Faculdade de Farmácia
ABIETANE CATIONIC AMPHIPHILES (ACA)-LOADED POLYMERIC BEADS TO
TACKLE RESISTANT BACTERIA
Íris Raquel Branco Neto
Dissertação orientada/o pelo(a) Professor(a) Doutor(a) Patrícia Dias Mendonça
Rijo e, coorientada pelo(a) Professor (a) Doutor(a) Célia Maria Cardona Faustino
Mestrado em Ciências Biofarmacêuticas
Faculdade de Farmácia
ABIETANE CATIONIC AMPHIPHILES (ACA)-LOADED POLYMERIC BEADS TO
TACKLE RESISTANT BACTERIA
Íris Raquel Branco Neto
Dissertação orientada/o pelo(a) Professor(a) Doutor(a) Patrícia Dias Mendonça
Rijo e, coorientada pelo(a) Professor (a) Doutor(a) Célia Maria Cardona Faustino
Mestrado em Ciências Biofarmacêuticas
The presented thesis project was performed at the Food Sciences and Phytochemistry research group at the Research Center for Biosciences & Health Technologies (CBIOS) of Universidade Lusófona de Humanidades e Tecnologias (ULHT), under the supervision of Professor Doctor Patrícia Dias Mendonça Rijo, Ph.D and co-supervision of Professor Doctor Célia Maria Cardona Faustino, Ph.D.
Additionally, some research was developed at the School of Sciences, University of Lisbon, under the supervision of Prof. Dr. Cristina Moiteiro,Ph. D.
Errata
page line Writen: Should read:
VIII 1 Desenvolvimento de novos ACAs Desenvolvimento de novos abietanos catiónicos anfifílicos (ACAs)
VIII 2 Química do ácido desidroabiético (DHA) Química do ácido dehidroabiético (DHA)
IX 4 Gram-positiva S aureus Gram-positiva S. aureus
IX 15 concentrações de 31,25, 62,50 e 93,75 μg/mL concentrações de 31.25, 62.50 e 93.75 μg/mL
IX 19 EE% de 99,49±0,05% EE% de 99.49±0.05%
IX 21 PP% = 60,00±0,05%; PE% = 88,12±0,05 % PP% = 60.00±0.05%; PE% = 88.12±0.05 % IX 24 19,69±7,40 μg/mL e 23,67±3,75 μg/mL 19.69±7.40 μg/mL e 23.67±3.75 μg/mL
X 4 Ácido desidroabiético Ácido dehidroabiético
XV 6 Universidade Lusófona Universidade Lusófona de Humanidades e Tecnologias
XIX 2 Table 2 – MICa values against a collection of Gram-negative bacteria and yeasts
Table 2 – MICa values against a collection of Gram-positive bacteria
51 70
17 6
The isomeric compounds (7a) and (7b) (Fig. 6) S. aureus CIP 106760 (VRE), S. aureus ATCC 6538, S. aureus ATCC 25923, S. aureus ATCC 700699, S. aureus FFHB 25923 (VRE), S. aureus 43300, S. aureus ATCC 43866, S. aureus ATCC 51299 (VRE), S. epidermidis ATCC 12228, Enterococcus faecalis ATCC 29212, E. faecalis ATCC 51299, E. faecalis V583 and Mycobacterium smegmatis ATCC 607], Gram-negative bacteria (Pseudomonas aeruginosa ATCC 9027, Escherichia coli ATCC 25922, E. coli 1323
The isomeric compounds (7a) and (7b) (Table 1) S. aureus CIP 106760 (MRSA), S. aureus ATCC 6538, S. aureus ATCC 700699, S. aureus FFHB 25923 (VRSA), S. aureus ATCC 43300 (MRSA), S. aureus ATCC 43866, S. aureus ATCC 51299 (VRSA), S. epidermidis ATCC 12228, Enterococcus faecalis ATCC 29212, E. faecalis ATCC 51299 (VRE), E. faecalis V583 (VRE) and Mycobacterium smegmatis ATCC 607], Gram-negative bacteria (Pseudomonas aeruginosa ATCC 9027, Escherichia coli ATCC 25922, clinical isolates: E. coli 1323
80 5 left for magnetic stirring for 2 left for magnetic stirring for 24h 85 1 Table 2 – MICa
values against a collection of Gram-negative bacteria and yeasts
Table 2 – MICa
values against a collection of Gram-positive bacteria
90 12 S aureus ATCC 43866 strong S. aureus ATCC 43866 a strong
90 18 E faecalis V583 E. faecalis V583
95 13 mean diameter of 2.37±0.20 and 2.31± 017 mm mean diameter of 2.37±0.20 and 2.31± 017 x 103 µm
Acknowledgements
This thesis was carried out at the Research Center for Biosciences & Health Technologies at Universidade Lusófona de Humanidades e Tecnologias (ULHT) under the supervision of Prof. Dr. Célia Faustino (Faculty of Pharmacy, University of Lisbon) and Prof. Dr. Patrícia Rijo (ULHT), whom I am deepest grateful for their continuous support, encouragement, inexhaustible optimism, constructive suggestions and discussions during my research. Finally, for the countless hours of attention they devoted throughout the course of my dissertation and for their help finishing this work.
I also want to express my gratitude:
To Prof. Dr. Catarina Pinto Reis and Prof. Dr. Marisa Nicolai for the help always given and for their contribution in the accomplishment of my work.
To ULHT/CBIOS for the warm welcome and the facilities provided throughout this work.
To all my laboratory mates, specially to Ana Mota, Catarina Garcia and Luis Roque, for their patience with me during the trial run of this work.
To my MSc colleague, Joana Andrade, for being an excellent colleague, friend and confidante in the last years. We have started a beautiful friendship and I hope it will last until the end of our days. I am here for you Mimi.
To Prof. Dr. Aida Duarte from Faculty of Pharmacy (University of Lisbon) for providing the original bacterial strains.
To Prof. Dr. Cristina Moiteiro from Faculty of Sciences (University of Lisbon), the solicitude expressed in performing the NMR spectra and elemental analyses.
To Prof. Dr. Milica Pesic from Institute for Biological Research "Siniša Stanković", Belgrade, Serbia for helping with the toxicological tests.
To FFUL and ULHT staff for the provided support for both the laboratory and the bureaucratic level.
To all those who supported me (you know who you are) for believing in me and giving me strength to follow my dreams.
To all those who doubted me, thank you for giving me even more strength to carry on my work and follow my dreams with even more eagerness.
To all my colleagues of Farmácia São João do Estoril, my deepest gratitude for all the patience, support, friendship and advice that you all have given me, especially in these final weeks. You are the best team I ever work with and I thank you for letting me be part of it.
To my friends Marta André, Susana Marques, João Almeida, Vera Janeiro, Rui Duarte, Teresa Gomes, David Portelada, Fernando Almeida, Paulo Silva and Francisco Serranito, some of you have accompanied me for years, others only more recently, but all of you gave me strength, support and fundamental advices at this stage of my life, and I know that you will surely be there in the future as I will be there for you.
And finally, I dedicate this work to my beloved parents, especially to my mother that with great love has given me the privilege of life and always supported me in difficult times with love, affection, lots of patience and especially confidence; and to my sisters and brother, that demonstrated all their love and support during this stage of my life. I love you all.
Abstract
This dissertation focused on the development of novel abietane cationic amphiphiles (ACAs) by chemical modification of dehydroabietic acid (DHA), leading to cationic amide derivatives by condensation of the DHA carboxyl function with the amine group of biogenic polyamines (spermine) and basic proteinogenic amino acids (arginine). After ACAs several synthesis procedures were performed, and subsequently TLC and NMR analyses, it was concluded that no molecular form was identified in the form of an amide, but rather the intermediary product and products of degradation.
The presented dissertation performed a screening of antimicrobial activities of the compounds DHA and ACA hemi-synthesis intermediary (ACA-Int) against a collection of Gram-positive, Gram-negative bacteria and yeasts; toxicological studies to assess if the compounds had antimicrobial and not citotoxic properties; and microencapsulation and stability studies to evaluate the chemical behavior and stability of the tested compounds.
The antimicrobial activity screening of DHA and ACA-Int showed that both compounds had inhibited the planktonic and biofilm growth of a collection of Gram-positive and Gram-negative bacteria and a yeast.
In addition, the MIC, MIC with organic interfering substances , MBC, MBIC, MBIC with organic interfering substances and time-kill kinetic study against standard and clinical isolates of sensitive and resistant bacterial strains was determined for DHA and ACA-Int.
In MICs studies DHA demonstrated to have higher efficacy than the ACA-Int in Gram-positive bacteria S. epidermidis ATCC 12228 and M. smegmatis ATCC 607 both with the lowest MIC value (7.81 µg/mL); and in Gram-negative bacteria, all clinical isolates of K.
pneumoniae and clinical isolate strain E. coli 3023 with a MIC value of 125.00 µg/mL. The
studies of MIC with organic interfering substances have showed that organic challenges have influence in MIC values thus increasing them.
Regarding to MBC values, the results showed low MBC/MIC ratios within each strain, which is typical for bactericidal agents.
In biofilm formation assay, most of the tested microorganisms were biofilm producer except for clinical isolate Ent. Cloacae 1264. Concerning the biofilm inhibition properties of DHA and ACA-Int, where the lowest MBICs value registered of DHA was achieved against Gram-positive S aureus ATCC 43866 (0.49 µg/mL; BI = 75.13±8.82%) which is a strong biofilm producer. In Gram-negative bacteria, the lowest MBIC was showed against clinical isolates K. pneumoniae 701 (0.98 µg/mL; BI = 92.75±5.69%) and K. pneumoniae 703 (0.98 µg/mL; BI = 94.13±2.37%). DHA and ACA-Int were also tested against C. albicans ATCC 10231 and they achieved MBIC values of 62.50 µg/mL; BI = 81.83±7.05% and 125.00 µg/mL; BI = 57.85±24.64%, respectively.
With MBIC organic interfering substances, in Gram-positive bacteria only HBS has more influence in MBIC and BI, yet in Gram-negative bacteria all challenges had influence in MBIC and BI values.
Time-kill kinetics profiles of DHA against the test organisms (S. aureus ATCC 25923) at concentrations of 31.25, 62.50 and 93.75 µg/mL showed that this compound has bacteriostatic properties. Nonetheless, as a future perspective regarding antimicrobial assays, it would be important to better understand the mechanisms of action of these diterpenes.
Regarding to the microencapsulation of the compounds, it was achieved a EE% of 99.49±0.05%, and the stability studies demonstrated that DHA remains stable within alginate beads (PP% = 60.00±0.05%; PE% = 88.12±0.05%).
In the Artemia salina toxicological study, both DHA and ACA-Int demonstrate not to have toxicity, but then again in SRB assay they demonstrate to have IC50 values of
19.69±7.40 µg/mL and 23.67±3.75 µg/mL, which demonstrates the need for further investigation in this area using other methodologies as well.
According to the references of this dissertation, this is the first report on these type of hemi-synthesis biological activities with these compounds, with preliminary scientific
validations upon their known ethnopharmacological uses. Perspective is to improve the studies of DHA and its derivatives, to scientifically validate their uses, understand their safety, and unravel new bioactive compounds with therapeutic potential and specific targets.
Resumo
Esta dissertação focalizou o desenvolvimento de novos abietanos catiónicos anfifílicos (ACAs) por modificação química do ácido dehidroabiético (DHA), que conduz a derivados catiônicos de amida por condensação da função carboxilo do DHA com o grupo amina de poliaminas biogênicas (espermina) e de aminoácidos proteinogénicos básicos (arginina).
Na presente dissertação foi realizado um rastreio das actividades antimicrobianas dos compostos DHA e intermediário da hemi-síntese dos ACAs (ACA-Int) contra uma coleção de bactérias resistentes Gram-positivas, Gram-negativas e leveduras; estudos toxicológicos foram efectuados de forma a avaliar se os compostos tinham propriedades antimicrobianas e não citotóxicas; e foi procedida uma microencapsulação e testes de estabilidade foram realizados de forma a avaliar o comportamento químico e a estabilidade dos compostos testados.
O rastreio da actividade antimicrobiana do DHA e ACA-Int demonstrou que ambos os compostos inibiram o crescimento planctónico e de biofilme numa colecção de bactérias Gram-positivas, Gram-negativas e numa levedura.
Além disso, foi determinado para o DHA e o ACA-Int os valores de MIC, MIC com desafio de bio-carga, MBC, MBIC, MBIC com desafio de bio-carga e foi efectuado o estudo cinético de time-kill contra estirpes bacterianas padrão e de isolados clínicos.
Nos estudos da MICs, o DHA demonstrou ter uma eficácia mais elevada do que o intermediário (ACA-Int) nas bactérias Gram-positivas S. epidermidis ATCC 12228 e M.
smegmatis ATCC 607, ambas com o menor valor de MIC (7.81 μg/mL). Já nas bactérias
Gram-negativas, todos os isolados clínicos de K. pneumoniae e o isolado clínico E. coli 3023 exibiram um valor de MIC de 125.00 μg/mL. Os estudos da MIC com bio-carga revelaram que os desafios orgânicos têm influência nos valores de MIC aumentando-os.
Em relação aos valores de MBC, os resultados expuseram baixas razões MBC/MIC dentro de cada estirpe, o que é típico para agentes bactericidas.
No ensaio de formação de biofilme, a maioria dos microrganismos testados foi produtora de biofilme com a excepção do isolado clínico Ent. Cloacae 1264. Em relação às propriedades de inibição do biofilme do DHA e do ACA-Int, o menor valor de MBIC obtido foi contra a estirpe Gram-positiva de S. aureus ATCC 43866 (0.49 μg/mL, BI = 75.13±8.82%), a qual é uma forte produtora de biofilme. Nas bactérias Gram-negativas, o menor valor de MBIC foi apresentado contra os isolados clínicos K. pneumoniae 701 (0.98 μg/mL, BI = 92.75±5.69%) e K. pneumoniae 703 (0.98 μg/mL, BI = 94.13±2.37%). O DHA e o ACA-Int foram também testados contra a levedura C. albicans ATCC 10231 e atingiram valores de MBIC de 62.50 μg/mL; BI = 81.83±7.05% e 125.00 μg/mL; BI = 57.85±24.64%, respectivamente.
No desafio da MBIC com bio-carga, nas bactérias Gram-positivas, apenas o HBS tem mais influência nos valores de MBIC e da BI, no entanto, nas bactérias Gram-negativas todos os desafios orgânicos tiveram influência nos valores de MBIC e da BI.
Os perfis cinéticos do teste time-kill do DHA contra o organismo de teste (S. aureus ATCC 25923) a concentrações de 31.25, 62.50 e 93.75 μg/mL revelaram que este composto tem propriedades bacteriostáticas. No entanto, e como uma perspectiva futura em relação aos ensaios antimicrobianos, seria importante compreender melhor os mecanismos de acção destes diterpenos.
Em relação à microencapsulação dos compostos, obteve-se uma EE% de 99.49±0.05%, e os estudos de estabilidade demonstraram que o DHA permanece estável dentro de microesferas de alginato (PP% = 60.00±0.05%; PE% = 88.12±0.05 %).
No estudo toxicológico da Artemia salina, tanto o DHA como o ACA-Int apresentaram ausência de toxicidade, contudo, no ensaio SRB demonstram ter valores de IC50 de
19.69±7.40 μg/mL e 23.67±3.75 μg/mL, respectivamente, o que demonstra a necessidade de uma investigação mais detalhada nesta área utilizando também outras metodologias.
De acordo com as referências desta dissertação, este é o primeiro relato sobre esse tipo de atividades biológicas de hemi-síntese com estes compostos, e com validações científicas preliminares sobre as suas conhecidas utilizações etnofarmacológicas. Perspectiva-se melhorar os estudos do DHA e dos seus derivados, validar cientificamente suas utilizações, entender a sua segurança e desvendar novos compostos bioativos com potencial terapêutico e alvos específicos.
Palavras-chave: Abietanos Catiónicos Anfifílicos; Ácido dehidroabiético; Resistência
Abbreviations and symbols
Abs – absorbance
ACA – abietane cationic amphiphiles AcOEt – ethyl acetate
A.D. – After Death
AHL – acyl homoserine lactone AMA – antimicrobial agents AMR – antimicrobial resistance B.C. – Before Christ
BHA – butylated hydroxyanisole BHT – butylated hydroxytoluene BI – biofilm inhibition percentage Boc – tert-butyloxycarbonyl Boc2O – di-tert-butyl carbonate
brs – broad singlet
BSA – bovine serum albumin °C – degree Celsius
13C NMR – 13C Nuclear Magnetic Resonance
CA – carnosic acid
CDCl3 – deuterated chloroform
CDI – carbodiimide
CFU – colony forming units
CLSI – Clinical Laboratory Standards Institute guidelines COSY – homonuclear correlation spectroscopy
CS – carnosol CV – coxsackie virus
d – duplet
DCC – dicyclohexylcarbodiimide DCM – dichloromethane
DHA – dehydroabietic acid DHAA – dehydroabioetylamine DMSO – dimethyl sulfoxide
DMSO-d6 – dimethyl sulfoxide deuterated EB – ethidium bromide
ECDC – European Centre for Disease Prevention and Control EE% – encapsulation efficiency
EPS – extracellular polymeric substances Et3N – triethylamine
et al. – and others
EU – European Union
GDP – Gross Domestic Product GI50 – growth inhibition of 50%
1H NMR – 1H Nuclear Magnetic Resonance
HAI – healthcare-acquired infections HBS – human blood serum
HMBC – heteronuclear multiple bond correlation HPLC – high performance liquid chromatography
HPLC-DAD – high performance liquid chromatography with coupled diode-array detection
HPLC-MS – high performance liquid chromatography with coupled mass spectrometry
HPLC-NMR – high performance liquid chromatography with coupled nuclear magnetic resonance
Hz – Hertz
IC50 – inhibition concentration of 50%
IR – infrared
J – coupling constant
LC50 – Median Lethal Concentration
LiAIH4 – Lithium aluminium hydride LPS – lipopolysaccharide
m – multiplet
M – molar concentration
MAPK – mitogen activated protein kinase
MBEC – minimum biofilm eradicating concentration MBIC – Minimum Biofilm Inhibitory Concentration MBC – Minimum Bactericidal Concentration MDR – multidrug resistance
MDRM – multidrug-resistant microorganisms mg – milligrams
MHA – Mueller-Hinton agar MHB – Mueller-Hinton broth MHz - megahertz
MIC – minimum inhibitory concentration mL – millilitres
MRSA – methicillin-resistant Staphylococcus aureus MSSA - methicillin-sensitive Staphylococcus aureus MS – mass spectrometry
NBT – nitro blue tetrazolium
ND – not determined or not described NF-κB – nuclear factor-kappa B
OM – outer membrane
PBP – penicillin-binding proteins PBS – phosphate buffered saline PCC – pyridinium chlorochromate ppm – parts per million
QS – quorum sensing
ROS – reactive oxygen species SDA – Sabouraud dextrose agar SDB – Sabouraud dextrose broth SEM – scanning electron microscopy SI – selective index
SRB – sulforhodamine B assay
TBTU – O-(benzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium tetrafluoroborate TEM – transmission electron microscopy
THF – tetrahydrofuran
TLC – thin layer chromatography THF – tetrahydrofuran
TNF-α – tumour necrosis factor α TSB – tryptic soy broth
UHLT – Universidade Lusófona de Humanidades e Tecnologias UV – ultra violet light
VRE – vancomycin-resistant Enterococcus
VRSA – vancomycin-resistant Staphylococcus aureus WHO – World Health Organization
w/v – weight/volume
Scientific production
Parts of this work have been published in the following conference presentations and publications:
Book chapters (state of art)
Í. Neto, C. Faustino and P. Rijo. 2015. Antimicrobial abietane diterpenoids against
resistant bacteria and biofilms. The Battle Against Microbial Pathogens: Basic Science,
Technological Advances and Educational Programs. A. Méndez-Vilas. Ed. Microbiology Book Series #5. Chapter: Vol. 1 Chapter 3. Formatex Research Center. pp.15-26.
International journals – articles (antimicrobial activity)
Íris Neto, Joana Andrade, A. S. Fernandes, Catarina Pinto Reis, Jagadish K.
Salunke, Arri Priimagi, Nuno R. Candeias, and Patrícia Rijo. 2016. Multicomponent
Petasis-borono Mannich Preparation of Alkylaminophenols and Antimicrobial Activity Studies.
ChemMedChem. 11:1 – 10. DOI: 10.1002/cmdc.201600244.
Filipa Siopa, Teresa Figueiredo, Raquel F. M. Frade, Íris Neto, Ana Meirinhos, Catarina P. Reis, Rita G. Sobral, Carlos A. M. Afonso, and Patrícia Rijo. 2017.
Choline-Based Ionic Liquids: Improvement of Antimicrobial Activity. ChemistrySelect. 1, 5909–5916.
DOI: 10.1002/slct.201600864
Íris Neto, Catarina P. Reis, Célia Faustino and Patrícia Rijo. 2017. Antimicrobial
activity and biofilm formation inhibition interfering substances challenge of dehydroabietic acid and nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics. In
preparation.
M. Braun, J. Siebert, L. Palma, Í. Neto, and P. Rijo. 2017. Antimicrobial and
National conferences – posters
Neto, Í., Reis, C., Faustino, C., Rijo, Patrícia. 2015. Ácido desidroabiético: um agente
natural antimicrobiano contra bactérias resistentes e biofilmes. 1ª Jornadas
Técnico-Científicas. ERISA. 24-25 Setembro.
Í. Neto, C.P. Reis, C. Faustino and P. Rijo. 2016. Antimicrobial Activity of Novel
Abietanes Cationic Amphiphiles Alginate Microspheres. III Simpósio de Nanociência e
Nanotecnologia Médica – 15 Abril– Auditório Agostinho Silva – Universidade Lusófona de Humanidades e Tecnologias – Lisboa.
International conferences – posters
Neto, I, Matias, D, Reis, CP, Faustino, C, Rijo, P. 2015. Antimicrobial activity and
biofilm formation inhibition of alginate microspheres of dehydroabietic acid. 11º Encontro
Nacional de Química Orgânica/4º Encontro Nacional de Química terapêutica – 1 a 3 de Dezembro – Porto.
Íris Neto, Catarina Garcia, Catarina Pinto Reis, Célia Faustino, Patrícia Rijo. 2016.
Synthesis and Efficacy of Abietane Cationic Amphiphiles from Dehydroabietic Acid. 6th
International Congress of Aromatic and Medicinal Plants. Coimbra, Portugal. 29th May – 1st June.
Célia Faustino, Íris Neto, Catarina Pinto Reis, Patrícia Rijo. 2016. Novel Antimicrobial
Abietane Cationic Amphiphiles for Dermatological Applications. International Society for
Biopysics and Imaging of the Skin. School of Sciences. Universidade de Lisboa. Campo Grande. 31ST May to 3rd June.
International conferences – virtual presentation
Í. Neto, C. Reis, C. Faustino and P. Rijo. 2016. Antimicrobial Activity Improvement of
Novel Abietane Cationic Amphiphiles from Dehydroabietic Acid. IV International Conference
Index of Figures
Fig. 1 – Sites of action and potential mechanisms of bacterial resistance to AMA [27]. ...30 Fig. 2 – Biofilm formation. Surface adhesion and proliferation of planktonic cells. An extracellular matrix and quorum sensing molecules are produced throughout biofilm maturation. Mature biofilm is characterized by a large number of conditions, slow-growing microbial cells in its centre, and fragmentation which leads to cell detachment and spread of the infection. Adapted from [38]. ...34 Fig. 3 – Deaths due AMR by 2050. Adapted from [16]. ...36 Fig. 4 – Several botanical families where diterpenes are present. ...40 Fig. 5 – Expected chemical structure of ACA I (30) (DHA + L-arginine methyl ester dihydrochloride). ...77 Fig. 6 – Expected chemical structure of ACA II (31). ...78 Fig. 7 – Expected chemical structure of ACA III (32). ...78 Fig. 8 – Equation to determine the cut-off OD value for biofilm formation ...80 Fig. 9 – Equation to determine the biofilm formation percentage. ...81 Fig. 10 – Encapsulation efficiency percentage equation. ...83 Fig. 11 – Protection percentage equation against acid stress reflux. ...83 Fig. 12 – Protection efficiency percentage equation against UV light. ...84 Fig. 13 – Formula to calculate the mortality percentage of Artemia salina toxicological study. ...85 Fig. 14 – Expected chemical structure of ACA-Int (33). ...88 Fig. 15 – Graphic representation of the time-kill kinetic study results of DHA (2a) against S.
aureus ATCC 25923. ...99
Fig. 16 – Digital images of a) the empty alginate beads and b) the DHA-alginate beads. ... 100 Fig. 17 – Mortality percentage of Artemia salina toxicological study. ... 103
Fig. 18 – Viability number of normal HaCaT cells after compound incubation. DHA = Dehydroabietic acid; ARG = arginine; SPER = spermine. ... 104
Index of Tables
Table 1 – Abietanes diterpenoids chemical structures. ...41 Table 2 – Bacterial strains used in antimicrobial tests. ...72 Table 3 – MICa values against a collection of Gram-positive, using the microdilution method,
for DHA (2a) and ACA-Int (33). ...89 Table 4 – MICa values against a collection of Gram-negative bacteria and yeasts, using the microdilution method, for DHA (2a) and ACA-Int (33). ...90 Table 5 – MBCa
and MICa values against a collection of Gram-negative bacteria and yeasts, using the microdilution method, for DHA (2a) and ACA-Int (33). ...91 Table 6 – MICa values against Gram-positive (S. aureus ATCC 25923 and S. aureus CIP 106760) and Gram-negative (P. aeruginosa ATCC 9027, using the microdilution method with organic interfering substances, for DHA (2a) and ACA-Int (33). ...92 Table 7 – Biofilm formation against a collection of Gram-Positive and Gram-negative bacteria and yeasts. ...93 Table 8 – MBICa values of DHA (2a) against a collection of Positive and Gram-negative bacteria and yeasts. ...95 Table 9 – MBIC values of DHA (2a) and ACA-Int against a collection of Gram-Positive and Gram-negative bacteria and yeasts. ...96 Table 10 – MBICa values against Gram-positive (S. aureus ATCC 25923 and S. aureus CIP
106760) and Gram-negative (P. aeruginosa ATCC 9027, using the microdilution method, for DHA with organic interfering substances challenge. ...98 Table 11 – Sulforhodamine B Assay IC50 valuesa for DHA (2a), arginine, spermine and
Table of Contents
Errata ...III Acknowledgements ...III Abstract... V Resumo ... VIII Abbreviations and symbols ... XI Scientific production ... XV
Book chapters (state of art) ... XV International journals – articles (antimicrobial activity) ... XV National conferences – posters ... XVI International conferences – posters... XVI International conferences – virtual presentation ... XVII Index of Figures ... XVIII Index of Tables ... XX Table of Contents ... XXI Chapter I – Introduction ...26 I.1 –Antimicrobial resistance ...27 I.1.1 – Antimicrobial resistance mechanisms ...29 I.1.1.1 – Enzymatic inactivation of antimicrobial agents ...31 I.1.1.2 – Alteration of the antimicrobial agent receptor or target site ...31 I.1.1.3 – Prevention of antimicrobial access to the target site ...31 I.1.2 – Bacterial biofilms ...32
I.1.4 – Antimicrobial drug discovery from natural products ...36 I.2 – Abietane diterpenoids ...40 I.2.1 – Chemical structure and occurrence ...40 I.2.2 – Antimicrobial activity ...48 I.2.2.1 – Abietic acid derivatives ...50 I.2.2.2 – Dehydroabietic acid derivatives ...51 I.2.2.3 – Dehydroabietylamine derivatives ...54 I.2.2.4 – Callitrisic acid derivatives ...55 I.2.2.5 – Pisiferic acid and carnosic acid derivatives ...56 I.2.2.6 – Ferruginol derivatives ...58 I.2.2.7 – Structure-activity relationship analysis ...60 I.2.2.8 – Mode of action ...61 I.2.2 – Anti-proliferative activity ...62 I.2.3 – Other biological activities ...63 I.3 – Aim of the dissertation ...65 Chapter II – Experimental Section ...68 II.1 – Materials ...69 II.1.1 – Reagents ...69 II.1.2 – Equipment ...69 II.1.3 – Chromatographic and spectroscopic methods ...69 II.1.3.1 – Thin liquid chromatography (TLC) ...69 II.1.3.2 – Preparative chromatography ...70 II.1.3.3 – Nuclear magnetic resonance spectroscopy (NMR) ...70 II.1.4 – Bacterial strains and culture media ...71 II.1.5 – Organic interfering substances challenge ...71
II.1.6 – Cell cultures ...71 II.1.7 – Statistical analysis, software and databases ...75 II.2 – Methods ...75 II.2.1 – Abietanes cationic amphiphiles hemi-synthesis from dehydroabietic acid ...75 II.2.1.1 – Dehydroabietic acid (2a) purification by recrystallization ...75 II.2.1.2 – Abietanes cationic amphiphiles hemi-synthesis ...75 II.2.1.2.1 – Hemi-synthesis of ACA I (30) with TBTU as coupling agent ...75 II.2.1.2.2 – Hemi-synthesis of ACA I (30) with DCC as coupling agent ...76 II.2.1.2.3 – Methylation of dehydroabietic acid (2a) for hemi-synthesis affording ACA I (30)...76
II.2.1.2.4 – Hemi-synthesis of ACA II (31) and ACA III (32) with TBTU as coupling agent ...77
II.2.3 – Antimicrobial tests ...78 II.2.3.1 – Minimum inhibitory concentration assay ...78 II.2.3.2 – Minimum bactericidal concentration assay ...79 II.2.3.3 – Minimum inhibitory concentration assay with organic interfering substances challenge ...79
II.2.3.4 – Biofilm formation assay ...79 II.2.3.5 – Inhibition properties of dehydroabietic acid (2a) and its derivatives on biofilm formation ...80
II.2.3.6 – Inhibition properties of dehydroabietic acid (2a) on biofilm formation with organic inteferers challenge ...81
II.2.3.7 – Time-kill kinetics studies ...81 II.2.4 – Microencapsulation of dehydroabietic acid ...82 II.2.4.1 – Production of dehydroabietic acid-loaded calcium alginate beads ...82
II.2.4.2 – Characterization of dehydroabietic acid-loaded calcium alginate beads ...82 II.2.4.3 – Stability Studies of dehydroabietic acid-loaded calcium alginate beads ...83 II.2.4.3.1 – Harsh conditions such gastric environment or low pH study ...83 II.2.4.3.2 – Photo stability studies ...83 II.2.5 – Toxicological studies ...84 II.2.5.1 – Artemia salina toxicological study ...84 II.2.5.2 – Sulforhodamine B Assay ...85 Chapter III – Results and discussion ...86 III.1 – Hemi-synthesis results ...87 III.1.1 – Dehydroabietic acid recrystallization ...87 III.1.2 – ACA hemi-synthesis ...87 III.2 – Antimicrobial tests ...88 III.2.1 – Minimum inhibitory concentration assay ...88 III.2.2 – Minimum bactericidal concentration ...90 III.2.3 – Minimum inhibitory concentration assay with organic interfering substances ...91 III.2.4 – Biofilm formation assay ...92 III.2.5 – Inhibition properties of dehydroabietic acid (2a) and ACA-Int (33) on biofilm formation ...94 III.2.6 – Inhibition properties of dehydroabietic acid (2a) on biofilm formation with organic interfering substances ...97
III.2.7 – Time-kill kinetic study...98 III.3 – Microencapsulation of dehydroabietic acid ...99
III.3.1 – Production and characterization of dehydroabietic acid-loaded calcium alginate beads ...99 III.3.2 – Stability studies of dehydroabietic acid-loaded calcium alginate beads ... 101 III.3.2.1 – Harsh conditions such gastric environment or low pH study ... 101 III.3.2.2 – Photostability studies ... 102 III.4 – Toxicological studies results ... 102 III.4.1 – Artemia salina toxicological study ... 102 III.4.2 – Sulforhodamine B assay ... 103 Chapter IV – Conclusions and future perspectives ... 105 References ... 110
I.1 –Antimicrobial resistance
According to the World Health Organization (WHO), “Antimicrobial resistance (AMR)
within a wide range of infectious agents is a growing public health threat of broad concern to countries and multiple sectors. Increasingly, governments around the world are beginning to pay attention to a problem so serious that it threatens the achievements of modern medicine. A post-antibiotic era—in which common infections and minor injuries can kill—far from being an apocalyptic fantasy, is instead a very real possibility for the 21st century”[1].
AMR is a global problem that affects both developing and developed countries, as a result of the use and misuse of antimicrobial agents over the past decades [1]. Infection diseases are still among the main causes of morbidity and mortality in developing countries while healthcare-acquired infections (HAI) with resistant microorganisms are a major cause of death worldwide [2]. In fact, the emergence of methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant S. aureus (VRSA), vancomycin-resistant Enterococcus (VRE) and multidrug-resistant (MDR) strains of these microorganisms have become a common occurrence in the hospital environment [3]–[5]. Moreover, one of the most potent class of antibiotics, the carbapenems, is being compromised by the growth, mainly in intensive care units, of MDR strains of Klebsiella pneumoniae, Acinetobacter baumanni, and Pseudomonas
aeruginosa [6].
A combination of increasing rates of AMR and a decline in the development of new antibiotics mainly due to a low return on investment contributed to the current crisis in fighting against antibiotic-resistant microorganisms, raising the spectre that once treatable infections may soon become untreatable [6]. Not surprisingly, the WHO identified antimicrobial resistance as a public health threat just at the beginning of the millennium [7], a consequence of the rapidly emergence and spread of antimicrobial resistant pathogens that cannot be treated with currently available antibiotics.
AMR to conventional antimicrobial agents mainly results from enzymatic inactivation of the drug, alteration of its receptor or target site, preventing the drug to gain access to the target site through altered membrane permeability or over-expression of efflux pumps [8]– [10]. Furthermore, many antibiotics were originally developed to target microorganisms growing in planktonic cultures, but it is now clear that many bacteria live as complex communities known as biofilms. Biofilms are complex microbial consortia surrounded by extracellular matrix primarily consisting of nucleic acids, proteins, lipids and exopolysaccharides that protects the microbial community from environmental stress, including bacteriophage, amoebae and biocide attack as well as against the host immune response(s) and antibiotic chemotherapy. Microbial biofilms are unequivocally responsible for the recalcitrance of many infections to conventional antimicrobial therapy, mostly due to the inability of the antimicrobial agents to penetrate the biofilm matrix [11]–[13]. Therefore, novel classes of antimicrobial agents with improved efficacy and new or combined mechanisms of action to reduce the likelihood of acquired resistance are an urgent requirement to contain the global threat of AMR.
Infection diseases are still among the main causes of mortality and morbidity in developing countries while HAI with resistant microorganisms are a major cause of death worldwide. Increasing rates of microbial resistance also invalidate the utility of even the most potent available antibiotics [1], [7], [14]–[16].
AMR is a natural biological phenomenon, which has become a threat to public health and a risk to the safety of patients, due to rapid growth in the emergence and spread of resistant pathogens while research and development of new antimicrobial therapeutic agents has been scarce. Also contributing to this crisis, is the fact that the development of new antimicrobials does not provide an economic return that justifies its investigation. The problem of antibiotic resistance worldwide is one of the foremost issues that we face in the coming decades and consequently there is a pressing need for novel classes of antimicrobial agents with new or combined mechanisms of action to reduce the likelihood of acquired resistance.
Antimicrobial cationic amphiphiles containing positively charged nitrogen functions (or other cationic groups) define a structurally diverse class of antibiotics with broad-spectrum activity and different modes of action. This class of antimicrobial agents comprise cationic antimicrobial peptides and lipopeptides, cationic lipids and cationic polymers [17]–[19].
Antimicrobial cationic amphiphiles do not target any single molecule or biochemical process, as conventional antibiotics do, but instead associate with cellular membranes by a combination of hydrophobic and electrostatic adsorption effects at the membrane/water interface. The positive charge of the antimicrobial cationic amphiphiles ensures accumulation at polyanionic microbial cell surfaces that contain acidic polymers, such as lipopolysaccharides in negative bacteria and wall-associated teichoic acids in Gram-positive bacteria. This phenomenon leads to membrane destabilization and enhanced permeability with subsequent disruption of the physical integrity of the membrane or translocation into the cell, compromising cellular processes such as DNA replication, protein folding and synthesis. This mode of action has been shown to limit the risk of cross resistance [17]–[19].
The antimicrobial activity of several terpenes and terpenoids, present in essential vegetable oils, resins and some extracts, has been associated with disruption of the cell cytoplasmic membrane by these hydrophobic secondary metabolites [20]–[24]. However, fatty acids and sterol derivatives, such as cholesterol and bile acids, remain the major source for the hydrophobic moiety of natural amphiphiles, and the potential of terpenoid components as raw materials for amphiphile preparation is a scarcely developed strategy [24], [25]. This is quite surprising as cationic terpenoid amphiphiles mimic the facially amphiphilic structures of most cationic antimicrobial peptides, while being stable to protease degradation.
I.1.1 – Antimicrobial resistance mechanisms
The antimicrobial agents (AMA) act through interaction with specific targets in microorganisms by inhibiting cell wall synthesis, protein synthesis, and/or replication of
nucleic acids. To achieve this interaction, the AMA have to access and connect to their target site (Fig. 1) [9], [26], [27].
Fig. 1 – Sites of action and potential mechanisms of bacterial resistance to AMA [27].
The development of AMR can be natural (intrinsic) or acquired and this can be transmitted within same or different species of microorganisms. Intrinsic resistance is achieved by spontaneous gene mutation and the acquired resistance is by mutation or acquisition of plasmids (DNA self-replicating, extrachromosomal), transposons (chromosomal integration or plasmid, transmitted DNA) or integrons (large mobile genetic elements) from one microorganism to another. Microorganisms gain AMR due to three main reasons namely: (i) modification of active site of the target resulting in reduction in the efficiency of binding of the AMA, (ii) direct destruction or modification of the AMA by enzymes produced by the organism (enzymatic inactivation) or, (iii) permeability barrier modification and efflux of AMA from the cell [9], [26], [27].
I.1.1.1 – Enzymatic inactivation of antimicrobial agents
Microorganisms spend a considerable amount of energy to resist AMA. One way the cells achieve AMR is by the synthesis of enzymes that selectively target and destroy or modify the chemical structure of AMA. The various enzymatic strategies that lead to AMA inactivation are through hydrolysis, group transfer or redox mechanisms. Hydrolytically susceptible chemical bonds (such as ester or amide bonds) are cleaved by enzymes (i.e. β-lactamase family of enzymes) that are expressed by the resistant organisms. The modification of the active group in the drug through acylation, phosphorylation, glycosylation, nucleotidylation or ribosylation by the organism could make the former innocuous. Redox mechanism involves the oxidation–reduction of the AMA leading to the formation of inactive compound [26], [27].
I.1.1.2 – Alteration of the antimicrobial agent receptor or target site
For the selective action of the AMA, target site has a vital role in this function. For the AMA to work they have to bind to a specific site of the microorganism, which vary with the class of AMA. The installation of mutations in target sites changes its conformation leading to reduced AMA activity. There are two types of modification of receptors (targets) which are: i) mutations in the RNA polymerase DNA gyrase, which inactivate for instance the fluoroquinolones; and ii) the modification of the structural conformation of penicillin-binding proteins (PBPs), which results in the development of resistance to penicillin [26], [27].
I.1.1.3 – Prevention of antimicrobial access to the target site
For the AMAs to bind to their target they must reach their target site in an appropriate concentration. Therefore, microorganisms developed another strategy to counteract the antimicrobial activity which prevents the access of AMA to its target site, thereby reducing the accumulation of AMA within the microorganism [26], [27]. This may occur due to the
membrane permeability [10], [26]–[29] or due to the presence of efflux pump mechanisms [26]–[31].
The cell wall of Gram-negative bacteria has an inner and an outer membrane (OM) acting as a permeable barrier. OM plays a crucial role in providing an extra protective barrier to the microorganism without compromising the exchange of compounds that are essential to life. By combining a highly hydrophobic lipid bilayer with proteins that form pores with specific size exclusion properties (porins), OM functions as a selective barrier. The permeation properties of this barrier have therefore a large impact on the susceptibility of microorganisms to AMA that have as targets intracellular processes. Mutations that cause changes in the structure of the OM can result in a permeability barrier that prevents access of AMA to their active site [10], [26], [27].
Instead of preventing penetration of AMA to the active site, some microorganisms have evolved an active efflux mechanism that pumps out antibiotics from the cytoplasm before they can bind to their target. These efflux pumps are also responsible for the resistance of AMA, and they can be found both in Gram-negative and in the Gram-positive bacteria. Some efflux pumps are specific for only one type of AMA, but others may be associated with multidrug resistance due to their ability to cast out several different classes of AMA. These efflux pumps either utilize ATP hydrolysis (i.e. (ATP) -binding cassette (ABC) superfamily) or ion gradient (i.e. small multidrug resistance family) to expel the AMA [26], [27], [30].
I.1.2 – Bacterial biofilms
The term „biofilm‟ was created and described in 1978 [32], [33]. Since then, it has been well documented that biofilm-associated microorganisms differ from their planktonic relatives in genetic transcription of resistance genes [34]. Microorganisms that develop biofilms can do it on several different surfaces, such as aquatic and soil environments, living tissues, medical devices or industrial or potable water piping systems [35], [36]. Availability of
key nutrients, chemotaxis towards surface, motility of bacteria, surface adhesions and presence of surfactants are some factors which influence biofilm formation [37]. Biofilms have been found to protect the microbial community from environmental stresses [35]. This is why the formation of biofilms in natural and industrial environments allow bacteria to develop resistance to bacteriophage, amoebae, chemically diverse biocides, host immune responses and antibiotics [35], [37].
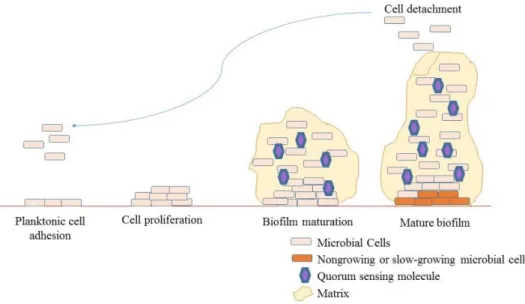
Biofilm formation can be divided into three main stages: early, intermediate, and mature. During the early stage, planktonic cells swim along the surface often using their flagella mode of movement or they can be transferred passively with the body fluids (Fig. 2). Next, the contact between microorganisms and a surface is made, resulting in the formation of a monolayer of cells. At this stage, the bacteria are still susceptible to antibiotics, and perioperative antibiotic prophylaxis can be critical for successful treatment. The next step involves irreversible adhesion to the surface, multiplication of the microorganisms, and the formation of microcolonies. During this stage, the polymer matrix is produced around the microcolonies and generally consists of a mixture of polymeric compounds, primarily polysaccharides (the matrix contributes 50%–90% of the organic matter in biofilms). The matrix consists mainly of water, which can be bounded within the capsules of microbial cells or can exist as a solvent. Apart from water and microbial cells, the biofilm matrix is a very complex material. The biofilm matrix consists of polymers secreted by microorganisms within the biofilm, absorbed nutrients and metabolites, and cell lysis products; therefore, all major classes of macromolecules (proteins, polysaccharides, and nucleic acids) are present in addition to peptidoglycan lipids, phospholipids, and other cell components. The third step is the formation of a mature community with mushroom-shaped microcolonies. During this stage, the biofilm structure can be disrupted, and microbial cells can be liberated and transferred onto another location/surface, causing expansion of the infection [38].
Fig. 2 – Biofilm formation. Surface adhesion and proliferation of planktonic cells. An extracellular matrix and quorum sensing molecules are produced throughout biofilm maturation. Mature biofilm is characterized by a large number of conditions, slow-growing microbial cells in its centre, and fragmentation which leads to cell detachment and spread of the infection. Adapted from [38].
Biofilm formation is regulated at different stages through diverse mechanisms, among which the best studied is quorum sensing (QS) [34], [38]. The QS mechanism involves the production, release, and detection of chemical signalling molecules, which allows communication between microbial cells [34], [38]. The QS process regulates gene expression in a cell-density-dependent manner; for biofilm production, the genes involved in biofilm formation and maturation are activated at a critical population density [34], [38]. There are three well-defined groups of signalling QS molecules in bacteria: oligopeptides, acyl homoserine lactones (AHLs), and autoinducer-2 (AI-2) [34], [38]. Gram-positive bacteria predominately use oligopeptides as a communication molecule, and AHLs are specific for Gram-negative bacteria [34], [38]. AI-2 is reported to be a universal signalling molecule that is used for both interspecies and intraspecies communication [38].
I.1.3 – Health, social and economic implications
The multidrug-resistant microorganisms (MDRM) are globally scattered and continue to develop new defence mechanisms, thereby threatening the ability to treat common
infectious diseases, resulting in the disability of individuals who could continue to have a normal life or to a subsequent death [15]. Infectious diseases caused by MDRM usually do not respond to standard treatment, resulting in a prolonged disease (prolonged hospitalization), patient and family suffering, increased hospital costs (first-line drugs resistance, more expensive therapies must be used; further diagnostic procedures; isolation) and an increased risk of death [15], [39]. For example, a patient infected with MRSA has a 64% higher probability of dying when compared to a patient who has an infection caused by a non-resistant strain [1], [15].
AMR puts at risk the achievements of modern medicine, for without efficient AMA to treat infections the success of interventions involving immunocompromised individuals or other surgeries may be compromised, because they might become too risky to undertake [1], [15], [16]. The global economic and trade growth and travel allow rapid propagation of MDRM between countries and continents through humans, animals, plants and food. Estimates show that AMR can cause an increase in losses in Gross Domestic Product (GDP) by more than 1% and that the indirect cost to society can affect more than 3 times the direct costs of health care [1], [15], [16].
AMR has been a growing threat already for several decades, and the magnitude and impact of this worldwide problem on the costs in the economics, health and social implications, are not yet fully known due to insufficient data [1]. To get a sense of the magnitude of this problem, it was estimated that only in the year 2013 in the USA (United States of America): the costs on treatments of HAI probably were greater than 16 billion dollars; there were 8 million additional days to the hospital internments; with an average of 2 million patients infected; and with a number of 100.000 deaths related with AMR [1], [39], [40].
According to European Centre for Disease Prevention and Control (ECDC) in 2013 in the EU, Norway and Iceland, 5–12% of hospital patients acquire an infection during their stay. Each year, an estimated 400 000 present with a resistant strain, of whom 25 000 die,
deaths and increased suffering, AMR has huge economic implications. MDRM in the EU are estimated to cause an economic loss of more than €1.5 billion each year [14], [39].
Currently, it is estimated that there are 700.000 deaths worldwide related to AMR, and that number will rise in 2050 approximately to 10 million deaths (Fig. 3), with an associated cost of 100 trillion dollars and a reduction of 2 to 3,5% in GDP [16].
Fig. 3 – Deaths due AMR by 2050. Adapted from [16].
I.1.4 – Antimicrobial drug discovery from natural products
Natural products have formed the basis of sophisticated traditional medicine systems that have been explored since thousands of years and a remarkable number of drugs have been developed from plants. The oldest data about plants used as drugs date from about 2600 B.C. from Mesopotamia, while Egyptian medicines date from 1550 B.C. with the pharmaceutical records being the famous Ebers Papyrus which documented over 700 drugs. Dating around 1100 B.C., the Chinese Materia Medica has been broadly documented over the centuries, with the first record comprising 52 prescriptions. Likewise, documentation of the Indian Ayurvedic system dates from approximately 1000 B.C. [41]–[44]. The Greeks and
Romans also made significant contributions to the development of herbal drugs practise, with Dioscorides, a Greek physician (100 A.D.), precisely recording the collection, storage, and use of medicinal herbs during his travels with Roman armies throughout the “known world”, and Galen (130–200 A.D.), a practitioner and teacher of pharmacy and medicine in Rome, being well-known for his complex prescriptions and formulas used in compounding drugs. The preservation of the Greco-Roman expertise during the Dark and Middle Ages (5th–12th centuries) may be attributed to the Arabs, who expanded it and included the use of their own resources together with Chinese and Indian herbs unknown to the Greco-Roman world [44]– [46]. The Portuguese maritime discoveries in the 16th century were also responsible for the dissemination of herbal medicines and many plants, such as plants from the genus
Plectranthus, widely distributed across the warm and tropical areas of Africa, Asia and
Oceania, have been introduced in the Mediterranean regions [47].
The role played by plant-based systems in the healthcare of many different cultures has been extensively investigated. WHO has estimated that around 65% of the world‟s population relies mainly on plant-derived traditional medicines for their primary health care [43], [46], [48].
Natural products are still being used directly or indirectly as sources to drugs against all classes of diseases. The goals of using plants as sources of therapeutics agents involve: the use of the whole plant or a part of it as a herbal medicine (e.g. cranberry, echinacea, garlic, ginkgo biloba, St. John‟s wort, Arnica); isolation of bioactive compounds for direct use as drugs (digoxin, morphine, reserpine, taxol, vinblastine, vincristine); isolation of bioactive compounds with novel or known structures to be used as lead compounds that could be altered by semi-synthesis to compounds with higher activity and/or lower toxicity (metformin, nabilone, taxotere, teniposide, verapamil); and the use of bioactive agents as pharmacologic tools (lysergic acid diethylamide, mescaline, yohimbine) [46], [48], [49].
Natural compounds offer massive structural diversity and in some cases great biological activity. Often chemical synthesis is not possible or is too time consuming. The
however, only about 15% have been investigated phytochemically and 6% have been studied for biological activity [46], [50]. With high throughput screening methods becoming more advanced and available, the number of investigated plants will rise [44], [46].
There are some broad starting points to select and obtain plant material of potential therapeutic interest. When screening for new bioactive compounds we should consider the past, present, and future value of employing information from plants used in traditional medicinal practices (ethnomedicine). Plants that have been used in traditional medicine are more likely to yield pharmacologically active compounds. In the field of antimicrobial activity, a correlation between biological activity and plants used in folklore has been demonstrated [51], [52].
The traditional processes to obtain a pharmacologically active pure constituent from a plant extract has always been a long and tedious process requiring substantial amount of material, the expertise of technicians and financial resources. In general, it comprehends several and consecutive steps of preparative chromatographic separation. Mostly, the process is expensive and time consuming. The separation performance is poor, at least in the initial fractionation steps which are typically performed by open column or preparative chromatography, however, these procedures have led to successful isolation of many bioactive molecules in the past. The loss of bioactivity in the course of purification processes is not uncommon and replication of fractionation is most of the times not practicable [44], [46].
Over the last decades, new advanced technologies and more effective strategies have been developed and adequate for a high throughput environment. The impact of HPLC-coupled spectroscopy and other types of methodology has been massive. The concerted use of HPLC-DAD, - MS and - NMR has opened entirely new possibilities for the characterization of secondary metabolites in biological extracts. These techniques can provide structural information or even absolute configuration of a molecule in few minutes, with relative low cost and time compared to the traditional methods [53], [54]. Developments in the field of NMR, in particular the advent of new probe technology at high magnetic fields, as well as the
miniaturization processes in crystallography facilitated structural elucidation and amounts of less than a milligram becoming a rather routine process [46], [55], [56].
Natural products remain an important and viable source of new drug candidates and lead molecules despite increasing competition from combinatorial and classical compound libraries, molecular modelling, and advances in synthetic chemistry technologies [57]–[61]. Moreover, natural products with low bioactivity or bioavailability can be synthetically modified to improve their pharmacological profiles. Many of the drugs in the market are natural products or natural product-derived, with approximately one-third of the world top-selling drugs being natural products or their synthetic derivatives [59]. Recently there has been a renewed interest in natural products research due to the failure of alternative drug discovery methods to deliver many lead compounds in key therapeutic areas, such as infectious diseases. The wide structural diversity of natural products aiming at providing the producer organism with a survival advantage in environments threatening its growth and/or survival often affords biologically and environmentally friendly lead molecules due to their co-evolution with the target sites in biological systems [62], [63]. Resistance-modifying properties of natural products have been observed, and synergism between natural products and antibiotics holds a promise in the fight against infectious diseases [26], [51], [64].
Medicinal plants are a valuable source of novel chemotypes and pharmacophores, with the plant-derived products still playing a major role in modern drug discovery and development efforts [24], [51], [52], [64]–[72]. About 80% of the population in developing countries relies on traditional medicine, mainly based on medicinal plants, for their primary healthcare, while in developed countries the use of herbal drugs is escalating in the form of complementary and alternative medicine [59], [68], [69].
Diterpenes, a class of natural products widespread in several botanical families such as Asteraceae, Celastraceae, Hydrocharitaceae, and Lamiaceae (Fig. 4), are promising sources of antimicrobial prototype compounds due to their structural diversity and wide range of oxidized patterns [20]–[22], [63], [70]–[78].
Fig. 4 – Several botanical families where diterpenes are present.
I.2 – Abietane diterpenoids
I.2.1 – Chemical structure and occurrence
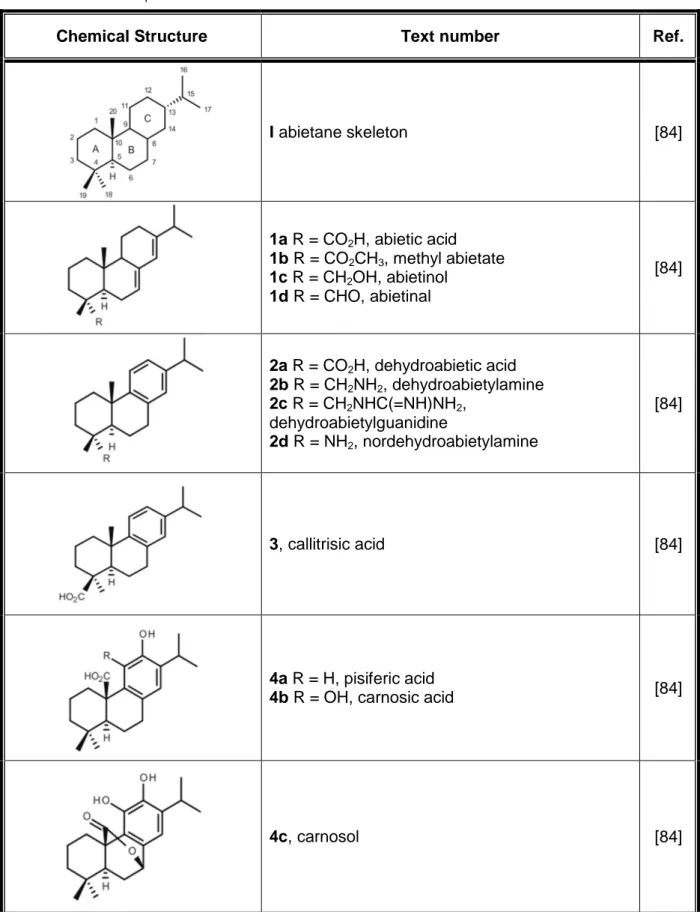
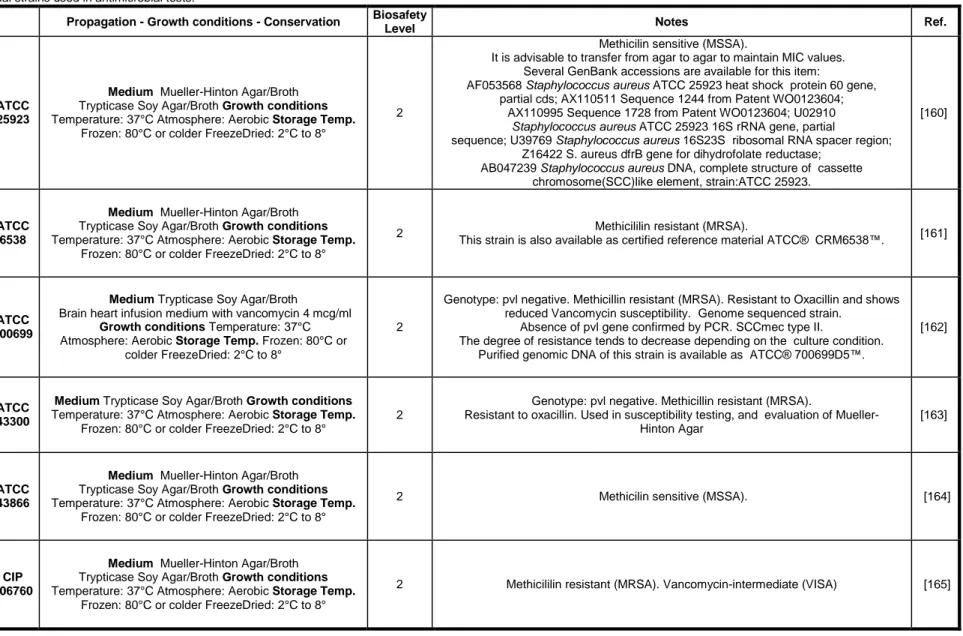
The abietanes are a family of natural occurring diterpenoids that have been isolated from a large variety of terrestrial plants [20]–[23], [63], [70]–[78]. These compounds are known to possess a broad range of biological activities, including, antimicrobial, antiulcer, antioxidant, anti-inflammatory, cardiovascular and cytotoxic activities, which have attracted a considerable interest from both the pharmaceutical and medical communities [20], [22], [23], [71], [73], [76]–[81]. During the last decades, many new members of the abietane diterpenoid family have been isolated and characterized, and research efforts have also been made regarding the chemical synthesis of these natural products and the development of synthetic derivatives with improved bioactivity and/or pharmacological profiles [23], [25], [80]–[83]. Abietanes (Table 1) have the characteristic carbon skeleton of 20 carbon atoms (I, abietane skeleton) exemplified by that of abietic acid (1a) which shows an equatorial carboxylic group (C-18) and two conjugated double bonds at positions 7 and 13.
Table 1 – Abietanes diterpenoids chemical structures.
Chemical Structure Text number Ref.
I abietane skeleton [84] 1a R = CO2H, abietic acid 1b R = CO2CH3, methyl abietate 1c R = CH2OH, abietinol 1d R = CHO, abietinal [84] 2a R = CO2H, dehydroabietic acid 2b R = CH2NH2, dehydroabietylamine 2c R = CH2NHC(=NH)NH2, dehydroabietylguanidine 2d R = NH2, nordehydroabietylamine [84] 3, callitrisic acid [84] 4a R = H, pisiferic acid
4b R = OH, carnosic acid [84]
5, ferruginol [84] 6a R1 = α-OH, R2 = H 6b R1 = H, R2 = OH 6c R1 = ═O, R2 = H [84] 7a R1 = CO2CH3, R2 = trans-CH3 7b R1 = CO2CH3, R2 = cis-CH3 7c R1 = CHO, R2 = cis-CH3 [84] 8a R = H 8b R = OH 8c R = OCH3 8d R = N(CH3)2 [84] 9a R = C6H5 9b R = p-CH3C6H4 9c R = p-CH3OC6H4 9d R = p-ClC6H4 9e R = p-BrC6H4 9f R = n-C4H9 9g R = cyclohexyl 9h R = o-CH3C6H4 [84]
10a R = C6H5 10b R = p-CH3C6H4 10c R = p-CH3OC6H4 10d R = p-ClC6H4 10e R = p-BrC6H4 10f R = n-C4H9 [84] 11a R = H 11b R = CH3 11c R = F 11d R = Cl 11e R = NO2 11f R = OCH3 [84] 12 [84] 13 [84] [84]
15 peptoid derivative of DHAA [84]
16 peptoid derivative of DHAA [84]
17 Schiff base derivatives 17a R = H 17b R = OCH3 17c R = Cl 17d R = F [84] 18a R1 = α-OH, R2 = H 18b R1 = H, R2 = OH 18c R1 = α-OH, R2 = OH [84] [84]
[84] 21a R1 = H, R2 = OH 15S 21b R1 = H, R2 = OH 15R 21c R1 = ═O, R2 = H [84] 22 Podocarpic acid [84] 23a R1 = R2 = H, R3 = α-OH 23b R1 = ═O, R2 = R3 = H 23c R1 = R3 = H, R2 = β-OCOCH3 [84] 24a R1 = H, R2 = ═O 24b R1 = ═O, R2 = H 24c R1 = β-OH, R2 = H [84]
25a R1 = OH, R2 = H 25b R1 = H, R2 = OH [84] 26a R1 = CH3, R2 = ═O 26b R1 = CH3, R2 = H 26c R1 = CO2CH3, R2 = H [84]
27 phenol abietanes related to ferruginol [82]
28 phenol abietanes related to ferruginol [82]
29 phenol abietanes related to ferruginol [82]
Aromatic abietanes, which comprise the largest group of naturally occurring abietanes, are characterized by an aromatic ring C, as exemplified by dehydroabietic acid (2a, DHA) (Table 1). There are about two hundred compounds up to now that belong to this group of natural products, being commonly known as dehydroabietic acid derivatives or dehydroabietanes [80], [81]. While DHA (2a) possesses an equatorial carboxylic group (C-18), in other natural congeners the carboxylic group adopts the axial configuration (C-19),
namely in callitrisic acid (3), or it represents C-20, such as in pisiferic (4a) and carnosic acids (4b) (Table 1). The carboxyl function is lacking in ferruginol (5) (Table 1), an aromatic abietane first isolated from the resin of the Miro tree (Podocarpus ferrugineus) endemic to New Zealand. Other functionalities are often present in dehydroabietanes, such as hydroxyl and carbonyl groups, and in some compounds an endoperoxide moiety can also be found [25], [80], [81].
The main source of abietane diterpenoids is colophony, also known as rosin, which is the distillation residue of pine oleoresins [80]. Rosin is chiefly a mixture of resin acids mainly composed of abietic (7,13-abietadien-18-oic) acid (1a), its equilibrium isomers levopimaric (8(14),12-abietadien-18-oic), palustric (8,13-abietadien-18-oic) and neoabietic (8(14),13(15)-abietadien-18-oic) acids, and dehydroabietic (8,11,13-abietatrien-18-oic) acid (2a), as well as some other non-abietane diterpenoids, such as pimaric and isopimaric acids [80], [81]. Dehydroabietylamine (2b, DHAA) (Table 1), an abietane diterpenic amine derived from abietic acid (1a), is the main component of disproportionated rosin amine [82]. There are three major types of colophony depending on whether the source of the oleoresin is gum, wood or tall oil. Gum rosin is recovered from the sap of living pine trees, wood rosin is extracted from pine stumps and tall oil is obtained as a by-product of paper pulp production. However, the abietanes can also be found in extracts or resins of other conifers belonging to different families such as Cupressaceae, Araucariaceae, Podocarpaceae, Phyllocladaceae, and Pinaceae, and they also occur in several Angiosperm species, particularly in the families
Asteraceae, Celastraceae, Hydrocharitaceae, and Lamiaceae [25], [80], [81], [85].
The secretion of oleoresin by coniferous plants is an important defence mechanism against injury. Oleoresin is a complex mixture of terpenoids consisting of turpentine (volatile terpenes) and rosin (mainly resin acids). When the plant is wounded, oleoresin is exuded into the damaged site. Once at the surface, evaporation of the volatile turpentine leaves the diterpenoid resin acids that polymerize, forming a protective barrier that seals the wound while simultaneously trapping insect invaders and microbial pathogens [25], [80], [81], [85].
Pine resin, as whole or its derivatives, have been used since ancient times as expectorants, vesicants and rubefacients, and as antiseptics for the treatment of wounds, pyodermas and boils. Several of the rosin acids, including abietic (1a) and dehydroabietic (2a) acids, their salts and their amides with aminoacids are employed in cosmetics and dermatological preparations, mainly due to their surfactant and antimicrobial properties [67], [80], [85], [86].
Studies on rosin and the resin acids demonstrated their antibacterial effects, mainly against Gram-positive bacteria [80], [87], [88]. The abietic acids were stronger antibacterial agents than pimaric and labdane acids, and among the individual resin acids, DHA (2a) was generally the most potent. The growth inhibiting capacity of zinc oxide combined with Portuguese rosin, abietic acid (1a) or DHA (2a) led to synergistic antibacterial effects [89]. Recently, rosin proved to be an effective microbicidal against a wide range of microorganisms, including methicillin-sensitive S. aureus (MSSA), MRSA, Bacillus subtilis,
Escherichia coli, P. aeruginosa, and Candida albicans, in the European Pharmacopoeia
challenge test [90].
I.2.2 – Antimicrobial activity
Since ancient times several herbal compounds have been reported to possess antimicrobial activity. The antimicrobial activity of many abietane diterpenes have been tested against standard bacterial strains such as S. aureus, Staphylococcus epidermidis,
Enterococcus faecalis, B. subtilis, E. coli, K. pneumoniae and P. aeruginosa. In general,
abietane diterpenes have shown antibacterial activity mainly against S. aureus.
The commonly found horminone and 7α-acetoxyroyleanone demonstrated to be active against S. aureus, S. epidermidis and B. subtilis. Horminone showed a minimum inhibitory concentration (MIC) against S. epidermidis in a range of 1.5 to 31.25 µg/ml; against

![Fig. 1 – Sites of action and potential mechanisms of bacterial resistance to AMA [27]](https://thumb-eu.123doks.com/thumbv2/123dok_br/18091194.866367/34.892.154.755.180.605/fig-sites-action-potential-mechanisms-bacterial-resistance-ama.webp)
![Fig. 3 – Deaths due AMR by 2050. Adapted from [16].](https://thumb-eu.123doks.com/thumbv2/123dok_br/18091194.866367/40.892.168.728.309.725/fig-deaths-amr-adapted.webp)