Cytotoxic activity and mechanism of action of organometallic complexes
Texto
(2)
(3)
(4)
(5) UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE QUÍMICA E BIOQUÍMICA. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. Daniel Mosebo Fernandes Bandarra. Dissertação orientada por: Doutora Margarida Meireles Doutora Mª José Calhorda Mestrado em Bioquímica Área de especialização em Bioquímica Médica 2010.
(6)
(7) Este trabalho foi realizado na Faculdade de Ciências da Universidade de Lisboa. Parte dos resultados incluídos no presente trabalho fazem parte do artigo científico já publicado [73]. No cumprimento do disposto no nº2 do artigo 8º do Decreto-lei 388/07, declaro que participei na concepção e na execução do trabalho que esteve na base do artigo, bem como na interpretação e redacção dos resultados..
(8) Dedico este trabalho a todos os que procuram fazer a diferença neste Mundo, colaborando com as suas ideias e experiências de modo a contribuir para a sua evolução. Jeg dedikerer dette arbejde til alle dem, der søger at gøre en forskel i denne verden, og som samarbejder med deres ideer og erfaringer til at bidrage til dens udvikling. I dedicate this work to all those who try to make a difference in this world, collaborating with their ideas and experiences in order to contribute to its evolution..
(9) How wonderful that we have met with a paradox, now we have some hope of making progress Niels Bohr.
(10)
(11) RESUMO O cancro é uma das doenças com maior mortalidade a nível mundial e, de acordo com a Organização Mundial de Saúde, prevê-se um aumento do número de mortes, podendo chegar a 12 milhões em 2030. Apesar desta tendência, muitos estudos têm sido feitos para a contrariar, nomeadamente ao nível da quimioterapia. Actualmente a quimioterapia e a radioterapia constituem as opções mais recorrentes para o tratamento de cancro, sendo exemplos de tratamentos que exploram a sensibilidade das células tumorais a danos no DNA. O uso de metais na medicina remota à Antiga China com o uso de ouro. Outros metais como a cisplatina têm sido usados desde a segunda metade do século XX. De facto a cisplatina e os seus análogos são os fármacos actualmente mais usados no tratamento de tumores sólidos, devido à sua elevada eficácia. No entanto, apresentam diversas limitações, como o desenvolvimento de resistência do organismo ou a sua elevada toxicidade. Por essa razão, novos complexos com outros centros metálicos, como o ruténio, o vanádio ou o molibdénio, têm sido testados. Vários estudos de agentes antitumorais com compostos com molibdénio têm sido feitos, tendo já sido comprovadas as suas propriedades citotóxicas. No entanto o seu mecanismo de acção encontrase por se esclarecer. O presente trabalho teve como objectivo o estudo das propriedades antitumorais de dois complexos organometálicos com molibdénio, [Mo(3-C3H5)Br(CO)2(1,10-fenantrolina)], B1, e [Mo(3C3H5)CF3SO3(CO)2(2,2’-bipiridil)], T2 (Figura 1), e o estudo do mecanismo de acção citotóxica destes complexos em linhas tumorais.. Figura 1 – Estrutura esquemática de B1 e T2.. A actividade citotóxica de B1 e T2 foi estudada em três linhas tumorais: células HeLa (cancro do colo do útero), células MCF-7 (cancro da mama) e RPE (epitélio pigmentado da retina imortalizada pela expressão da proteína telomerase humana), através do ensaio do MTT (brometo de 3-(4,5dimetiltiazon-2-il)-2,5-difeniltetrazólio), um ensaio metabólico universalmente usado. Para o complexo B1 foram obtidos valores de IC50 (inibição da viabilidade celular em 50%) entre 1 e 9 M e para T2 entre 13 e 46 M para as três linhas celulares estudadas (Tabela 1). Estes valores encontram-se na gama de valores considerados muito bons ao nível da citotoxicidade em linhas tumorais, em partícular B1, que apresenta valores de IC50 semelhantes aos da cisplatina.. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. | xi.
(12) Quadro 1 – Estudos in vitro da actividade citotóxica dos complexos de molibdénio T2 e B1 em células Hela, MCF-7 [74] e RPE (os valores correspondem à média ± desvio padrão de três replicados). IC50 (M) Compostos HeLa. MCF-7. RPE. T2. 23,7 ± 0,01. 44,5 ± 0,7. 13,1 ± 2,4. B1. 5,1 ± 1,0. 8,9 ± 0,5. 0,7 ± 0,1. Para compreender o mecanismo da acção citotóxica destes complexos em linhas tumorais, começouse por estudar a interacção dos complexos com a membrana plasmática através da determinação do coeficiente de partição octanol/água dos complexos em solução. Ambos os complexos apresentam um comportamento hidrofóbico em solução, sendo B1 mais hidrofóbico que T2. De forma a perceber melhor a interacção composto-membrana plasmática, procedeu-se à quantificação de molibdénio em células tumurais HeLa após a incubação destas com os compostos, durante 48 horas e com diversas concentrações. Para além disso, nas mesmas condições, procedeu-se à quantificação de molibdénio em fracções citosólicas e nucleares. Relativamente às células controlo (sem composto), observou-se um aumento dos níveis de molibdénio quer no citosol, quer no núcleo, sendo este aumento dependente da concentração de composto. Estes resultados parecem indicar que B1 e T2 possuem propriedades que lhes permitem entrar na célula e fundamentalmente no núcleo. Face aos resultados obtidos, colocou-se a hipótese de que os complexos pudessem exercer o seu efeito citotóxico através da interacção com o DNA, inibindo assim o crescimento das células tumorais. Procedeu-se a uma série de ensaios visando a detecção da interacção entre os compostos e o DNA. Começou-se por estudar a interacção dos complexos com DNA plasmídico, através da realização de electroforeses, em gel de agarose, de soluções de DNA plasmídico após incubação com os compostos. Observou-se que ambos os complexos levam a alterações na mobilidade do DNA palsmídico, sendo este efeito mais evidente para B1. Estes resultados sugerem que há interacções dos compostos com o DNA. Efectuaram-se também estudos de titulação de calf thymus DNA (ctDNA) por espectrofotometria UV-Vis e por dicroísmo circular. Nos ensaios por espectrofotometria UV-Vis mediram-se variações de absorvência após a adição de quantidades crescentes de ctDNA, fixando-se a concentração de composto, com os respectivos controlos. Nos ensaios de dicroísmo circular fixou-se a concentração de DNA e adicionaram-se quantidades crescentes de composto. Os resultados obtidos sugerem que a interacção entre os compostos e o DNA seja maioritariamente por intercalação, não podendo no entanto ser excluídos outros tipos de interacções. A partir da titulação determinaram-se as constantes xii | CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES.
(13) de ligação entre ctDNA e os complexos, tendo-se obtido valores de 2,08 (±0,98) × 105 e 3,68 (±2,01) × 105 M-1 para B1 e T2, respectivamente. Os valores das constantes de ligação DNA-composto encontram-se na mesma gama de valores obtidos para o brometo de etídeo, um intercalador clássico, confirmando assim a natureza intercalativa do tipo de interacção entre os complexos e o DNA. A microscopia de força atómica (AFM) de soluções de DNA plasmídico com e sem B1 permitiu estudar possíveis transições estruturais do DNA face a interacções do complexo. Obtiveram-se imagens topográficas onde se observaram alterações a nível da estrutura terciária do DNA plasmídico aquando da presença de B1. Estes resultados são comparativos com os obtidos anteriormente, onde é demonstrado a interacção dos complexos com o DNA. Infelizmente, devido a limitações experimentais, não foi possível efectuar os estudos de AFM para o complexo T2. Em suma, a realização deste trabalho permitiu, a partir dos estudos de actividade citotóxica, identificar dois compostos como potenciais agentes antitumorais e esclarecer um dos possíveis mecanismos responsáveis pela sua acção citotóxica em células tumorais. Tendo em conta os resultados obtidos, conclui-se que ambos os compostos apresentam propriedades que lhes permitam entrar na célula e chegar ao núcleo. Neste compartimento, os complexos levam à inibição do crescimento celular através da sua interacção, maioritariamente por intercalação, com o DNA, levando, possivelmente, a alterações ao nível da sua conformação e ultimamente à morte celular. O presente trabalho mostra a potencialidade do uso de B1 e T2 na quimioterapia, podendo trazer novas perspectivas nesta área, de modo a ultrapassar as limitações existentes actualmente, uma vez que se trata de compostos metálicos com diferentes propriedades químicas relativamente aos actualmente usados. A utilização destes ligandos e a possibilidade de combinar outros novos, constituem uma mais valia no tratamento do cancro. É de referir ainda a oportunidade que este trabalho proporcionou a publicação de um artigo científico com o título: “Mo(II) complexes: A new family of cytotoxic agents?”, na revista Jounal of Inorganic Biochemistry [73] (doi:10.1016/j.jinorgbio.2010.07.006).. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. | xiii.
(14) Abstract Antitumor properties of two molybdenum complexes, [Mo(3-C3H5)Br(CO)2(1,10-phenanthroline)] (B1) and [Mo(3-C3H5)CF3SO3(CO)2(2,2’-bipyridyl)] (T2) have been tested in vitro against human cervical cancer cell line (HeLa), human breast cancer cell line (MCF-7), and human telomerase reverse transcriptase – retinal epithelial cells (RPE) using a metabolic activity test, MTT (3-(4,5dimethylthiazon-2-yl)-2,5-diphenyltetrazolium bromide), with IC50 values ranged from 1 to 9 M, and from 13 to 46 M, approximately, for B1 and T2, respectively. In order to understand the mechanism of action against cancer cell lines several studies have been made. Cellular uptake of molybdenum and octanol/water partition assays revealed that both B1 and T2 exhibit a selective uptake by cells with the ability to reach the nucleus, and a hydrophobic behavior in solution, B1 being more hydrophobic than T2. The interaction of the complexes with DNA was also studied. According to gel electrophoresis studies, both complexes seem to interact with plasmid DNA. The binding constants of B1 and T2 with calf thymus DNA (ctDNA), as determined by absorption titration, are 2.08 (±0.98) × 105 and 3.68 (±2.01) × 105 M-1, respectively. These results together with data obtained from circular dichroism suggest that the complexes interact with DNA, mainly by intercalation, changing its conformation and possibly inducing cell death. Preliminary studies of structural transitions in the tertiary structure of plasmid DNA using atomic force microscopy showed that B1 seems to induce structural changes on the plasmid, plectonemic supercoiling being the predominant form adopted by the plasmid. These results show that in future both complexes may provide a valuable tool in cancer chemotherapy.. Keywords: Molybdenum, antitumor activity, interaction with DNA, chemotherapy. xiv | CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES.
(15) Index 1.. Index of figures ............................................................................................................................3. 2.. Index of tables ..............................................................................................................................5. 3.. Abbreviations list.........................................................................................................................6. 4.. Introduction .................................................................................................................................7 4.1.. The current state of cancer research ......................................................................................7. 4.2.. The hallmarks of chemotherapy ............................................................................................7. 4.3.. The limit to chemotherapy.....................................................................................................9. 4.4.. Medicinal inorganic chemistry ............................................................................................11. 4.5.. Mode of action of metal anticancer compounds..................................................................12. 4.6.. Experimental strategies .......................................................................................................13. 4.7.. Electronic absorption titration .............................................................................................13. 4.8.. Circular dichroism ...............................................................................................................14. 4.9.. Atomic force microscopy (AFM) ........................................................................................14. 5.. Aim..............................................................................................................................................17. 6.. Experimental..............................................................................................................................19. 7.. 6.1.. Instrumentation and materials .............................................................................................19. 6.2.. Synthesis of molybdenum(II) complexes ............................................................................19. 6.2.1.. [Mo(3-C3H5)(CF3SO3)(CO)2(2,2’-bpy)] (T2)............................................................19. 6.2.2.. [Mo(3-C3H5)(Br)(CO)2(1,10-phenanthroline)] (B1)..................................................20. 6.3.. Cell cultures.........................................................................................................................20. 6.4.. Subculture of cells ...............................................................................................................20. 6.5.. Cell quantification ..............................................................................................................20. 6.6.. Cryopreservation of cells.....................................................................................................21. 6.7.. Resuscitation of frozen cells................................................................................................21. 6.8.. Cytotoxic activity assay in vitro ..........................................................................................21. 6.9.. Octanol/water partition coefficient......................................................................................22. 6.10.. Conductimetry .................................................................................................................22. 6.11.. Cellular molybdenum uptake...........................................................................................22. 6.12.. DNA binding studies .......................................................................................................23. 6.12.1.. Electronic absorption titration .....................................................................................23. 6.12.2.. Circular dichroism .......................................................................................................23. 6.12.3.. Gel electrophoresis studies ..........................................................................................23. 6.12.4.. Atomic force microscopy ............................................................................................24. Results and discussion...............................................................................................................25 7.1.. Cytotoxic activity assay in vitro ..........................................................................................25. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. |1.
(16) 7.2.. Octanol/water partition coefficient......................................................................................29. 7.3.. Conductimetry .....................................................................................................................30. 7.4.. Cellular molybdenum uptake ..............................................................................................31. 7.5.. DNA Binding Studies..........................................................................................................32. 7.5.1.. Electronic absorption titration .....................................................................................32. 7.5.2.. Circular dichroism .......................................................................................................34. 7.5.3.. Gel electrophoresis studies ..........................................................................................35. 7.5.4.. Atomic force microscopy ............................................................................................35. 8.. Conclusions ................................................................................................................................37. 9.. Acknowledgements ....................................................................................................................39. 10. References ..................................................................................................................................41 11. Annex..........................................................................................................................................47. 2 | CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES.
(17) 1.. Index of figures. Figure 1 - Cisplatin and its analogues (Adapted from [17]). ............................................................................. 8 Figure 2 - Dose-response relationships and proposed resistance mechanisms (Adapted from [24])............... 10 Figure 3 – Drawing schemes of the transitions in plasmid DNA tertiary structure in response to an intercalator agent: (a) predominantly relaxed; (b) toroidally supercoiled; (c) mixed toroidal and plectonemic supercoils; (d) complete plectonemic supercoiling (adapted from [59])............................................................................... 15 Figure 4 - Schematic illustration of the overview of the work plan. Mo(II) complexes were tested and acted as potent cytotoxic drugs, interacting with DNA in vitro. Do they enter the cell and directly damage DNA to inhibit cell growth? ...................................................................................................................................... 18 Figure 5 – Haemocytometer (adapted from [62]) ......................................................................................... 21 Figure 6 - Schematic structure of B1 and T2.................................................................................................. 25 Figure 7 - In vitro cytotoxic assays for T2 against HeLa cells (left). Dose-response curve obtained by nonlinear regression analysis for HeLa cells treated with T2 (right). ............................................................................. 25 Figure 8 - In vitro cytotoxic assays for B1 against HeLa cells (left). Dose-response curve obtained by nonlinear regression analysis for HeLa cells treated with B1 (right). ............................................................................. 26 Figure 9 - In vitro cytotoxic assays for B1 against RPE cells (left). Dose-response curve obtained by nonlinear regression analysis for RPE cells treated with B1 (right)................................................................................ 26 Figure 10 - In vitro cytotoxic assays for T2 against RPE cells (left). Dose-response curve obtained by nonlinear regression analysis for RPE cells treated with T2 (right). ............................................................................... 27 Figure 11 - Dose-response curve obtained by nonlinear regression analysis for HeLa cells treated with B1 for 1, 2, 24, and 48 hours (left). In vitro cytotoxic activity for B1 against HeLa cells at 1, 2, 24, and 48 hours (right). .................................................................................................................................................................... 28 Figure 12 - Dose-response curve obtained by nonlinear regression analysis for HeLa cells treated with T2 for 1, 2, 24, and 48 hours (left). In vitro cytotoxic activity for T2 against HeLa cells at 1, 2, 24, and 48 hours (right).29 Figure 13 - Mean log octanol/water partition coefficients (Log P) of the molybdenum compounds............... 29 Figure 14 - Specific conductivity of B1 in 0,5% DMSO in water solution......................................................... 30 Figure 15 - Specific conductivity of T2 in water solution................................................................................ 30 Figure 16 - Comparison of the intracellular molybdenum concentration in HeLa cells after 48 h of exposure to compounds T2 and B1. ................................................................................................................................ 31 Figure 17 - Molybdenum concentration in cytosolic (a) and nuclear (b) extracts of HeLa cells after 48 h of exposure to compounds T2 and B1. ............................................................................................................. 32 Figure 18 - Left: UV-Vis absorption spectra of T2 (20 M) in Tris buffer in the presence of increasing amounts of ctDNA. [DNA] = 0, 10, 20, 30, 40, 50 M. The arrow indicates the absorbance changes upon increasing DNA concentration; right: plot of D/ap vs. D for the titration of DNA to complex. Absorbance was monitored at 299 nm. ....................................................................................................................................................... 33 Figure 19 - Left: UV-Vis absorption spectra of B1 (20 M) in Tris buffer in the presence of increasing amounts of ctDNA. [DNA] = 0, 10, 20, 30, 40, 50 M. The arrow indicates the absorbance changes upon increasing DNA concentration; right: plot of D/ap vs. D for the titration of DNA to complex. Absorbance was monitored at 272 nm. ....................................................................................................................................................... 34 Figure 20 – Circular Dichroism of ctDNA incubated with B1 (left) and T2 (right). [Complex] = 0, 10, 25, 50, 75, 100, 250 M. The arrow indicates the signal changes upon increasing complex concentration. .................... 35 Figure 21 - Electrophoretic mobility pattern of pYES2 plasmid DNA (C) incubated with complex B1 (right) and T2 (left) at 10, 50 and 100 M...................................................................................................................... 35 Figure 22 - A Selection of topographic images recorded of plasmid pYES2 (a) and plasmid pYES2 incubated with T2 (b) adsorbed to AP-mica. The scale bars correspond to 250 nm........................................................ 36 3 Figure 23 - ESI+ mass spectrum of the [Mo( -C3H5)Br(CO)2(1,10-phenanthroline)] (B1) complex dissolved in DMSO with no incubation time (0 hours)...................................................................................................... 47 3 Figure 24 - ESI+ mass spectrum of the [Mo( -C3H5)Br(CO)2(1,10-phenanthroline)] (B1) complex dissolved in DMSO after 2 hours of incubation time at 37 ºC. .......................................................................................... 48. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. |3.
(18) 3. Figure 25 - ESI+ mass spectrum of the [Mo( -C3H5)Br(CO)2(1,10-phenanthroline)] (B1) complex dissolved in DMSO after 24 hours of incubation time at 37 ºC. ........................................................................................ 49 3 Figure 26 - ESI+ mass spectrum of the [Mo( -C3H5)Br(CO)2(1,10-phenanthroline)] (B1) complex dissolved in DMSO after 48 hours of incubation time at 37 ºC. ........................................................................................ 50 3 Figure 27 - ESI+ mass spectrum of the [Mo( -C3H5)CF3SO3(CO)2(2,2’-bipyridyl)] (T2) complex dissolved in DMSO with no incubation time (0 hours)...................................................................................................... 51 3 Figure 28 - ESI+ mass spectrum of the [Mo( -C3H5)CF3SO3(CO)2(2,2’-bipyridyl)] (T2) complex dissolved in DMSO after 2 hours of incubation time at 37 ºC. .......................................................................................... 52 3 Figure 29 - ESI+ mass spectrum of the [Mo( -C3H5)CF3SO3(CO)2(2,2’-bipyridyl)] (T2) complex dissolved in DMSO after 24 hours of incubation time at 37 ºC. ........................................................................................ 53 3 Figure 30 - ESI+ mass spectrum of the [Mo( -C3H5)CF3SO3(CO)2(2,2’-bipyridyl)] (T2) complex dissolved in DMSO after 48 hours of incubation time at 37 ºC. ........................................................................................ 54 3 Figure 31 - Absorption spectra of B1 ([Mo( -C3H5)Br(CO)2(1,10-fenantrolina)]). .......................................... 55 3 Figure 32 - Absorption spectra of T2 ([Mo( -C3H5)CF3SO3(CO)2(2,2’-bipiridil)])............................................. 55. 4 | CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES.
(19) 2. Index of tables Table 1 - Drugs of Choice for Different Types of Cancer (Adapted from [5])..................................................... 9 Table 2 - In vitro cytotoxicity assays for molybdenum complexes T2 and B1 against HeLa, MCF-7 [74], and RPE cells (data are mean ±SD of three replicates each)........................................................................................ 27. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. |5.
(20) 3. Abbreviations list AFM – Atomic force microscopy ALL – Acute lymphoblastic leukaemia AML – Acute myelogenous leukemia B1 – [Mo(3-C3H5)Br(CO)2(1,10-phenanthroline)] BC – Before Christ CD – Circular dichroism ctDNA – calf thymus DNA DCR – Dose-response curve DMSO – Dimethyl sulfoxide DNA – Desoxyribonucleotic acid DTT – Dithiothreitol EDTA – Ethylenediamine tetraacetic acid FBS – Fetal bovine serum FDA – Food and drug administration HeLa – Human cervical cancer cell line HEPES – 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid IC50 – Inhibitory concentration (dose causing 50% inhibition of cell growth) ICP-MS – Inductively coupled plasma mass spectrometry MCF-7 – Human breast cancer cell line MDR – Multidrug resistance MOPP – Mustargen-oncovin-procarbazine-prednisone MTT – 3-(4,5-dimethylthiazon-2-yl)-2,5-diphenyltetrazolium bromide NCI – American national cancer institute PBS – Phosphate buffered saline RNA – Ribonucleic acid ROS – Reactive oxygen species RPE – Human telomerase reverse transcriptase – retinal epithelial cells RPMI – Roswell park memorial institute medium T2 – [Mo(3-C3H5)CF3SO3(CO)2(2,2’-bipyridyl)] TAE buffer – Buffer solution containing a mixture of tris base, acetic acid and EDTA TE buffer – Buffer solution containing tris and EDTA WHO – World health organization. 6 | CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES.
(21) 4. Introduction 4.1.. The current state of cancer research. Cancer is one of the major causes of death worldwide [1]. According to World Health Organization (WHO) cancer is the world’s second biggest killer, despite cancer being one of the research fields where most money has been invested. As a result, many developments have been achieved regarding the understanding of the pathogenesis of cancer [2]. Furthermore, properties such as unlimited proliferation capacity, self-sufficiency in growth signals, insensitivity to growth-inhibitory signals, evasion of programmed cell death (apoptosis), sustained angiogenesis, tissue invasion and metastasis are considered to be a set of characteristics acquired during tumor development [3,4]. Even though remarkable progress is being made in understanding the molecular, biochemical and cellular basis of cancer, such knowledge has not been sufficient to cure the disease efficiently [5,6]. Improvements in cancer treatment are however being achieved, namely on chemotherapy, which is the treatment of choice for patients diagnosed in the late stages of locally and advanced widespread metastatic cancers [4,5,7].. 4.2.. The hallmarks of chemotherapy. Chemotherapy emerged in the late 1940s and 1950s with the classical alkylating agents, nitrogen mustard. First used as sulfur mustard gas during the First World War, it was applied to treat patients with non-Hodgkin’s lymphoma, whose remission only lasted few weeks [5,8,9]. Cyclophosphamide and chlorambucil were later developed to treat patients with lymphomas, leukaemias and solid tumors; however these alkylating agents quickly proved inefficient, as tumors became resistant to these drugs [8,9,10]. After the Second World War, new approaches arose with the synthesis of antimetabolites, like methotrexate, a folate analogue which, as single agent, exhibited antitumor activity against epithelial cancer, along with breast, ovarian, bladder, head and neck cancers. Folate analogues also became the first drugs to induce successfully remission in patients with acute lymphoblastic leukaemia (ALL), although the effect did not last long [5,8,10,11]. Methotrexate is still mainly used on patients with ALL as well as other lymphomas. The application and research done with these compounds have provided an important model for understanding mechanisms of resistance for other agents. Further research led to the development of new drugs, such as vincristine, a Vinca alkaloid, which was found to inhibit cell division [8,12,13]. In the 1960s several experiments have shown that killing cancer cells might be cell cycle dependent, whereas some DNA synthesis inhibitors (methotrexate) were more effective against rapidly dividing cells. On the other hand, alkylating drugs, such as cyclophosphamide, that physically damage DNA, could kill cells in any stage of the cell cycle. Furthermore, it was shown that cytotoxic activity was dose dependent, and it was also important to use several therapies together to prevent drug resistance [8]. In the late 1960s nitrogen mustard, vincristine, procarbazine and prednisone – known as the MOPP regimen – was. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. |7.
(22) successfully used as combination chemotherapy to treat Hodgkin’s and non-Hodgkin’s lymphoma, proving that drugs were most efficient when used as combination therapy and against tumors of small volume [8,10,13, 14]. Barnett Rosenberg, a Michigan State University researcher, discovered in 1964 that cisdiamminedichloroplatinum(II), known as cisplatin, could be used as a successful agent in the therapy of testicular and ovarian cancer, after having received approval for clinical use by Food and Drug Administration (FDA) [5,9,15,16] (Figure 1).. Figure 1 - Cisplatin and its analogues (Adapted from [17]).. Despite several successful reported cases using chemotherapy, cancer drug discoveries gained a reputation of low efficacy and high toxicity risk, leading FDA to approve for marketing only 10% (29 of 280) of new agents into Phase I (dose-finding) clinical trials in patients in the period of 1975 to 1994 [18]. During those years several new drugs were used in chemotherapy, such as anthracyclines and epipodophyllotoxins, which are related to the topoisomerase II inhibition, an enzyme essential for DNA replication, transcription and repair. In the early 1980s chemotherapy progression decreased, which led the American National Cancer Institute (NCI), division of cancer treatment, to create an innovative screening system, where panels of 60 human tumor cell lines were used to test new drugs. This methodology is now widely used by industry, including a rapid colorimetric cell viability assay, the MTT (3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay, and high-throughput automated screening [8]. Recently, a new era arose with targeted-therapy, whereas targets such as growths factors, signaling molecules, cell-cycle proteins, apoptotic signals and molecules related to angiogenesis became a priority in chemotherapy (Table 1) [4].. 8 | CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES.
(23) Table 1 - Drugs of Choice for Different Types of Cancer (Adapted from [5]). Drug Class. Principal mechanism of action. Alkylating agents. To form covalent bonds with DNA and prevent DNA replication. Antimetabolites. To block or subvert pathways in DNA synthesis To inhibit dihydrofolate reductase, preventing generation of tetrahydrofolate, thereby interfering with thymidylate synthesis To convert into nucleotides and inhibit thymidylate synthesis 6-thiol analogues of the endogenous 6-OH purine bases that become converted into nucleotides To interfere with topoisomerase II action, inhibiting DNA and RNA synthesis To cause fragmentation of DNA chains To intercalate in DNA, interfere with RNA polymerase and inhibit transcription To act as an alkylating agent. Folate antagonist. Pyrimidine analogues Purine analogues Antitumor antibiotics. Plant alkaloids Vinca alkaloids Podophyllotoxins Taxoids Camptothecins. To inhibit mitosis at metaphase by binding to tubulin To inhibit DNA synthesis by interfering with topoisomerase II, and also mitochondrial function To promote the polymerization of tubulin and inhibit the disassembly of microtubules To inhibit topoisomerase I and DNA and RNA synthetases, and also microtubule formation. Example(s) Cyclophosphamide Carmustine Cisplatin. Types of tumors commonly used for Non-Hodgkin’s lymphoma Brain gliobastoma Ovarian, head, neck, lung and testicular. Methotrexate. Acute lymphocytic leukemia (ALL). Fluorouracil. Colorectal and gastric. Mercaptopurine Thioguanine. Acute myelogenous leukemia (AML). Doxorubicin Bleomycin. Osteogenic sarcoma, Hodgkin’s disease, CML, soft tissue sarcoma Cervical. Dactinomycin. Wilms’ tumor. Mitomycin. Lung. Vincristine, vinblastine Etoposide. Lung, Non-Hodgkin’s lymphoma Lung, Kaposi’s sarcoma. Taxol. Ovarian, breast and lung. Irinotecan, topotecan. Refractory colorectal and advanced ovarian. Nowadays better drugs have been developed and knowledge regarding cancer has grown, so that the new challenge is to design new strategies where targeted-therapy and cytotoxics can be combined in the most effective manner [8].. 4.3.. The limit to chemotherapy. Cytotoxic agents of chemotherapy are conditioned by several factors. Drug metabolizing enzymes and drug transporters are essential in the understanding of the overall process where antitumor compounds are involved [19,20]. It is also important to understand the side effects of these agents in order to make them less toxic to the organism without significant cytotoxic activity loss [21]. Thus it would be crucial to achieve a balance between drug sensitivity and resistance displayed by target tumor cells to maximize the efficacy and minimize the toxicity of treatment [21,20]. Although the use of chemotherapy has improved quality of life and prolonged survival for many cancer patients,. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. |9.
(24) its potential to induce toxicity is also predictable. Several side effects arise, the most common being gastrointestinal toxicity, alopecia (such as hair loss) and myelosuppression [21]. Such malignancies are due to the fact that the target of most chemotherapeutic agents consists of rapidly dividing cells, leading adverse effects into normal cells with a high growth proliferation, such as hair cells. Patients whose tumor does not respond to the therapeutic agents seem to possess resistance mechanisms, which are considered to be the major limitation for successful cancer treatment [22,72]. Chemotherapeutic resistance can be classified as ‘acquired’ or ‘intrinsic’. Intrinsic resistance limits the usefulness of therapeutic agents, while acquired resistance might be related to a resistance achieved after an initial successful progress, which somehow became powerless [22,23,72]. These mechanisms of resistance can also be reflected by dose-response relationships: if the dose-response curve (DCR) has a “shoulder” it will be classified as ‘active’, otherwise ‘passive’ results in a DRC terminal plateau (saturable passive) or in a decreased DRC slope (non-saturable passive) (Figure 2, [24]).. Figure 2 - Dose-response relationships and proposed resistance mechanisms (Adapted from [24]).. Examples of active resistance would be efflux pumps, DNA repair systems, anti-apoptotic factors, etc. Passive resistance would include drug uptake or activating systems, proapoptotic factors or factors that are part of the apoptotic cascade, cells in a sensitive phase of the cell cycle, etc. Furthermore, several factors may contribute to resistance: blood flow and drug delivery, extracellular environment, drug efflux, drug uptake, drug detoxification, drug binding, DNA repair, decreased DNA mismatch repair, reduced apoptotic response, apoptosis inhibitors, etc [22,24,25]. Although these mechanisms can act individually, they can also act synergistically, resulting in multidrug 10 | CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES.
(25) resistance (MDR) [26,72]. To diminish side-effects and reduce propensity to induce drug resistance, novel antitumor compounds have been designed in order to overpass chemotherapy limitations.. 4.4.. Medicinal inorganic chemistry. Metals are essential to many life-related processes and their use as therapeutic agents can be traced back to 2500 BC by the ancient Chinese, who have attributed medicinal proprieties to gold. Paracelsus (1493-1541), considered to be the ‘true father of modern metallotherapy’, used several heavy metals to treat patients with different malignancies, such as cancer [9,27,28]. Since then several reports have described the use of metals in medicine. As already mentioned, the First World War has changed the era of chemotherapy with the cytotoxic action of nitrogen mustards established years after, and further investigation led to the development of efficient antitumor drugs, such as cisplatin and its derivatives [8,9,15]. Nowadays they continue to be the most frequently used cytostatic drugs worldwide, constituting the main component of combination chemotherapy for the treatment of solid carcinomas [29]. This knowledge led to the development of other metal compounds with cytostatic activity, the groups 13 to 15 of the periodic table and certain transition metals of groups 4 to 11 being very active [9]. Metals can bind to organic fragments affording organometallic compounds, whose chemistry offers a rich field in medicine with a high potential to develop effective antitumor agents [30]. Furthermore, the limited selectivity and toxicity of metals alone can be overcome by the formation of organometallic compounds. Features such as the metal coordination numbers, the coordination geometries, the redox potential and the thermodynamic and kinetic properties have to be considered on the design of organometallic agents suitable for successful interaction with biological molecules [15,29]. This variety of options provides unlimited possibilities of combination that results in an extensive spectrum of mechanisms open to organometallic compounds. Although platinum complexes are widely used for the treatment of cancer, the appearance of drug resistance among the drug toxicity induced and its side-effects have led to the development of other non-platinum drugs, metal-based compounds, in order to overcome platinum compounds limitations, namely ruthenium, vanadium and molybdenum compounds [9,15,29,30]. Molybdenum is an essential trace element commonly used in the cell as cofactor by important enzymes (e.g. xanthine oxidase/dehydrogenase). In 1979, Köpf and Köpf-Maier reported the antitumor action of several metal-based complexes with Mo [31]. Since then a number of molybdenum containing molecules have been described to display cancerostatic activity. These include Na2MoO4, heteropolyacid Mo salts, polyoxomolybdenum complexes, Mo complexes bound to small carborane ligands, and chiral octahedral complexes [32,33,34,35]. Portuguese investigators in 2005 studied several molybdenum(II) compounds, concluding that they were very efficient cytotoxic agents against six cell lines, and filed a patent [36]. The mechanisms of action of most of the organometallic complexes of molybdenum are far from being understood. Since cell growth is. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. | 11.
(26) slowed down by those compounds, it seems conceivable that the inhibitory activity might in some way be related to DNA damage. This can be due to a direct action on the DNA molecule (eg. intercalation in the double helix) or by the oxidative action of oxygen free radicals generated by the chemical agents (chapter 4.5).. 4.5.. Mode of action of metal anticancer compounds. Metal compounds have a high propensity to bind and to interact with many important biological molecules, e.g. DNA, which is considered to be the primary intracellular target of the antitumor agents [17,27,28,37]. On the other hand, organometallic compounds can act as enzyme inhibitors or modulators blocking substrate interaction [38]. Other possible mechanism can be the generation of endogenous reactive oxygen species (ROS), which can directly or indirectly induce oxidative DNA damage [38,39]. Therefore covalent and noncovalent interactions can be considered in the interaction of the organometallic compounds with DNA. Thus, covalent interactions lead to complete inhibition of DNA processes and they usually are irreversible, contrarily to noncovalent bindings which are reversible [40,41]. One example of a covalent binder is the chemotherapeutic agent cisplatin and its analogues (Figure 1), which form covalent bonds between platinum and the nitrogen atom of some of the DNA base pairs [17]. Additionally three major noncovalent interactions can be found: minor groove binding, major groove binding and intercalation. However, one complex can participate in more than one mode of interaction, as actinomycin D, a well-known transcription inhibitor [41,42]. These metal compounds-DNA interactions, ultimately, trigger programmed cell death [27,37]. A classification of metal anticancer compounds based on their mode of action has been suggested [43]. This classification relies on the metal compounds, rather than the nature of the compound targets (e.g. proteins, DNA, enzymes, transporters, cellular transduction pathways, etc.), since the knowledge about their interaction is poorly understood and unreliable. Thus five classes were created: 1) The metal as a functional role 2) The metal as a structural role 3) The metal as a carrier of ligands 4) The metal as a catalyst 5) The metal as a photosensitizer One of the most successful metals antitumor compounds, cisplatin, with an antitumor activity associated with interaction with DNA, forming intrastand crosslinks to adjacent guanosine residues, leading to cell apoptosis [17,28,37], belongs to the first class. The cytotoxic activity of compounds belonging to this class depends essentially of the thermodynamic and kinetic parameters of the metal center and the nature of the ligand. The main disadvantage of these compounds is related to the high toxicity caused by uncontrolled reactivity with molecules other than their targets. The second class. 12 | CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES.
(27) compounds should not bind directly to the biological molecules (e.g. non-covalent interactions with DNA) and be less toxic than functional compounds [43]. One of the most successful drugs belonging to this class is the antimalarial ferroquine. Several other platinum compounds can intercalate into DNA rather than forming covalent adducts as cisplatin does. Compounds of the third class carry a ligand, which is delivered in vivo. The ligand is protected by the metal until its delivery. The metal as a catalyst is associated to the generation of ROS that cause cell damage [28,39]. Several metal compounds need to be primarily induced to be antitumor active (e.g. ruthenium complexes with polypyridine ligands) [17,43]. Although several classes have been created, all the compounds used in clinical practice belong to the first class, and no compounds, from any of the others classes, are expected to enter in clinical use hereafter, even though a lot of research in this field is being made in order to develop new drugs [43].. 4.6.. Experimental strategies. Several approaches can be used in order to study possible mechanisms by which antitumor metal compounds inhibit cell proliferation, particularly by interactions with DNA [44,45]. Gel electrophoresis studies constitute a rapidly and economical mode to evaluate the effect of metal complexes on DNA tertiary structure. Absorption titration and circular dichroism are universally used to examine the binding mode of DNA-complexes [42,46]. The analysis of the transitions in the tertiary structure of plasmid DNA induced by metals complex constitutes a complementary approach to the DNA-complex interaction study and it can be achieved by using atomic force microscopy (AFM) [47].. 4.7.. Electronic absorption titration. Electronic absorption titration spectroscopy is one of the most useful techniques and widely used in DNA binding studies [44]. The mode of interaction can be evaluated by the changes on the absorption spectra. Thus, if the complex intercalates in DNA, a red-shift (bathochromism) along with a decrease in the intensity of the complex spectral band (hypochromism) can be observed. The existence of a bathochromism is also indicative of the stabilization of DNA duplex [48,49]. On the other hand, the increase in the intensity of the complex absorption band (hyperchromism) is related to the damage of the secondary structure of DNA. Then, hyperchromism and hypochromism are related to spectral features of the double helix structure of DNA, where hypochromism means that the DNA-binding mode of complex may be due to electrostatic effect or intercalation and hyperchromism is related to the loss of secondary structure of DNA. The complex-DNA binding strength can be evaluated by the intrinsic binding constant, K, which represents the binding constant per DNA base pair [50]. In order to determine the binding constant, the neighbor exclusion model was primarily used by Benesi and Hildebrand [76]. It was limited to molecules that occupy only one binding site, and does not correspond to most biological ligands. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. | 13.
(28) which interact with more than one base pair in the DNA [41]. Schemechel and Crothers [77] adapted the previous model and Wolf et al applied it successfully to determine K according to the following equation (Eq 1) [48,50,52]: [DNA]. ( a. f). [DNA]. =(. b. f). +. ( b. f). (Eq 1). where [DNA] is the concentration of DNA in base pairs, the apparent absorption coefficients a, f and b correspond to Absorbance observed/[complex], the extinction coefficient for the free complex and the extinction coefficient for the free complex in the fully bound form, respectively. K is given by the ratio of slope to intercept in plots of [DNA]/(a-f) versus [DNA].. 4.8.. Circular dichroism. Circular dichroism (CD) results from the difference between left- and right-handed polarized components of the incident light absorbed by chiral molecules. Those components are absorbed differently by the sample, leading to a difference in the absorption coefficients: = left-right (M-1 cm-1). The signal is measured in units of cm2g-1 although it is widely used as ellipticity () [53]. CD can be used to follow rapidly and efficiently DNA conformational transitions, which constitute an important tool to evaluate with high selectively the behavior of complexes that recognize DNA structures or sequences [49,53]. For these reasons CD represents a further source of useful information in the study of the interactions of metals complexes with DNA. The most common DNA conformation observed is the B-form, which is characterized by positive bands at about 260-280 nm and a negative band at around 245 nm. Since the base pairs of DNA are perpendicular to the doublehelix axis in the B-form, the peak intensities are relatively small, considering the weak chirality of the molecule [49,54,55]. Nevertheless, CD being an extremely sensitive method, small conformational changes (e.g. due to the interaction with metal complexes) can be measured efficiently [53]. The intercalation of the complex in DNA enhances the intensities of both the bands stabilizing the DNA conformation, as is commonly observed for the classical intercalators [46]. On the other hand, simple groove binding and electrostatic interactions would lead to no perturbations or minimal perturbations in both bands of B-DNA, because these binding modes do not influence the secondary structure of DNA [45,49,55].. 4.9.. Atomic force microscopy (AFM). Atomic force microscopy has become a successful technique to investigate DNA structure and dynamics at very high resolution [56]. AFM is an ultra-high resolution microscopic technique that does not require complicated sample preparation or any other complex treatment [47]. It can be used to study DNA tertiary structure as well as the influence of factors that induce conformational transitions, such as intercalator complexes (e.g. ethidium bromide) [57]. Recent publications have. 14 | CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES.
(29) shown the potential of AFM to investigate the interactions of DNA with anti-tumor agents of metal complexes (e.g. cisplatin), where conformational structure changes were evaluated [58]. Furthermore studies carried out in order to study the effect of ethidium bromide, a classical DNA intercalator on DNA plasmid has led to the definition of some schematic structures (Figure 3) [59]. Four structures were considered: predominantly relaxed, toroidally supercoiled, mixed toroidal and plectonemic supercoils, and complete plectonemic supercoiling. Hence, with the interaction of metal complexes intercalators the DNA plasmid would become increasingly supercoiled.. Figure 3 – Drawing schemes of the transitions in plasmid DNA tertiary structure in response to an intercalator agent: (a) predominantly relaxed; (b) toroidally supercoiled; (c) mixed toroidal and plectonemic supercoils; (d) complete plectonemic supercoiling (adapted from [59]).. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. | 15.
(30) 16 | CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES.
(31) 5. Aims Several metal-based complexes with molybdenum were reported as having antitumor and cancerostatic activity [31,36]. The mechanisms of action of most of the organometallic complexes of molybdenum are far from being understood, although they might in some way be related to DNA damage [43]. The present study aimed at evaluating the antitumor activity of two molybdenum complexes, B1 ([Mo(3-C3H5)Br(CO)2(1,10-phen)]) and T2 ([Mo(3-C3H5)CF3SO3(CO)2(2,2’-bpy)]), and study their mechanism of action against cancer cell lines. To evaluate the cytotoxic activity of the complexes a metabolic activity test (MTT) was used. Octanol/water partition assays and the determination of intracellular molybdenum were carried out in order to understand how easily the complexes could pass through the membrane and where may start the process that triggers cell death. Several other studies regarding the interactions between the complexes and DNA were also performed, namely absorption titration and circular dichroism. Complementary approaches, such as gel electrophoresis studies and atomic force microscopy, were used to contribute to the study of the binding mode of DNA-complexes. Understanding the mechanism of action of B1 and T2 will potentially constitute a valuable tool in cancer chemotherapy in order to reduce drug resistance of the chemotherapeutic agents used nowadays and to overcome some chemotherapy limitations. An overview of the work plan is illustrated in Figure 4.. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. | 17.
(32) Figure 4 - Schematic illustration of the overview of the work plan. Mo(II) complexes were tested and acted as potent cytotoxic drugs, interacting with DNA in vitro. Do they enter the cell and directly damage DNA to inhibit cell growth?. 18 | CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES.
(33) 6. Experimental. 6.1.. Instrumentation and materials. Commercially available reagents and all solvents were purchased from standard chemical suppliers. Octanol was purchased from Riedel-de Haën, Germany. The RPMI 1640 cell culture medium, fetal bovine serum (FBS) were purchased from LONZA Co. MTT (3-(4,5-dimethyl-thiazol-2-yl)-2,5diphenyl tetrazolium bromide) was purchased from Sigma Chemical Co, USA. Calf thymus DNA (ctDNA) was purchased from Sigma Chemical Co. Ltd. and a stock solution was prepared by dilution in a buffer solution (50 mM NaCl/5 mM Tris-HCl, pH 7.1) followed by stirring at 4 ºC for two days. This solution was stored at 4 ºC. The stock solution of ctDNA gave a ratio of UV absorbance at 260 and 280 nm (A260/A280) > 1.8, indicating that the DNA was sufficiently free of protein contamination [60]. The DNA concentration was determined by the UV absorbance at 260 nm after 1:10 dilution using = 6600 M-1 cm-1 [61]. MTT was dissolved (5 mg/ml) in phosphate buffer saline pH 7.2. Infrared spectra were measured on a Mattson 7000 FT spectrometer. Samples were run as KBr pellets. NMR spectra were recorded on a Bruker Avance-400 spectrometer in CDCl3 or deuterated DMSO. Elemental analyses were carried out at the University of Vigo, Spain. UV-Vis spectra were recorded on a Shimadzu UV-2450 equipped with a Peltier cell for temperature control.. 6.2.. Synthesis of molybdenum(II) complexes 6.2.1. [Mo(3-C3H5)(CF3SO3)(CO)2(2,2’-bpy)] (T2). Thallium triflate (TlCF3SO3) (0.353 g, 1 mmol) was added to a solution of [MoBr(3C3H5)(CO)2(2,2’-bipyridyl)] (0.429 g, 1 mmol) in acetonitrile (20 ml), and the mixture was refluxed for 5 hours. A white solid of TlBr was formed and filtered with celite. The solid was washed 3 times with acetonitrile. The filtrate was evaporated and the solid residue dissolved in dichloromethane. Addition of n-hexane resulted in the formation of red crystals after a few days [73]. Yield: 72% (0.359 g) IR (KBr disc) (cm-1): 3436; 3069; 1947; 1863; 1602; 1573; 1495; 1474; 1441; 1389; 1312; 1302; 1287; 1237; 1219; 1174; 1158; 1127; 1109; 1077; 1034; 930; 795; 764; 734; 657; 650; 630; 577; 570; 516; 504; 439; 418. 1. H NMR (400 MHz, DMSO-d6): 1.63 (d, Hanti); 3.76 (d, Hsyn); 4.06 (m, Hmeso); 7.69 (t, H3/H6); 8.08. (t, H2/H7); 8.16 (d, H4/H5); 9.2 (s, H1/H8).. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. | 19.
(34) 6.2.2. [Mo(3-C3H5)(Br)(CO)2(1,10-phenanthroline)] (B1) A solution of 1,10-phenantroline (2 mmol, 0.3965 g) was added to a stirring solution of (3C3H5)(CO)2(MeCN)2] (0.7100 g, 2 mmol) in dicholoromethane (20 ml) and the mixture was kept stirring overnight. The red precipitate formed was washed with dichloromethane (10 ml), diethyl ether (10 ml) and dried under vacuum. Yield: 87% (0.7875g) IR (KBr disc) (cm-1): 1926; 1863; 1832; 1626; 1463, 1426; 1. H NMR (400 MHz, CDCl3): 1.51 (d, Hanti); 2.86 (m, Hmeso); 3.14 (d, Hsyn); 4.15 (m Hmeso); 7.82 (m,. H7); 7.96 (s, H4/H5); 8.01 (s, H2); 8.5 (m, H3/H6); 9.23 (t, H8); 9.4 (s, H1); 13. C NMR (400 MHz, CDCl3): 53 (Csyn); 54 (Canti); 62 (Cmeso); 124 (C7); 127 (C2/C4/C5); 136 (C3/C6); 151 (C8).. 6.3.. Cell cultures. Different cell lines were used in order to study intracellular processes, as the cytotoxic activity of the molybdenum complexes synthesized (Chapter 6.2), as well as their mechanism of action. Thus HeLa (cervical carcinoma), MCF-7 (breast carcinoma) and hTERT-RPE1 (human telomerase reverse transcriptase – retinal carcinoma) were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 200 U/ml penicillin, 100 µg/ml streptomycin and 0.3 g/ml L-glutamine in a humidified atmosphere of 95% air /5% CO2 at 37º C. All cells culture procedures were carried out in a culture cabinet under sterile conditions, as well as all the material used sterilely.. 6.4.. Subculture of cells. In order to maintain the cells in a healthy and viable state they were subcultured, process also known as passaging. Thus the adherent cells were harvested enzymatically from culture dishes occupied 80 to 90% of the surface with cells (confluent state). Thus, trypsin was used to detach the monolayer of adherent cells and, after diluted in phosphate buffered saline (PBS), it was inactivated by the addition of medium containing serum. The suspension of cells was then distributed to new culture dishes and incubated in a humidified atmosphere of 95% air /5% CO2 at 37º C.. 6.5.. Cell quantification. Seed with the appropriate seeding density is crucial to reach the optimum growth. One way to quantify cells is by using a haemocytometer, which is both simple and cheap. It contains 9 large squares and inside it has 16 small squares. Each large square measures 1 mm x 1 mm and is 0.1 mm deep (Figure 5).. 20 | CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES.
(35) Figure 5 – Haemocytometer (adapted from [62]). Furthermore each square has a volume of 0.1 mm3 and from this it is possible to determine the concentration of cells and the total number of cells per cubic centimeter. Then cells are counted in each large square and the average of cells is calculated to increase the accuracy. The count can be converted to the number of cells per mL of suspension according to the following equation (Eq 2). Cells (cells/mL) = Average of the number of cells counted × 10. 6.6.. (Eq 2). Cryopreservation of cells. One way to preserve cells is by freezing stocks in liquid nitrogen. This process, known as cryopreservation, grants a renewable source of cells for later use. To avoid the formation of ice crystals inside the cell and changes in pH, DMSO is used to lower the freezing point. In order to accomplish a successful cell cryopreservation a freezing chamber (‘Mr Frosty’) was used to cool down slowly from room temperature to -80 ºC at a rate of 1-3 ºC per minute. Cells were then harvested and resuspended in a solution of 60% medium and 40% of serum, from which was added DMSO (10%) and stored in vials in a ‘Mr Frosty’ cryo freezing container. After that it was placed in a -80 ºC freezer at overnight and hereafter transferred to liquid nitrogen.. 6.7.. Resuscitation of frozen cells. In order to revive frozen stocks from cryogenic vials stored in liquid nitrogen it is necessary to warm up the vials at 37 ºC for 1-2 min, avoiding that cells warm up to 37 ºC, otherwise they may rapidly die. The cell suspension is then added to fresh growth medium pre-warmed and placed in a culture flask, which is incubated in a humidified atmosphere of 95% air /5% CO2 at 37º C.. 6.8.. Cytotoxic activity assay in vitro. In order to determine the cell viability towards molybdenum complexes, cytotoxic activity assay was performed by the MTT (3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide) method CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. | 21.
(36) previously described with some modifications [63]. Exponentially growing cells were seeded at a density of approximately 4x105 cells/ml, in a 96-well flat-bottomed microplate, and incubated for 48 h in an atmosphere of 5% CO2/95% air at 37ºC. Then the cells were treated with the complexes and incubated for 48 h. Compounds were dissolved in the culture medium with 0.5% DMSO and tested in concentrations ranging from 1 to 1000 M. Control wells contained supplemented media with 0.5% DMSO. After incubation time MTT solution (100 L, 0,5 mg/mL) was added into each well, and incubated for 2 hours at 37ºC h in an atmosphere of 5% CO2/95% air. After medium removal, 100 L DMSO was added to dissolve the formazan crystals. The optical density was measured at 570 nm using a 96-well multiscanner autoreader. The IC50 values (concentration that caused 50% growth inhibition) were calculated by non-linear regression analysis. For the kinetic studies, cell cultures were exposed to different concentrations of compounds for different periods of time (1, 2, 24, and 48 h), after which the medium with the drug was removed and was replaced by fresh medium. Each experiment included ten replicates for the different concentrations of complexes and results represent at least 3 independent experiments. UV-Vis and mass spectrometry spectra of solutions with appropriate concentrations were measured during this period and showed no decomposition of the complexes being studied (Annex).. 6.9.. Octanol/water partition coefficient. Water-saturated octanol and octanol-saturated water were prepared by shaking equal volumes of octanol and water for 5 hours and allowing the mixture to separate into the respective phases for 24 hours. Solutions of molybdenum complexes (20 M) were prepared in water-saturated octanol and their absorbance was analyzed by UV spectrophotometry. Three and six milliliters of complex solution were then added to 40 ml of octanol-saturated water. These solutions were shaken vigorously for 2 hours. The aqueous phase was separated ensuring that there was no contamination from the octanol phase, and each of these solutions was analyzed by UV spectrophotometry to obtain the absorbance of the compounds.. 6.10. Conductimetry The specific conductivity of molybdenum complexes solutions was measured at 25ºC using a Radiometer Copenhagen – Meterlab CMD 230 conductimeter. The relative uncertainty in determining the specific conductivity of the compounds solution was within 0.5%. The repeatability of the conductivity measurement, estimated from two successive runs, was about ± 3. The conductance reading was checked every 20 s until it reached a steady value.. 6.11. Cellular molybdenum uptake The protocol used was as previously described with some modifications [64]. HeLa cells were seeded in 100 mm dishes at 4x105 cells/ml and incubated at 37 ºC in an atmosphere of 5% CO2/95%. 22 | CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES.
(37) air for 48 h. The culture medium was removed and replaced with medium containing the molybdenum complex at a concentration of 0, 10, 50 and 100 M for 48 h. Following treatment, the cell monolayer was scraped off the culture dishes and the molybdenum content was determined by inductively coupled plasma mass spectrometry (ICP-MS) by standard protocol at the University of Vigo, Spain. Molybdenum content of cytosolic and nuclear extracts was also analyzed. Cells were washed twice with PBS buffer and lysed with cytosolic lysis buffer (HEPES 50 mM, pH 7.2, EDTA 2 mM, NaCl 10 mM, sucrose 250 mM, DTT 2 mM). Cells were then scraped up and centrifuged (3000 g, 4 min, 4ºC) (supernatant - cytosolic fraction). The pellet was washed with cytosolic buffer and ressuspended with nuclear lysis buffer (HEPES 50 mM, pH 7.2, EDTA 2 mM, NaCl 400 mM, glycerol 20% (v/v), DTT 2 mM) (pellet - nuclear fraction). The molybdenum content of both fractions was determined by ICP-MS at the University of Vigo, Spain.. 6.12. DNA binding studies 6.12.1.Electronic absorption titration Calf thymus DNA (ct DNA) solutions of various concentrations (0 – 100 M) were added to 20 M buffered solutions (5 mM Tris, 50 mM NaCl, pH 7.2) of the metal complexes. Absorption spectra were recorded after equilibration at 37.0 ºC for 10 min. The intrinsic binding constant, K, was determined according to Eq 1 (Chapter 4.7).. 6.12.2.Circular dichroism The CD spectra of ctDNA (500 M) in the absence and presence of molybdenum complexes at various concentrations (0, 10, 25, 50, 75, 100, 250 M) were recorded on a Jasco J810 spectropolarimeter at 37ºC (Julabo F25 temperature control unit). The region of wavelength between 220-360 nm was scanned for each sample using a 1 mm path quartz cell, and the result was displayed in millidegreed (deg).. 6.12.3.Gel electrophoresis studies E. coli. Bacteria were transformed with pYES2 DNA plasmid (5856 nucleotides, multiple cloning site, ampicillin resistance gene) by prior treatment with Ca2+ at 4ºC in order to become competent. DNA was added to the suspension of competent cells and taken up during a brief increase in temperature (heat shock). After a brief incubation to allow expression of the antibiotic resistance genes the cells were plated onto medium containing the antibiotic, ampicillin. The plasmid was then isolated from the bacteria using a kit from GE Healthcare and stored at 4ºC. The pYES2 was incubated with various concentrations (0, 10, 50 and 100 M) of molybdenum complexes (B1 and T2) in TE buffer (Tris HCl 10 mM, EDTA 1 mM, pH 8.0). DNA digested with Hind III and plasmid. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. | 23.
(38) without complexes were used as marker and control, respectively. Samples were incubated for 10 min. After incubation samples were run on an agarose gel (1% in TEA Buffer [Tris acetate 40 mM, EDTA 1 mM, pH 8.0]) stained with ethidium bromide, and imaged with a common transilluminator.. 6.12.4.Atomic force microscopy Squares of mica were stuck to steel discs ready for mounting samples onto the AFM instrument. The mica squares were cleaved with adhesive tape immediately prior to use. A method involving divalent cations to bridge between the negatively charged mica substrate and DNA backbone was used. The plasmid, pYES2, was diluted in a solution of Tris-HCl (20 mM, pH 7.5), MgCl2 (5 mM), and B1 (20 M). Solution of plasmid diluted in Tris-HCl and MgCl2 was used as control. The solution was then spotted directly onto freshly cleaved mica and, following a 2 min incubation period, the mica was gently rinsed with H2O and blown dry with compressed N2.. 24 | CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES.
(39) 7. Results and discussion. 7.1.. Cytotoxic activity assay in vitro. To evaluate the potential antiproliferative activity of the molybdenum complexes T1 and B2 (Figure 6), human cervical cancer cell line (HeLa), human breast cancer cell line (MCF-7) [74], and human telomerase reverse transcriptase – retinal epithelial cells (RPE) were incubated for 48 hours with varying. concentrations. of. compounds. and. the. MTT. (3-(4,5-dimethylthiazol-2-yl)-2,5-. diphenyltetrazolium bromide) assay was used .. Figure 6 - Schematic structure of B1 and T2.. The well known MTT assay measures cell viability in terms of metabolic turnover, as indicated by the oxidation of MTT to purple formazan by mitochondria. The relation between cell viability and compound concentration obtained for HeLa and RPE cells treated with compound T2 and B1 was determined (Figure 7 - 10, left).. % Cell Viability. 100. 50. 0 0. 1. 10. 25. 50 100 250 500 1000. [T2] ( M) Figure 7 - In vitro cytotoxic assays for T2 against HeLa cells (left). Dose-response curve obtained by nonlinear regression analysis for HeLa cells treated with T2 (right).. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. | 25.
(40) % Cell Viability. 100. 50. 0 0. 1. 10. 25. 50 100 250 500 1000. [B1] ( M) Figure 8 - In vitro cytotoxic assays for B1 against HeLa cells (left). Dose-response curve obtained by nonlinear regression analysis for HeLa cells treated with B1 (right).. From the relation between cell viability and compound concentration, dose-response curves obtained by non linear regression analysis for HeLa and RPE cell lines treated with the complexes were done in order to determine the IC50 value (final concentration ≤0.5% DMSO), which corresponds to the concentration of compound required to inhibit cell proliferation by 50% (Figure 7-10, right).. % Cell Viability. 100. 50. 0 0. 1. 2.5. 5 10 20 30 50 100 [B1] ( M). Figure 9 - In vitro cytotoxic assays for B1 against RPE cells (left). Dose-response curve obtained by nonlinear regression analysis for RPE cells treated with B1 (right).. 26 | CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES.
(41) % Cell Viability. 100. 50. 0 0. 1. 2.5. 5 10 20 30 50 100 [T2] ( M). Figure 10 - In vitro cytotoxic assays for T2 against RPE cells (left). Dose-response curve obtained by nonlinear regression analysis for RPE cells treated with T2 (right).. The IC50 values of B1 and T2 against HeLa, MCF-7 [74], and RPE were then calculated from the dose-response curves (Table 2). Table 2 - In vitro cytotoxicity assays for molybdenum complexes T2 and B1 against HeLa, MCF-7 [74], and RPE cells (data are mean ±SD of three replicates each).. IC50 (M) Compounds HeLa. MCF-7. RPE. T2. 23.7 ± 0.01. 44.5 ± 0.7. 13.1 ± 2.4. B1. 5.1 ± 1.0. 8.9 ± 0.5. 0.7 ± 0.1. As demonstrated by the IC50 values (Table 2), the molybdenum complexes showed to be very effective as cytotoxic agents against the in vitro growth of various cancer cell lines. B1 exhibited activities with IC50 values ranging from 1 to 9 M, approximately, and for T2 ranging from 13 to 46 M, approximately, for the cell lines studied. The complexes exhibited significant potency against RPE cells, but less toxic toward MCF-7. Thus, it is clear that the RPE cells are the most sensitive, whereas MCF-7 cells turned out to be the most resistant against the cytotoxic agents. It is also evident that B1 is more effective than T2. This interesting behavior may be related to the ligand, 1,10-phenanthroline, which already has been shown to be very effective as cytotoxic agent [44]. Furthermore, both the complexes achieved almost total inhibition (<10% cell viability) at the maximum concentration tested (100 M) for all cell lines. In fact, B1 values are comparable to. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. | 27.
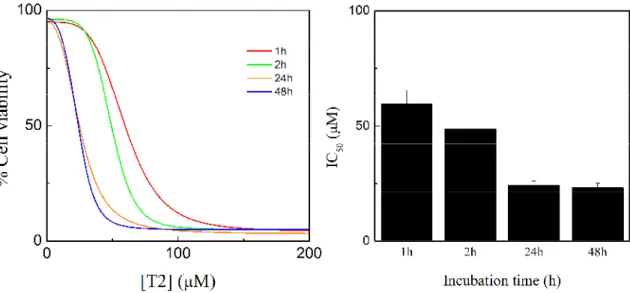
(42) cisplatin, which has IC50 values below 10 M with a range of cell lines [65,66]. For further comparison between these two complexes and the two ligands, complementary studies have been performed. The effect of the incubation time of cells with the compounds was also investigated. Thus doseresponse curves (Figure 11-12, left) and cytotoxic activity (Figure 11-12, right) for the treatment of HeLa cells with the compounds for 1, 2, 24, and 48 hours were determined.. Figure 11 - Dose-response curve obtained by nonlinear regression analysis for HeLa cells treated with B1 for 1, 2, 24, and 48 hours (left). In vitro cytotoxic activity for B1 against HeLa cells at 1, 2, 24, and 48 hours (right).. These results indicate that the molybdenum complexes inhibit cell proliferation in a durationdependent manner. The IC50 value of both complexes decrease as the exposure time increases, although the difference between 24 h and 48 h does not seem relevant. Indeed, it seems that some time is needed in order to achieve the maximum cytotoxic effect, which may be related to the formation of new species from the molybdenum complexes. In fact, observing the MS spectra for both. the. complexes. (Annex,. Figure. 23-30),. a. cationic. complex. is. formed. ([(Mo)(C12H8N2)(C3H5)(O)2]+ , for B1 and [(Mo)(C10H8N2)(O)2]+ for T2). For B1 it is formed after two hours of incubation time and for T2 is formed immediately. Thus, it is reasonable to admit that this cationic form may be related to the cytotoxic effect of both the complexes, since it appears after 24 hours with a significative relative abundance, as the cytotoxic activity starts to achieve the maximum effect. This relation can be seen clearly with B1, which seems to inflict more cytotoxic effect as the cationic form is created. T2 seems to form more of this cationic complex at 24 hours, the maximum effect; after that, no relative abundance increase is observed. This may be related to the stabilizing landing observed for the cytotoxic effect after 24 hours (Figure 12). The increase of the cationic form may be the key to achieve the maximum cytotoxic effect.. 28 | CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES.
(43) Figure 12 - Dose-response curve obtained by nonlinear regression analysis for HeLa cells treated with T2 for 1, 2, 24, and 48 hours (left). In vitro cytotoxic activity for T2 against HeLa cells at 1, 2, 24, and 48 hours (right).. 7.2.. Octanol/water partition coefficient. Partition coefficients, P, determinations were carried out to estimate how easily the compounds T2 and B1 are able to pass through a biological membrane. The P measurements are based on the difference in solubility that a given compound exhibits in an aqueous versus a hydrophobic medium [67].. Complex. B1. presented. a. hydrophobic behavior (log P = 0.760 ±. 1. 0.039). For complex T2 it was not possible to determine a conclusive value hydrophobic; however, less than B1 (Figure 13). In order to study further the. Log P. of log P, nevertheless T2 seems to be 1/2. behavior of the complexes in solution, the determination of the conductance were carried out (Chapter 4.3). 0. T2. B1. Figure 13 - Mean log octanol/water partition coefficients (Log P) of the molybdenum compounds.. CYTOTOXIC ACTIVITY AND MECHANISM OF ACTION OF ORGANOMETALLIC COMPLEXES. | 29.
Imagem
![Figure 1 - Cisplatin and its analogues (Adapted from [17]).](https://thumb-eu.123doks.com/thumbv2/123dok_br/19175340.942920/22.865.155.739.275.637/figure-cisplatin-analogues-adapted.webp)
![Figure 2 - Dose-response relationships and proposed resistance mechanisms (Adapted from [24]).](https://thumb-eu.123doks.com/thumbv2/123dok_br/19175340.942920/24.865.157.720.475.922/figure-dose-response-relationships-proposed-resistance-mechanisms-adapted.webp)


Documentos relacionados
Este tipo de ensaio só pode ser realizado em enrolamentos cujos terminais sejam exteriores à cuba do transformador. A tensão de ensaio deve corresponder a uma onda completa
[r]
Antitumor activity assays of the new complexes using the invasive MDA-MB-231 and non-invasive MCF-7 human breast tumor cell lines indicated a good degree of cytotoxicity for
4 em inglês pode contribuir para uma inclusão mais segura deste gênero e seus conteúdos nas aulas, pois se torna uma opção viável devido aos temas extraídos de contextos reais,
Assim, as festas e eventos patrocinados são oportunidades indispensáveis a este fim e identificamos a cavalgada não só como festa popular de cunho cultural e
Schematic representation of complexes of the complex iron sulfur molybdenum (CISM) family and related complexes, including ACIII (A), and separation of the complexes in the three
This work reports the in vitro activity against Plasmodium falciparum blood forms (W2 clone, chloroquine-resis- tant) of tamoxifen-based compounds and their ferrocenyl
![Figure 5 – Haemocytometer (adapted from [62])](https://thumb-eu.123doks.com/thumbv2/123dok_br/19175340.942920/35.865.322.600.80.374/figure-haemocytometer-adapted-from.webp)


![Table 2 - In vitro cytotoxicity assays for molybdenum complexes T2 and B1 against HeLa, MCF-7 [74], and RPE cells (data are mean ±SD of three replicates each)](https://thumb-eu.123doks.com/thumbv2/123dok_br/19175340.942920/41.865.226.675.633.838/table-vitro-cytotoxicity-assays-molybdenum-complexes-hela-replicates.webp)