion (RSD) of 0.80%. Then, a reagent blank has been measured 11 times (n = 11), and the standard devia-tion of the reagent blank (σ) is 0.0025. Therefore, a detection limit for three times standard deviation divided by the slope of the linear regression equation (3σ/k) is 0.015 μg/mL.
THE STUDY OF REACTION MECHANISM
Analysis of product by XRD. To investigate the prod-uct, XRD measurements were carried out at room temperature (Fig. 7). All the peaks can be indexed to Prussian Blue with lattice constant comparable to the values of Joint Committee on Powder Diffraction Standard (JCPDS) card number 01-0239 (JCPDS01-0239). The spectrum show various diffraction peaks at 2θ values of 17.37, 24.71, 35.16, 39.49, 50.67, 53.88, and 57.16°. The peaks are identified to originate from (100), (110), (200), (210), (220), (300) and (310) planes of Prussian Blue. The obtained d-values are in accordance with the d-values of JCPDS01-0239.
Discussion of reaction mechanism. Due to the reduc-ibility of the sulfhydryl group, Fe(III) is reduced to Fe(II) by methimazole in the presence of potassium
fer-SO42–, NO3–, 0.60
0.45
0.30
0.15
2.5 2.0 1.5 1.0 0.5 0
V, mL
A CTMAB
SDS Tween-20
Fig. 6. Effect of surfactants. Methimazole (30 μg/mL): 1.00 mL; FeCl3 (1.5 × 10–2 M): 1.00 mL; K3[Fe(CN)6]
(1.5 × 10–2 M): 1.00 mL; CTMAB, SDS: 0.1% (m/v); tween-20: 0.1% (v/v); reaction time: 40 min; total volume: 25 mL.
500
400
300
200
100
0
60 50
40 30
20 11
In
te
n
si
ty
(
a
.
µ
)
2-Theta/degree 100
110
200 210
220 300 310
ricyanide, and methimazole is oxidized to form disul-fide [18]. Subsequently, the in situ formed Fe(II) reacts with potassium ferricyanide to form soluble Prussian Blue (KFeIII[FeII(CN)
6]). Based on the electronic
transfer number, the reaction stoichiometric ratio of Fe(III) and methimazole is 1 : 1. Hence, it seems rea-sonable that the reaction mechanism is as follows:
SAMPLE ANALYSIS
Determination of methimazole in pharmaceutical sam-ples. Different pharmaceutical sample solutions were analysed, and the results are shown in Table 1. The analytical results agree well with the reference method [10]. Low RSD and high recoveries have been obtained. As the sample in the experiment is real tab-let, it also shows that other components of the sample do not affect the determination of methimazole with potassium ferricyanide-Fe(III) and the results are sat-isfactory.
Analysis of recovery of methimazole from serum and urine samples. The serum and urine samples were prepared for the analysis of recovery of methimazole with the proposed method (The serum samples were obtained from a healthy volunteer. The urine samples were centrifuged for 15 min at 3000 r/min to remove
the suspended matter before determination). The serum and urine samples were added according to the proposed procedure. The results are presented in Table 2. High accuracy and good recoveries are obtained, which indicates that the proposed method can be successfully applied to recover methimazole in the serum and urine samples.
It is the first time that the determination of methi-mazole was carried out using potassium ferricyanide-Fe(III) system with spectrophotometry control.
The reaction occurs at room temperature during 40 min, and the amounts of both FeCl3 and
K3[Fe(CN)6] are 1.00 mL of 1.5 × 10–2M solutions.
The linear range for methimazole is 0.02–6.00 μg/mL, the detection limit (3σ/k) is 0.015 μg/mL, and the apparent molar absorption coefficient is 5.7 × 104L/mol cm. A simple, sensitive, reliable and selec-N
N CH3
SH
I. 2 + 2Fe(III) + 2Fe(II) + 2 H
+
N N S
H3C
N N
S
CH3
Fe(II) + K2[FeIII(CN)6] KFeIII[FeII(CN)6] II.
Table 1. The recovery of methimazole in tablets (n = 5, t0.05, 4 = 2.78)
Sample No Sample con-tents, μg/mL
By proposed method,
μg/mL
By reference method,
μg/mL
Added, μg/mL
Found,
μg/mL Recovery, %
RSD, % (n = 5) 1
2 3 4 5
1.200 1.200 2.500 2.500 4.000
1.212 1.208 2.492 2.507 3.994
1.209 1.203 2.505 2.511 3.991
0.500 0.500 0.500 1.000 1.000
1.705 1.720 2.993 3.503 4.985
98.6 102.4 100.2 99.6 99.1
0.44 0.54 0.63 0.69
0.90
Table 2. The recovery of methimazole in urine and serum (n = 5, t0.05, 4 = 2.78)
Sample volume, mL Added, µg/mL Found, µg/mL Recovery, % RSD, %
Serum (2.5%) 1 2 3
1.500 2.000 3.000
1.533 2.016 2.986
102.2 100.8 99.5
0.86 0.63 0.77 Urine (5%)
1 2 3
1.500 2.000 3.000
1.489 2.024 3.013
99.3 101.2 100.4
tive method of the determination of methimazole is developed. The proposed method can be successfully applied to the determination of methimazole in phar-maceuticals, serum and urine samples with satisfac-tory results. Compared with other methods mentioned in this paper, this method needs neither any compli-cated apparatus, nor new reagents to be synthesized. Therefore, the determination of methimazole with potassium ferricyanide-Fe(III) has a good prospect.
REFERENCES
1. Hua, L.J., Han, H.Y., and Chen, H.B., Electrochim. Acta, 2009, vol. 54, no. 5, p. 1389.
2. Sie ko, D., Gugala, D., Nieszporek, J., Jankowska, J., and Saba, J. Electrochim. Acta, 2006, vol. 51, no. 11, p. 2273.
3. Smith, C.M., and Reynard, A.M., Textbook of Pharma-cology, Philadelphia: W.B. Saunders Company, 1992, p. 652.
4. Aletrari, M., Kanari, P., Partassides, D., and Loizou, E., J. Pharm. Biomed. Anal., 1998, vol. 16, no. 5, p. 785.
5. Blanchflower, W.J., Hughes, P.J., Cannavan, A., McCoy, M.A., and Kennedy, D.G., Analyst, 1997, vol. 122, no. 9, p. 967.
6. DeWasch, K., Brabander, H.F.D., Impens, S., Vande-wiele, M., and Courtheyn, D., J. Chromatogr. A, 2001, vol. 912, no. 2, p. 311.
7. Buick, R.K., Barry, C., Traynor, I.M., McCaughey, W.J., and Elliott, C.T., J. Chromatogr. B, 1998, vol. 720, no. 1–2, p. 71.
8. Liu, X.L., Yuan, H., Pang, D.W., and Cai, R.X., Spec-trochim. Acta A, 2004, vol. 60, no. 1–2, p. 385. 9. China Pharmacopoeia (Part II), Beijing: Chemical
In-dustry Press, 2005, p. 128.
10. Sánchez-Pedreño, C., Albero, M.I., Garcia, M.S., and Ródenas, V., Anal. Chim. Acta, 1995, vol. 308, no. 1–3, p. 457.
11. Martinez, N.A., Messina, G.A., Bertolino, F.A, Sali-nas, E., and Raba, J., Sensor. Actuat. B-Chem., 2008, vol. 133, no. 1, p. 256.
12. Aslanoglu, M., and Peker, N., J. Pharm. Biomed. Anal., 2003, vol. 33, no. 5, p. 1143.
13. (a) Inorganic Chemistry Studio of Beijing Normal University, Huazhong Normal University and Nanjing Normal University, Inorganic Chemistry (Part II), Beijing: Higher Education Press, 1992, p. 887.
(b) Inorganic Chemistry Studio of Beijing Normal Uni-versity, Huazhong Normal University and Nanjing Normal University, Inorganic Chemistry (Part II), Beijing: Higher Education Press, 1992, p. 389.
14. Wuhan University, Analytical Chemistry (Part IV); Beijing: Higher Education Press, 2000; p. 162.
15. Pan, J.M., Chen, Y.S., and Yan, H.T., Applications of the Chromogenic Reagent in Metallurgical Analysis, Shang-hai: Shanghai Science and Technology Press, 1981, p. 53.
16. Talens-alesson, F.I., Hall, S.T., Hankins, N.P., and Az-zopardi, B. J. Colloid. Surface. A, 2002, vol. 204, no. 13, p. 85.
17. Zhang, H., Wu, L.L., Li, Q.M., and Du, X.Z., Anal. Chim. Acta, 2008, vol. 628, no. 1, p. 67.
18. Hu, H.W., Organic Chemistry (Part II), Beijing: Higher Education Press, 1990, p. 581.
![Fig. 2. Effect of FeCl 3 concentration. Methimazole (30 μg/mL): 1.00 mL; FeCl 3 : 1.5 × 10 –2 M; K 3 [Fe(CN) 6 ] (1.5 × 10 –2 M): 1.00 mL; reaction time: 40 min; total vo-lume: 25 mL.](https://thumb-eu.123doks.com/thumbv2/123dok_br/16983076.763052/2.918.99.406.86.321/fig-effect-fecl-concentration-methimazole-fecl-reaction-total.webp)