compatible solutes of hyperthermophilic
microorganisms
Eva Correia Lourenço
Supervisor: Dr. Maria Rita Ventura
Co-supervisor: Professor Helena Santos
Dissertation presented to obtain the Ph.D in Biotechnology
Instituto de Tecnologia Química e Biológica António Xavier
Universidade Nova de Lisboa
To Doctor Maria Rita Ventura for believing in my scientific skills and giving me the opportunity to develop my scientific career. Her support, guidance and availability during these four years made this work possible.
To Professor Helena Santos for the important scientific contribution given throughout the PhD, assertiveness and critical analysis of my work, and for the prompt response to my questions.
To Professor Christopher Maycock for the enthusiasm in discussing science, and for always encouraging me to go forward.
To Dr. Sofia Miguel for all the discussions about our work, the friendship and support which greatly contributed to overcome less positive days.
To my colleagues from the Bioorganic Chemistry Lab and Organic Synthesis Lab, Paula Rodrigues, Vanessa Miranda, Saul Silva and Osvaldo Ascenso for the friendly and supportive environment.
To my colleagues in the Cell Physiology & NMR lab, especially Ana Mingote, Ana Esteves and Marta Rodrigues for always making me feel welcome and their patience for teaching me new techniques.
To the CERMAX for the use of the NMR facilities, especially to Helena Matias for her efforts to carry out the HR-MAS work.
To Fundação para a Ciência e a Tecnologia for the financial support provided by the Ph.D. grant, and Instituto de Tecnologia Química e Biológica António Xavier, for providing the conditions for conducting quality scientific work.
To the family I have chosen, my friends. To Aurélien and Vasco.
Acknowledgments ... III Table of Contents ... VII Abbreviations ... XV Abstract ... IXX Resumo ... XXV Chapter 1
Introduction ... 1
Hypersolutes ... 5
Carbohydrates Synthesis ... 8
References ... 12
Chapter 2 Stereoselective 1,2-cis Glycosylations - Towards the Synthesis of Protein Stabilisers ... 15
Abstract ... 19
Introduction ... 19
Results and Discussion ... 25
1,2-cis Stereoselective Glucosylations ... 25
1,2-cis Stereoselective Galactosylations ... 30
1,2-cis Stereoselective 2-Azido-2-DeoxyGlucosylations ... 41
Conclusion ... 47
Acknowledgements ... 49
References ... 49
Chapter 3 Synthesis of MGG: A Natural Compatible Solute ... 55
Abstract ... 59
Introduction ... 59
Results and Discussion ... 63
Solution-phase Synthesis ... 63
Solid-supported Synthesis ... 65
Conclusion ... 70
Acknowledgements ... 71
References ... 72
Abstract ... 79
Introduction ... 79
Results and Discussion ... 81
Chemical Synthesis ... 81
Performance of MGlyG as stabilizer for model enzymes ... 85
Conclusion ... 89
Acknowledgements ... 90
References ... 90
Chapter 5 Synthesis of a Solute Library and Assessment as Protein Thermostabilisers ... 93
Abstract ... 97
Introduction ... 97
Results and Discussion ... 100
Synthesis of the New Analogues of Compatible Solutes ... 100
Assessment of the ability of the new analogues as thermostabilisers ... 107
Conclusion ... 118
Acknowledgements ... 120
References ... 120
Chapter 6 Conclusion ... 123
Chapter 7 Experimental Procedures ... 129
Chemical Synthesis ... 133
Materials and Analysis ... 133
Solvent and Reagent Purification ... 133
Graphical Index of Compounds and Experiments ... 133
Experimental Procedures ... 150
Differential scanning fluorimetry ... 240
Materials ... 240
DSF assay ... 241
List of Figures:
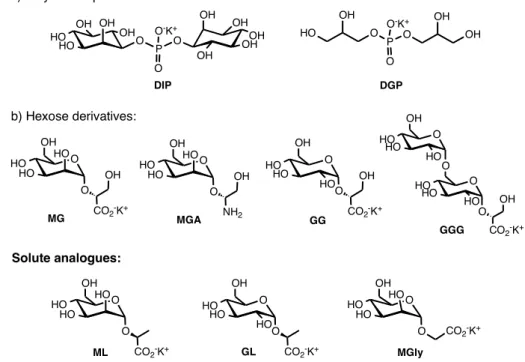
Figure 1. Examples of natural compatible solutes and analogues. ... 6
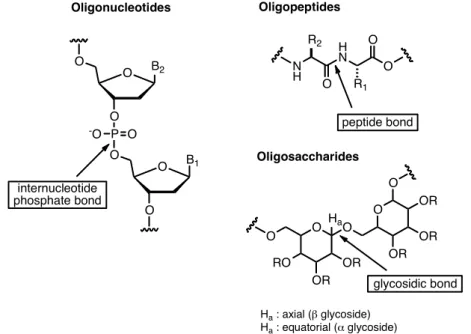
Figure 2. The three major classes of biooligomers. ... 9
Figure 3. Glycosyl acceptors. ... 26
Figure 4. Glycosyl acceptors. ... 31
Figure 5. MGG, 101. ... 60
Figure 6. MGlyG, 124. ... 79
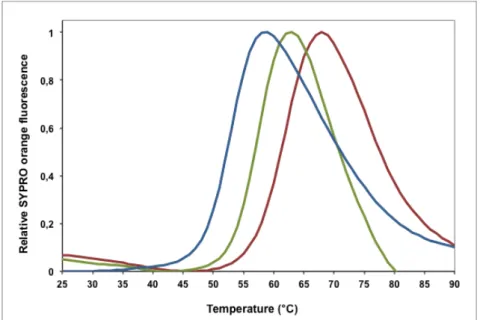
Figure 7. Curves obtained for the fluorescence data of SNase, comparing the stabilisation effect of different concentrations of MGlyG - 0.1 M (green) and 0.25 M (red) - with the control experiment (absence of solute (blue)). ... 86
Figure 8. Increment in the melting temperature (TM) of malate dehydrogenase (MDH, blue bars), staphylococcal nuclease (SNase, red bars) and lysozyme (green bars) in the presence of 0.25 M of different solutes. The melting temperature (TM) in the absence of solutes was 50 ºC for MDH, 52 ºC for SNase and 71ºC for lysozyme. ... 87
Figure 9. Dependece of MDH, SNase and lysozyme melting temperatures on the concentration of MGlyG. ... 88
Figure 10. Dependece of SNase melting temperature on the concentration of MGlyG (squares), GGG (triangles), MG (circles) and GG (diamonds). . 88
Figure 11. b-Galactopyranosyl-5-hydroxylysine (GalHI). ... 98
Figure 12. Di-N-acetyl-glucosamine phosphate (DAGAP). ... 99
Figure 13.Glycosyl acceptors. ... 99
Figure 14. Increment in the melting temperature (TM) of malate dehydrogenase (MDH, red bars), staphylococcal nuclease (SNase, green bars) and lysozyme (blue bars) in the presence of 0.5 M of different solutes. The melting temperature (TM) in the absence of solutes was 50 ºC for MDH, 52 ºC for SNase and 71ºC for lysozyme. ... 111
Figure 15. Stabilising effect of different compounds against thermal denaturation of malate dehydrogenase (MDH), staphylococcal nuclease (SNase) and lysozyme. In the abscissa axis the increment in the melting temperature of MDH induced by 0.5 M of several compounds, and in the ordinate axis the increment in the melting temperature of SNase (solid symbols) and lysozyme (open symbols) are plotted. ... 112
Figure 16. Stabilising effect of different glucose derivatives against thermal denaturation of malate dehydrogenase (MDH), staphylococcal nuclease (SNase) and lysozyme. In the abscissa axis the increment in the melting temperature of MDH induced by 0.5 M of several compounds, and in the ordinates axis the increment in the melting temperature of SNase (solid symbols) and lysozyme (open symbols) are plotted. ... 113 Figure 17. Stabilising effect of different galactose derivatives against thermal
temperature of MDH induced by 0.5 M of several compounds, and in the ordinates axis the increment in the melting temperature of SNase (solid
symbols) and lysozyme (open symbols) are plotted. ... 114
Figure 18. Stabilising effect of different lactate derivatives against thermal denaturation of malate dehydrogenase (MDH), staphylococcal nuclease (SNase) and lysozyme. In the abscissa axis the increment in the melting temperature of MDH induced by 0.5 M of several compounds, and in the ordinate axis the increment in the melting temperature of SNase (solid symbols) and lysozyme (open symbols) are plotted. ... 115
Figure 19 Stabilising effect of different malate derivatives against thermal denaturation of malate dehydrogenase (MDH), staphylococcal nuclease (SNase) and lysozyme. In the abscissa axis the increment in the melting temperature of MDH induced by 0.5 M of several compounds, and in the ordinate axis the increment in the melting temperature of SNase (solid symbols) and lysozyme (open symbols) are plotted. ... 115
Figure 20. Stabilising effect of different galactosyl glycerate derivatives against thermal denaturation of malate dehydrogenase (MDH), staphylococcal nuclease (SNase) and lysozyme. In the abscissa axis the increment in the melting temperature of MDH induced by 0.5 M of several compounds, and in the ordinate axis the increment in the melting temperature of SNase (solid symbols) and lysozyme (open symbols) are plotted. ... 116
Figure 21. Dependence of SNase melting temperature on the concentration of solutes. ... 117
Figure 22. Dependence of MDH melting temperature on the concentration of solutes. ... 118
Figure 23. Dependence of lysozyme melting temperature on the concentration of solutes. ... 118
List of Schemes: Scheme 1. Glycosylation reaction. ... 10
Scheme 2. Full spectrum of mechanisms from SN2 all the way to pure SN1. 2 20 Scheme 3. Glycosylation Reaction: a) C-2 Group Participation – Disarmed donor; b) C-2 Non-participative group - Armed donor. ... 21
Scheme 4. Glycosylation reaction scheme according to Crich.15 ... 23
Scheme 5. Solvent coordination to the glycosyl donor. ... 24
Scheme 6. Synthesis of thioglucosyl donor 3. ... 26
Scheme 7. Synthesis of methyl (2R)-O-tert -butyldimethylsilyl-2,3-dihydroxipropanoate 8. ... 30
Scheme 8.Synthesis of thiogalactosyl donors 19 and 21. ... 31
Scheme 9. Synthesis of super armed thiogalactosyl donor 50. ... 36
Scheme 10. Glycosylation reaction of super armed thiogalactosyl donor 50. 36 Scheme 11. Synthesis of thiogalactosyl donors 57 and 58. ... 38
Scheme 13. Solid support synthetic strategies (X, Y: leaving group). ... 61
Scheme 14. Glycosylation reactions for the solution phase synthesis of MGG, 105. ... 63
Scheme 15. Deprotection strategy to obtain MGG, 101. ... 64
Scheme 16. Synthesis of thioglucoside 111. ... 66
Scheme 17. Optimisation for the solid supported synthesis of MGG. ... 67
Scheme 18. Deprotection strategy for the synthesis of MGG, 101. ... 68
Scheme 19. Solid supported synthesis of MGG. ... 69
Scheme 20. Synthesis of partially protected MG, 126. ... 81
Scheme 21. Glycosylation of acceptor 126 with thioglucoside donor 3. ... 83
Scheme 22. Deprotection scheme for the synthesis of MGlyG, 124. ... 85
Scheme 23. General deprotection scheme for the glucose and galactose analogues. ... 102
Scheme 24. General deprotection scheme for the mannose analogues. .... 105
Scheme 25. Hydrogenation of the 2-azido-2-deoxyglucoside derivatives. .. 107
Scheme 26. Synthesis of the N-acetyl glucosamine derivatives. ... 107
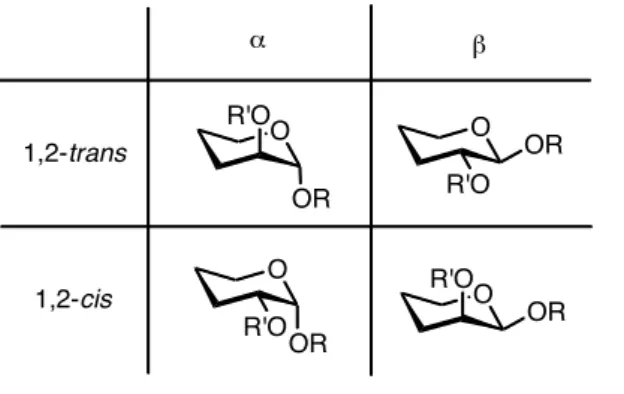
List of Tables: Table 1. Stereochemistry of the anomeric bond. ... 10
Table 2. Effect of solvent and temperature on the stereoselectivity of the glycosylation of donors 1 and 3. ... 27
Table 3. Effect of solvent and temperature on the stereoselectivity of the glycosylation of donor 19 and 21. ... 32
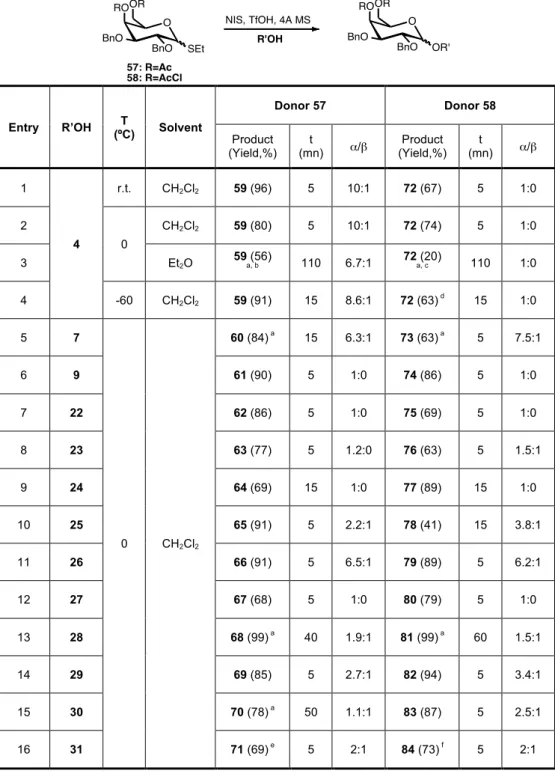
Table 4. Effect of solvent and temperature on the stereoselectivity of the glycosylation of donor 57 and 58. ... 39
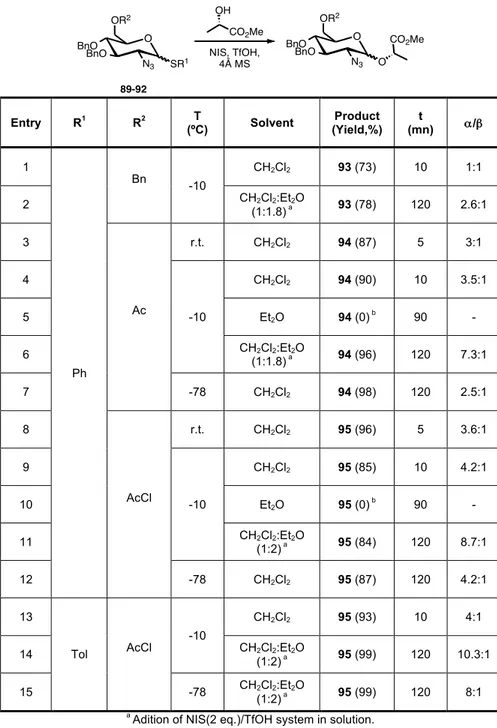
Table 5. Effect of solvent, temperature and protecting groups on the stereoselectivity of the glycosylation of donors 89-92. ... 44
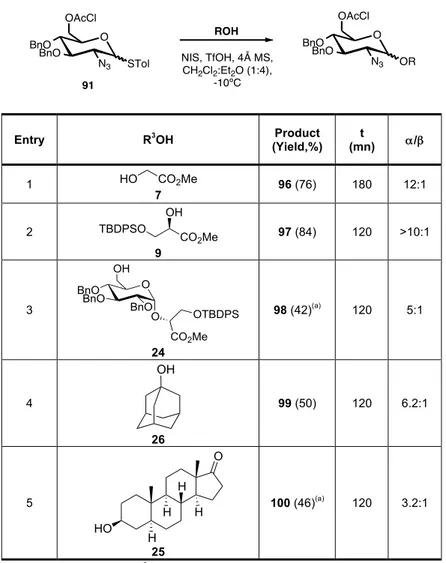
Table 6. Glycosylation of donor 91 with different acceptors. ... 46
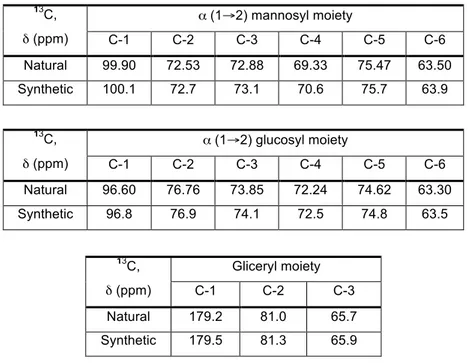
Table 7. Comparison of 13C NMR chemical shifts for the synthetic potassium salt of MGG 101 with data from the natural product1. ... 65
Table 8. Optimisation of the glycosylation reaction of acceptor 127. ... 82
Table 9. Glycosylation of donor 1 with different acceptors. ... 84
Table 10. Results obtained for the glycosylation reaction with the thioglycoside donors 1 and 19. ... 100
Table 11. Final products and overall yieldsa for glucose and galactose derivatives. ... 102
Table 12. Results obtained for the glycosylation reaction with the mannose trichloroacetimidate donor 104. ... 104
Table 13. Final products and overall yieldsa for mannose derivatives. ... 105
Table 14. Results obtained for the glycosylation reaction with the 2-azido-2-deoxythioglucoside donor 90. ... 106
Ac Acetate
Ac2O Acetic anhydride
AcOH Acetic acid
BnBr Benzyl bromide
CPME Cyclopentyl methyl ether DIC N,N'-diisopropylcarbodiimide DIP di-myo-inositol phosphate
DGP di-Glycerol phosphate
DMAP 4-Dimethylaminopyridine
DMF Dimethylformamide
DSF Differential Scanning Fluorimetry
Et Ethyl
Et2O Diethyl ether
EtOAc Ethyl acetate
GG α-D-Glucosyl-D-glycerate
GGG α-D-glucopyranosyl-(1→6)-α-D-glucopyranosyl-(1→2)-D -glycerate
GL α-D-Glucosyl-S-lactate
Hex Hexane
HOBt Hydroxybenzotriazole
HR-MS High Resolution Mass Spectrometry HR-MAS NMR High Resolution Magic Angle Spining NMR HSQC Heteronuclear Single Quantum Correlation NMR
iPr2NEt N,N-Diisopropylethylamine
MDH Malate dehydrogenase
Me Methyl
MeOH Methanol
MG α-D-Mannosyl-D-glycerate MGA α-D-Mannosyl-D-glyceramide
MGG α-D-Mannopyranosyl-(1→2)-α-D-glucopyranosyl-(1→
2)-D-glycerate
MGly α-D-Mannosyl-glycolate
-glucopyranosyl)-glycerate ML α-D-Mannosyl-S-lactate
NaOMe Sodium methoxide
NIS N-Iodosuccinimide
NMR Nuclear magnetic resonance
Ph Phenyl
TLC Thin layer chromatography SNase Staphylococcal nuclease TBAF Tetra-n-butylammonium fluoride TBDPSCl tert-Butylchlorodiphenylsilane TBDMS tert-Butyldimethylsilane TFA Trifluoroacetic acid
TfOH Trifluoromethanesulfonic acid
THF Tetrahydrofuran
In response to osmotic or heat stress, marine hyperthermophiles (thriving optimally at or above 80ºC) accumulate ionic compatible solutes such as α -D-mannosyl-D-glycerate (MG). It has been proposed that these hypersolutes stabilise intracellular components, such as proteins and enzymes, allowing them to withstand high growth temperatures. Actually, the efficacy of these solutes in the stabilisation of a number of model proteins has been demonstrated in vitro. Since several human pathologies such as Alzheimer’s, Creutzfeldt-Jacob’s, cystic fibrosis and Parkinson’s diseases have been associated with the structural instability of proteins, and consequent protein aggregation, the development of reliable strategies to improve protein stability is of great importance and could lead to several pharmaceutical and biotechnological applications.
The main goals of this doctoral work were the development of strategies for the synthesis of newly isolated natural solutes, such as α -D-mannopyranosyl-(1→2)-α-D-glucopyranosyl-(1→2)-D-glycerate (MGG) and (2R)-2-(1-α-D -mannopyranosyl)-3-(1-O-α-D-glucopyranosyl)-glycerate (MGlyG), and the construction of a solute library inspired by the structure of natural hypersolutes, mainly MG.
1,2-cis glycosides. In the case of galactose this effect could be enhanced by the presence of an additional ester group at the C-4 position of the donor. In the present work, the natural MGG was efficiently synthesised in solution and on solid support in a stereocontrolled manner. Different strategies using two different thioglucosides bearing a free C-2 hydroxyl group were designed and tested in solution. These thioglucosides were used as glycosyl acceptors in the first glycosylation reaction with the tetraacetylmannosyl trichloroacetimidate, and as donors in the second glycosylation reaction with the glycerate acceptor. The glycosylation reactions with both thioglucosides were highly stereoselective affording exclusively the α-anomer. For the solid supported synthesis, a thioglucoside was efficiently immobilized on the solid support (Tentagel MB-NH2) by the C-6 hydroxyl group using a succinate linker, and successfully used in the synthesis of MGG. The stereoselectivity of the glycosylation reactions was reproducible on solid support, affording the desired α-product. Characterisation of the products while attached to the solid support was performed using HR-MAS NMR.
A synthetic strategy for the synthesis of the recently isolated MGlyG was successfully developed based on the glycosylation reaction of ethyl 6-O-acetyl-2,3,4-tri-O-benzyl-1-thio-α/β-D-glucopyranoside donor with a partially protected mannosyl glycerate acceptor. Optimisation studies were performed changing the solvent and temperature for these reaction in order to improve the 1,2-cis stereoselectivity. The effectiveness of MGlyG for the protection of model enzymes against heat induced inactivation was evaluated, using differential scanning fluorimetry (DSF). For comparison, the protection induced by natural compatible solutes, like MG, GG or GGG, was assessed by the same method. The results demonstrated that for the studied enzymes (malate dehydrogenase, staphylococcal nuclease and lysozyme) MGlyG was the best stabiliser, and that the extent of protein stabilisation rendered by the solute depends on the specific solute/enzyme examined.
mannose and glucosamine have been synthesised and their thermostabilisation properties assessed using DSF. The comparative analysis of the results obtained reinforces the idea of the importance of charge for the stabilisation effect, and that the extent of stabilisation is determined by specific interactions between solutes and proteins. Furthermore, the importance of the nature of the sugar and the non-glycosidic moiety of the molecule for the stabilisation effect were studied, and results suggested that the structure of the non-glycosidic moiety had more influence on the stabilisation effect than the nature of the hexose.
Hipertermófilos marinhos capazes de proliferar optimamente acima dos 80ºC acumulam solutos compatíveis iónicos (hipersolutos), tais como o α -D-manosil-D-glycerato (MG), como resposta a condições de stress osmótico e de temperatura. Dada a capacidade destes hipersolutos de estabilizarem componentes intracelulares, nomeadamente proteínas, foi proposta a acumulação destes compostos intracelularmente como estratégia destes organismos para suportar temperaturas elevadas. Para além disso, testes in vitro demonstraram a capacidade estabilizadora destes solutos numa variedade de proteínas modelo. Uma vez que a instabilidade estrutural das proteínas e a sua consequente agregação tem sido associada a uma série de doenças humanas, tais como a Alzheimer, a doença Creutzfeldt-Jacob, a fibrose quística e a doença de Parkinson, o desenvolvimento de estratégias que visam o aumento da estabilidade das proteínas é de grande interesse e poderá ter inúmeras aplicações farmacêuticas e biotecnológicas.
Os principais objectivos desta tese de doutoramento foram o desenvolvimento de estratégias para a síntese de compostos naturais recentemente isolados, tais como o α-D-manosil-(1→2)-α-D-glucosil-(1→2)-D -glicerato (MGG) e (2R)-2-(1-O-α-D-manosil)-3-(1-O-α-D-glucosil)-glicerato (MGlyG), e a construção de uma biblioteca de compostos baseada na estrutura de solutos produzidos naturalmente pelos hipertermófilos, nomeadamente o MG.
na posição C-6 dos dadores têm uma influência directa na selectividade anomérica da reacção, favorecendo a formação de 1,2-cis glicósidos. No caso da galactose, este efeito pode ser acentuado pela presença adicional de um grupo éster na posição C-4 do dador glicosídico.
No presente trabalho, o soluto natural MGG foi eficientemente e esterosselectivamente sintetizado em solução e em suporte sólido. Foram desenvolvidas e testadas duas vias de síntese em solução utilizando dois dadores tioglucosídicos contendo o grupo hidroxilo do C-2 livre. Estes tioglucósidos foram utilizados como aceitadores numa primeira glicosilação com o dador tetraacetilmanosil tricloroacetamidato, e posteriormente como dadores na reacção de glicosilação com o glicerato como aceitador. As reacções de glicosilação com ambos os tioglucósidos foram estereosselectivas, tendo-se formado exclusivamente o anómero α. A síntese em suporte sólido foi conseguida com sucesso através da imobilização do tioglucósido na resina Tentagel MB-NH2 através do C-6 e utilizando como
“linker” o succinato. A estereosselectividade das reacções em solução foi reproductível em suporte sólido, tendo-se obtido exclusivamente o produto α
pretendido. A caracterização dos compostos em suporte sólido foi realizada através de RMN HR-MAS.
Chapter 1
Introduction
Introduction
Hypersolutes ... 5 Carbohydrates Synthesis ... 8 References ... 12
Hypersolutes
Halotolerant and moderately halophilic microorganisms accumulate compatible solutes in the cytoplasm to face fluctuations in the osmotic pressure of their environment.1 Compatible solutes are low molecular weight and highly soluble organic compounds, namely amino acids and derivatives, sugars and polyols that are able to protect cell structures under stress conditions and do not interfere with normal cell function.
In response to osmotic or heat stress, marine hyperthermophiles (microorganisms thriving optimally at or above 80ºC) use the same strategy and accumulate ionic compatible solutes also known as hypersolutes.1 In contrast with neutral or zwitterionic compounds, more commonly found in mesophiles, hypersolutes are in general negatively charged and most fall into two categories: hexose derivatives, like α-D-mannosyl-D-glycerate (MG) and polyol-phosphodiesters such as di-myo-inositol phosphate (DIP) (Figure 1).1 Although the preference for the accumulation of negatively charged compounds is still unclear, in vitro studies have proved not only the efficacy of these solutes in the stabilisation of a number of model proteins, but also that charged compounds rendered better protection against aggregation, unfolding and inactivation of proteins than neutral compounds.2-5 The increase in the concentration of these solutes not only with the increase of the osmotic pressure of the medium but also with the growth temperature led to the proposal that they would play a role in thermoadaptation by stabilising intracellular components, such as proteins and enzymes, allowing them to withstand high growth temperatures.1, 6
-glucopyranosyl-(1→2)-D-glucopyranosyl-(1→2)-D-glycerate (GGG) (Figure 1) with a more complex structure, have been synthesised.7
O HO HO HO OH O
CO2-K+ OH O HO HO HO OH O
CO2-K+ OH MG GG HO HO O O HO O
CO2-K+ OH O HO HO HO OH GGG HO HO OH OH O O P O O-K+
OH OH OH OH OH OH DIP O HO HO HO OH O
CO2-K+
O HO HO HO OH O
CO2-K+
ML GL O HO HO HO OH
O CO2-K+
MGly HO OH O O OH OH P O O-K+
DGP a) Polyol-Phosphodiesters:
b) Hexose derivatives:
Solute analogues: O HO HO HO OH O NH2 OH MGA Natural Solutes:
Figure 1. Examples of natural compatible solutes and analogues.
shown that, in the presence of the solute, the protein backbone motions were restricted in a concentration-dependent manner.10 Also, the fact that different solutes stabilise proteins to different extents suggests the presence of weak interactions between the solutes and the protein surface9, without interfering with the biological function of the protein.
The success of many industrial and pharmaceutical processes is highly dependent on the preservation of the native structure and activity of enzymes and other proteins. Moreover, since several afflicting human pathologies have been associated with structural instability of proteins, and consequent protein aggregation, like Alzheimer’s, Creutzfeldt-Jacob’s, Huntington’s, cystic fibrosis and Parkinson’s, the development of reliable strategies to improve protein stability is of great importance.
applications as stabilising agent of biomaterials, which have been disclosed in a number of patent applications18-21.
Recently in another study3, several molecules chemically related to MG were synthesised and in some cases (like α-D-mannosyl-(S)-lactate (ML), Figure 1) showed a superior ability to protect model enzymes against heat-induced denaturation, aggregation and inactivation. This finding showed that an apparently small structural change, the glycerate moiety being replaced by a lactate, had positive effect in the protein thermostabilisation and has stimulated the production of new synthetic analogues. The possibilities of solute engineering having MG as the reference compound are very large, so in this work a solute library was constructed, and the diversity of these analogue structures was introduced in three levels: the nature of the sugar (mannose, glucose, galactose and glucosamine), both anomers for each sugar and the glycosyl acceptor (like, for example methyl (2R)-D-glycerate, methyl (S)-lactate, glycerol, methyl glycolate, methyl butyrate or dimethyl malate) used in the glycosylation reaction. The solute library provided a wide range of structurally different sugar derivatives that could give insight into the key features necessary for protein stabilization, and synthetic analogues with improved stabilisation properties.
Carbohydrates Synthesis
besides the sequence of the monomeric structures, other aspects such as the functional groups and their stereochemistry, the conformation of the sugars and the stereoselective formation of glycosidic linkages must be considered. In order to perform biophysical and biochemical studies of biologically active carbohydrates to understand their biological function, sufficient quantities of defined oligosaccharides are required and which are difficult to obtain from their natural sources due to their microheterogeneity and low concentrations and low biomass yields. The chemical synthesis of oligosaccharides for biological studies is a powerful tool to provide pure, structurally defined and homogeneous samples in substantial quantity, and simultaneously allows for synthesising several structures including non-natural ones.
B2
O O
O P O
-O
B1
O O
O Oligonucleotides
N H
H N
O R2
O O
R1
Oligopeptides
internucleotide phosphate bond
peptide bond
O
OR OR RO
O O
O
OR OR OR O
Ha
Ha : axial (β glycoside)
Ha : equatorial (α glycoside)
glycosidic bond Oligosaccharides
Figure 2. The three major classes of biooligomers.
The structural complexity of carbohydrates can be translated in two major chemical challenges: the multitude of functional groups with similar reactivity that need to be distinguished, and the creation of a new stereogenic center when the glycosidic bond is formed.
reaction plays a central role in carbohydrates chemistry. The glycosidic bond is formed by a nucleophilic attack by an alcohol (R’OH), or by the hydroxyl group of a partially protected sugar moiety – the glycosyl acceptor - to the anomeric center of the glycosyl donor often through a unimolecular SN1
mechanism. The reaction is generally performed in the presence of an activator called promoter (E) that assists the departure of the leaving group (X) and in some cases, other additives such as molecular sieves or any base that may act as acid scavenger are used (Scheme 1).22
O
X
RO RO O
E+ R'OH
O
OR' RO
Scheme 1. Glycosylation reaction.
Despite the glycosylation reaction being known for centuries and the existence of many glycosylation methods available for glycosidic bond formation, none of them is general, as there are many factors to take in consideration. Although 1,2-trans glycosides (Table 1) can be stereoselectively prepared in the classical manner by using the C-2 acyl neighbouring group participation, the stereoselective formation of 1,2-cis
glycosides (Table 1), like α-glucopyranosides, α-galactopyranosides and β -mannopyranosides, still represents a challenge and proceeds with poorer stereocontrol, which results in mixtures of diastereomers.
Table 1. Stereochemistry of the anomeric bond.
O R'O
OR
O R'O
OR O
R'O OR
O
R'O OR 1,2-trans
1,2-cis
For example, this can be easily illustrated by comparing the selectivities obtained for the synthesis of the 1,2-cis glucosides, GG (>10:1 1,2-cis
/1,2-trans isomers) and GL (4:1 1,2-cis/1,2-trans isomers), which are difficult to attain in a stereocontrolled manner, with the selectivities obtained for the synthesis of the 1,2-trans mannosides, MG and ML (1:0 1,2-trans/1,2-cis
isomers).3, 7
Many factors, such as the nature of the leaving and protective groups on the donor, the steric hindrance of the glycosyl acceptor, the reaction solvent, temperature, and the promoting system can influence the outcome of the reaction and should be taken into account.22 So improved and more general methods for the control of the 1,2-cis stereoselectivity of the glycosylation reaction are needed.
When planning a synthetic strategy for carbohydrates synthesis another crucial task is the preparation of properly functionalized building blocks and deprotection strategies. This involves the use of multistep transformations for differentiation of functional groups (amino and hydroxyl) of similar reactivity and, in most cases, purification by chromatography is needed after each step. In order to simplify the labour-intensive, time consuming and expensive solution phase synthesis of these compounds, many research efforts have been directed towards the development of efficient strategies and methodologies for solid phase synthesis.
Using solid supports for the synthesis of compatible solutes will allow a more efficient preparation of these compounds in a relatively short time, essentially by using well established solution phase reactions and taking advantage of the main feature of solid supports - the purification step, which can be accomplished by a simple filtration, allowing the use of excess reagents to drive reactions to completion.
The development of improved and more efficient chemical synthesis of these compounds is crucial for the expansion of the industrial applications of hypersolutes.
References
1. Costa, M. S.; Santos, H.; Galinski, E. A., An overview of the role and diversity of compatible solutes in Bacteria and Archaea. In Biotechnology of Extremophiles, Antranikian, G., Ed. Springer Berlin Heidelberg: 1998; Vol. 61, pp 117-153.
2. Ramos, A.; Raven, N. D. H.; Sharp, R. J.; Bartolucci, S.; Rossi, M.; Cannio, R.; Lebbink, J.; vanderOost, J.; deVos, W. M.; Santos, H., Stabilization of enzymes against thermal stress and freeze-drying by mannosylglycerate. Applied and Environmental Microbiology 1997, 63, 4020-4025.
3. Faria, T. Q.; Mingote, A.; Siopa, F.; Ventura, R.; Maycock, C.; Santos, H., Design of new enzyme stabilizers inspired by glycosides of hyperthermophilic microorganisms. Carbohydrate Research 2008, 343, 3025-3033.
4. Borges, N.; Ramos, A.; Raven, N. D. H.; Sharp, R. J.; Santos, H., Comparative study of the thermostabilizing properties of mannosylglycerate and other compatible solutes on model enzymes. Extremophiles 2002, 6, 209-216.
solute from the hyperthermophile Archaeoglobus fulgidus. Applied and Environmental Microbiology 2000, 66, 1974-1979.
6. Santos, H.; da Costa, M. S., Compatible solutes of organisms that live in hot saline environments. Environmental Microbiology 2002, 4, 501-509. 7. Lourenco, E. C.; Maycock, C. D.; Ventura, M. R., Synthesis of potassium (2R)-2-O-alpha-D-glucopyranosyl-(1 -> 6)-alpha-D -glucopyranosyl-2,3-dihydroxypropanoate a natural compatible solute. Carbohydrate Research
2009, 344, 2073-2078.
8. Lentzen, G.; Schwarz, T., Extremolytes: natural compounds from extremophiles for versatile applications. Applied Microbiology and Biotechnology 2006, 72, 623-634.
9. Pais, T. M.; Lamosa, P.; dos Santos, W.; LeGall, J.; Turner, D. L.; Santos, H., Structural determinants of protein stabilization by solutes - The importance of the hairpin loop in rubredoxins. Febs Journal 2005, 272, 999-1011.
10. Pais, T. M.; Lamosa, P.; Garcia-Moreno, B.; Turner, D. L.; Santos, H., Relationship between protein stabilization and protein rigidification induced by mannosylglycerate. Journal of Molecular Biology 2009, 394, 237-250.
11. Ohtake, S.; Wang, Y. J., Trehalose: Current use and future applications. Journal of Pharmaceutical Sciences 2011, 100, 2020-2053. 12. Luley-Goedl, C.; Nidetzky, B., Glycosides as compatible solutes: biosynthesis and applications. Natural Product Reports 2011, 28, 875-896. 13. Mascellani, N.; Liu, X. P.; Rossi, S.; Marchesini, J.; Valentini, D.; Arcelli, D.; Taccioli, C.; Citterich, M. H.; Liu, C. G.; Evangelisti, R.; Russo, G.; Santos, J. M.; Croce, C. M.; Volinia, S., Compatible solutes from hyperthermophiles improve the quality of DNA microarrays. Bmc Biotechnology 2007, 7.
16. Jorge, C. D.; Ventura, R.; Maycock, C.; Outeiro, T. F.; Santos, H.; Costa, J., Assessment of the efficacy of solutes from extremophiles on protein aggregation in cell models of Huntington's and Parkinson's diseases.
Neurochemical Research 2011, 36, 1005-1011.
17. Cruz, P. E.; Silva, A. C.; Roldao, A.; Carmo, M.; Carrondo, M. J. T.; Alves, P. M., Screening of novel excipients for improving the stability of retroviral and adenoviral vectors. Biotechnology Progress 2006, 22, 568-576. 18. Santos, H. R., A.; da Costa, M. S. Utilização de manosilglicerato na termoestabilização, osmoprotecção e protecção contra a desidratação de componentes celulares e células. INPI (Instituto Nacional de Propriedade Industrial) - Invention patent, submited in June 1996.
19. Santos, H. R., A.; da Costa, M. S. Thermostabilization, osmoprotection, and protection against desiccation of enzymes, cell components, and cells by mannosylglycerate. European patent submitted in 14/03/97 with nº 97670002.1 and published in 07/01/98 Bulletin 1998/02. 20. Lamosa, P. F., T. Q.; Ventura, M. R.; Maycock, C. D.; Santos, H. Derivados sintéticos de manosil-glicerato para a estabilização e/ou preservação de biomateriais. Patent: PAT 103442 P; 24/02/2006.
21. Santos, H. d. C., M. S.; Empadinhas, N.; Albuquerque, L. Identification of a bifunctional gene for mannosylglycerate synthesis and development of a high-scale-production heterologous system based on Saccharomyces cerevisiae. International patent submitted in 17/10/2003, 2003 (process nº 3398007Y).
22. Demchenko, A. V., Handbook of Chemical Glycosylation. Wiley: 2008.
Chapter 2
Stereoselective 1,2-cis
Glycosylations -
Towards
the
Synthesis
of
Protein
Stabilisers
This chapter contains data published in:
Eva C. Lourenço, M. Rita Ventura; The synthesis of compatible solute analogues - solvent effects on selective glycosylation. Carbohydrates Research2011, 346, 163-168.
Eva C. Lourenço, M. Rita Ventura; The effect of electron withdrawing protecting groups at positions 4 and 6 on 1,2-cis galactosylation. Tetrahedron
Stereoselective 1,2-cis Glycosylations - Towards the Synthesis of Protein Stabilisers
Abstract ... 19
Introduction ... 19
Results and Discussion ... 25 1,2-cis Stereoselective Glucosylations ... 25 1,2-cis Stereoselective Galactosylations ... 30 1,2-cis Stereoselective 2-Azido-2-DeoxyGlucosylations ... 41
Conclusion ... 47
Acknowledgements ... 49
References ... 49
Abstract
Thioglycosides are extremely versatile and have been widely employed in the synthesis of complex carbohydrates and carbohydrate libraries. Since obtaining high stereoselectivity in 1,2-cis glycosylation reactions is challenging in this chapter the use of thioglycosides as donors in this reaction was explored. In order to improve the α anomeric selectivity of the glycosylation reaction different thioglucosides, thiogalactosides and 2-azido-2-deoxythioglucosides were prepared as donors and the NIS/TfOH system used as the promoter. The influence of the nature of the protective groups on different positions of the donor, solvent and temperature in this reaction was studied using a wide range of acceptors (from unhindered primary hydroxyl groups to other sugars). Studies showed that the α-stereoselectivity of thioglucosides and 2-azido-2-deoxythioglucosides could be improved by the use of an electron-withdrawing group (acetyl or acyl) at the C-6 position of these donors, and in the case of thiogalactosides by the use of an acyl group both at the C-4 and C-6 position. In general, it was observed that donors possessing stronger electron-withdrawing groups at these positions have a higher α-directing effect. Glycosylations of the different thioglycosides with several acceptors showed that careful optimisation of the solvent system and temperature is a powerful way to obtain high yields and α-stereoselectivity. These glycosides are key intermediates for the synthesis of new analogues of compatible solutes.
Introduction
be considered simply in terms of pure unimolecular (SN1) or pure bimolecular
mechanism (SN2), and was described by Crich 2
as “continuum of mechanisms” ranging between the pure SN2 mechanism at the one extreme
and the SN1 mechanism with free oxocarbenium ions at the other (Scheme 2).
Where according to a given donor-acceptor pair, the reaction conditions may favour either a bimolecular or a monomolecular rate-determining step (Scheme 2).2
O X
PO PO O X
O X'
PO PO O X' O
OR
PO O
OR PO
O PO
X
-E+
Activation Activation
ROH ROH
ROH ROH
E+
activated donor
activated donor
β-glycoside α-glycoside
oxocarbenium ion
β-donor
α-donor
SN1 SN1
SN2 SN2
Scheme 2. Full spectrum of mechanisms from SN2 all the way to pure SN1. 2
When considering that the new glycosidic linkage creates a chirality center, many factors influence the stereochemical outcome of this reaction, like:
• the nature of the protecting groups, specially the group bound to the C-2 of the sugar ring;
• the reactivity of the anomeric centre of the glycosyl donor, the acceptor and the promoter;
• the solvent;
• the steric hindrance of the acceptor;
• the temperature at which the reaction takes place;
need to be taken into account when designing the glycosidic donors to accomplish the desired product.
and thereby the rate of attack of the anomeric group on the electrophilic promoter species. And they determine the stability of the partial positive charge on the anomeric center, which develops upon expulsion of the anomeric leaving group. A glycoside donor is deactivated by electron-withdrawing protecting groups (such as acyl functions) and termed “disarmed”, since they decrease the electron density, therefor, the nucleophilicity of the anomeric function and destabilise the oxocarbenium ion transition state.3 Whereas electron releasing protective groups (such as aryl or alkyl functions) are more reactive and described “armed”.
The protecting group on C-2, adjacent to the anomeric center, has the highest impact in the outcome of the glycosylation reaction as it can participate by formation of an intermediate if it is an ester (like acetyl, benzoyl, 2-phthalimido, Scheme 3. a), or not if it is an ether (Scheme 3. b).1
O OR O R O O OR O R O R'OH O OR O R O OR' O RO R'OH O RO OR' b O RO OR'
R: alkyl, aryl. X: Leaving Group. E+: Promoter. a a b O RO X E+ O RO X E+
1,2-trans glycosides
1,2-cis glycosides 1,2-trans glycosides a) b) RO RO RO RO RCOO
Scheme 3. Glycosylation Reaction: a) C-2 Group Participation – Disarmed donor; b) C-2 Non-participative Group - Armed donor.
The most commonly applied nonparticipating groups are benzyl for neutral sugars and azide for 2-amino-2-deoxy sugars, but other protective groups have also been used. In this case, since the nucleophilic attack would be almost equally possible from either the upper (β product) or the bottom face (α
(Scheme 3. b)). This is why the formation of 1,2-trans glycosides can easily be accomplished by neighbouring group participation of the 2-acyl group, while 1,2-cis glycosides it is far more complicated.
Even though the effect of substituents remote to the anomeric center is less important for the stereochemical outcome of the glycosylation reaction than those at C-2, their significant contribution needs to be addressed.1 Several reports have been speculating about remote group participation by esters from C-34-7, C-45, 6, 8-11 (axial and equatorial) and C-64, 9, 10 positions. These assumptions are mostly based on the observation of changes in stereoselectivity when esters replace ethers at those positions. Distinguish between the electron-withdrawing effect from the effect of remote participation of the protecting groups on the outcome of the reaction is a controversial and a non-unanimous subject.2, 4, 11, 12
In a recent study, several molecules chemically related to MG were synthesised and in some cases showed a superior ability to protect model enzymes against heat-induced denaturation, aggregation and inactivation.13 This finding has stimulated the production of new synthetic analogues from different hexoses, such as mannose, glucose, galactose and glucosamine. In this work, although it would be interesting to test both anomers of each solute analogue, since in nature these compounds are present in the α-form the main challenge would be the stereoselective synthesis of the α-glucosides, the α-galactosides and the α-glucosamine analogues.
Despite the several methods available for the formation of 1,2-cis glycosides none of them is general and when applied to different acceptors the stereoselectivity is not predictable in many cases. Lemieux14 and Crich15 have both developed methods using “armed” donors - benzyl ethers - bearing non-participative groups on C-2 and obtained preferentially the 1,2-cis
isomers. Crich’s method15 is based on the use of thioglucosides that upon treatment and activation with triflic anhydride lead to the formation of triflate intermediates (Scheme 4). He proposed the existence of a system where the
substitution. This leads to the formation of the α-glucoside and directs the system equilibrium in that direction. Crich also found that size and steric hindrance of the acceptor had a great influence in this reaction. With smaller and less hindered glycosyl acceptors, like methanol, a α/βmixture is obtained, which can be explained by the fast direct displacement of the α -triflate (more stable) that directs the system equilibrium in that direction.
O O
BnO O
SEt BnO Ph
O O
BnO O
OTf BnO Ph
O O
BnO O
OTf BnO Ph
O O
BnO O
OR BnO Ph
O O
BnO O
OR BnO Ph
ROH ROH
Promoter, TfO
-Scheme 4. Glycosylation reaction scheme according to Crich.15
thioglucoside 1 was used for the synthesis of the GG analogue, GL, under the same conditions with a less bulky, more reactive acceptor, like (S)-lactate, a
α/β ratio of 4:1 was obtained.13 Reactive alcohols are stronger nucleophiles and react faster, which turns difficult to control the selectivity and tend to give lower 1,2-cis stereoselectivity.1
The importance of solvents for the stereochemical outcome of the glycosylation reaction is well known in literature.1 In general, polar solvents have a tendency to direct the glycosylation in an β-selective way while non-polar solvents, like dichloromethane or toluene, increase the proportion of α -glycoside. Moreover there are other contributions that can be manipulated in order to favour the synthesis of the desired products, like the participating properties of some ethereal and nitrile solvents. In the case of diethyl ether, a
β−diethyl oxonium-ion intermediate may be formed due to steric reasons, which after nucleophilic displacement with inversion of configuration would give α-product (Scheme 5).1 When using nitrile solvents, on contrary, the nitrilium cation formed in situ adopts axial orientation, leading towards the formation of the β-glycosides (Scheme 5).1
O RO
O
RO O
O RO
OR' R'OH
Et2O
MeCN
O RO
N
O
RO OR'
R'OH
Scheme 5. Solvent coordination to the glycosyl donor.
Boons and co-workers19 studied the α-directing effect of ethers as solvents in the glycosylation of a armed tetrabenzylated β-thioglucoside, and found that by performing the reaction in a mixture of toluene:1,4-dioxane obtained higher
toluene than in dichloromethane, were worst than with 1,4-dioxane. Dichloromethane was the worst solvent affording more β-anomer (α/β 0.7:1) and THF was not used in this study.
Bonnaffé and co-workers20 performed an optimisation study of the solvent system for the α-selective glycosylation reaction of a 2-azido trichloroacetimidate disaccharide derived from glucose. Ethyl ether and dichloromethane showed the worst results both in terms of diastereoselectivity (α/β 70:30) and yield (<74%). They found that THF enhanced the α -stereoselectivity (α/β 93:7) and lowered the yield (66%) of the reaction, but when used as mixture THF/Et2O (9:1) they obtained the best results (α/β92:8,
90%).
In another study, Ito and co-workers21 described the optimisation of the solvent system during the construction of three sequential α-glucosidic bonds using 4,6-O-cyclohexylidene-protected thioglucoside donors. It was shown that the best solvent systems were 1:1 mixtures of CHCl3/Et2O and
CHCl3/CPME.
A common point in these studies is that all of the acceptors used were other glycosides, mono and higher saccharides, and no smaller or more reactive alcohols were studied.
In order to improve the α-selectivity of the glycosylation reaction for the synthesis of new synthetic 1,2-cis analogues from glucose, galactose and glucosamine, several thioglycosides were prepared as donors. Using the NIS/TfOH16 system as the promoter, the influence of the nature of the protective groups on different positions of the donor, as well as the solvent and the temperature on these glycosylations, were studied using a wide range of acceptors.
Results and Discussion
1,2-cis Stereoselective Glucosylations
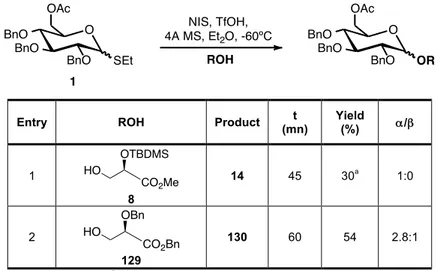
according to literature in 3 steps from methyl glucose and used to study the influence of the solvent and temperature on the glycosylation reaction. To further investigate the influence of protecting groups thioglucoside donor 3
was synthesised with a more electron-withdrawing group at the 6-position (chloroacetyl) from donor 1 (Scheme 6).
O BnO BnO OAcCl SEt BnO O BnO BnO OH SEt BnO O BnO BnO OAc SEt BnO a 1 2 b 3
a) NaOMe, MeOH, 0ºC, 96%. b) Chloroacetyl chloride, Py, 0ºC, 86%.
Scheme 6. Synthesis of thioglucosyl donor 3.
CO2Me
OH
CO2Me
OH
CO2Bn
OH
CO2Me
OH TBDPSO CO2Me
OTBDMS HO
HO CO2Me
4 Methyl (S)-lactate
7 Methyl glycolate
8
Methyl (2R)-O-tert -butyldimethylsilyl-2,3-dihydroxipropanoate
5 Methyl (R)-lactate
6 Benzyl (S)-lactate
9
Methyl (2R)-3-O-tert -butyldiphenylsilyl-2,3-hidroxypropanoate
Figure 3. Glycosyl acceptors.
Table 2. Effect of solvent and temperature on the stereoselectivity of the glycosylation of donors 1 and 3.
NIS, TfOH, 4A MS O BnO BnO OR SEt BnO R'OH O BnO BnO OR OR' BnO 1: R=Ac 3: R=AcCl
Entry R’OH T
(ºC) Solvent
Donor 1 Donor 3
Product (Yield,%)
t
(mn) α/β
Product (Yield,%)
t
(mn) α/β
1
4 0
CH2Cl2 10 (91) 10 4:1 16 (90) 5 5.5:1
2 CH2Cl2
(THF, 2 eq) 10 (83) 10 4:1
3 Tol/Dioxane (1:3) 10 (43) a 80 6:1
4 Tol/Dioxane
(1:3)b 10 (78) 20 6.3:1 16 (85) 5 7.8:1
5 THF/Et2O
(1:9) 10 (87) 10 6:1
6 THF/Et2O
(9:1) 10 (60) 10 5.8:1
7 Et2O 10 (85) 10 7.5:1 16 (90) 5 9.3:1
8 THF 10 (72) 45 6.5:1 16 (85) 5 6.8:1
9
-60
CH2Cl2 10 (83) 10 7:1 16 (94) 20 8.5:1
10 Et2O 10 (81) 45 9:1 16 (90) 25 14.2:1
11
5 0
CH2Cl2 11 (85) 10 4:1
12 Et2O 11 (76) 15 7.9:1
13
-60
CH2Cl2 11 (82) 10 6.5:1
14 Et2O 11 (83) 45 8.3:1
15
6 0
CH2Cl2 12 (93) 10 4:1
16 Et2O 12 (82) 15 8.3:1
18 Et2O 12 (81) 45 10:1 17 (85) 20 11:1
7
7 0
CH2Cl2 13 (93) 10 4.2:1
20 Et2O 13 (85) 45 4.4:1
21
-60
CH2Cl2 13 (96) 10 4.2:1
22 Et2O 13 (88) 45 13:1 18 (70) 20 11:1
23 8 -60 Et2O 14 (30) b,c 45 1:0
24 9
0 CH2Cl2 15 (96) 30 >10:1
25 0 Et2O 15 (94) 45 1:0 a
Hydrolysis product also recovered. b TMSTOf was used instead of TfOH. c Initial donor was recovered.
As expected, ethereal solvents like ethyl ether, 1,4-dioxane and THF, gave higher α-selectivity than dichloromethane that is a halogenated solvent. This study showed that by using the standard glycosylation conditions - dichloromethane at 0ºC - the worst results were obtained in terms of selectivity for the two donors with all of the acceptors.
Glycosylation of donor 1 with methyl (S)-lactate 4 in the toluene/dioxane (1:3) mixture used by Boons19 improved the α/β ratio (6:1), however the reaction was slower (80 min), we obtained only 43% yield and recovered the corresponding reducing monosaccharide as by-product (Table 2, entry 3). Changing the promoter to NIS/TMSOTf increased the yield (78%), but the α/β
ratio remained almost the same (α/β6.3:1, Table 2, entry 4). The THF/Et2O (9:1) mixture employed by Bonnaffé
20
was not beneficial in this case and resulted in a 5.8:1 α/β ratio and only 60% yield (Table 2, entry 6). By reversing the proportions of both solvents in the mixture, THF/Et2O
7). However, when using only THF both selectivity and yield were lower, and the reaction was slower (Table 2, entry 8).
Concerning the study of the effect of temperature, conducting the reaction in dichloromethane and ethyl ether at -60ºC afforded in both cases an enhance in the α-anomeric selectivity (Table 2, entries 9 and 10). In the case of dichloromethane (α/β7:1) the yield was slightly lower (83%) but the reaction time remained the same (10 min) (Table 2, entry 9). With ethyl ether the donor was consumed more slowly (45 min) but this was compensated by improvement of the α/βratio 9:1 and the good yield (81%, Table 2, entry 10). Regarding the glycosylation with methyl (R)-lactate 5 in dichloromethane and ethyl ether both at 0 and 60ºC (Table 2, entries 11-14) the results were very similar to those obtained for methyl (S)-lactate 4. With the bulkier benzyl (S )-lactate 6 the highest αanomeric selectivity was achieved in ethyl ether at -60ºC (α/β 10:1, Table 2, entry 18). When comparing the α/β ratio obtained for the glycosylation reaction in dichloromethane at 0ºC using methyl (S)-lactate
4, methyl (R)-lactate 5 and benzyl (S)-lactate 6 as acceptors the results were the same (α/β 4:1, Table 2, entry 1, 11 and 15), meaning that under this conditions neither the (R),(S)-stereochemistry and bulkiness of the ester moiety of the lactate acceptor was relevant for the anomeric selectivity. Glycosylation with the primary hydroxyl group of the small methyl glycolate 7
acceptor in ethyl ether at -60ºC afforded excellent results both in terms of α/β
secondary hydroxyl group total migration occurs, and the same phenomenon was observed when using a benzoyl group at this position.
CO2Me OH TBDPSO
CO2Me OAc TBDPSO
CO2Me OH AcO
CO2Me OTBDMS AcO
CO2Me OTBDMS HO
a b
c d
9
8
a) Ac2O, Py, DMAP, 0ºC-r.t., 94%. b) TBAF, THF, 0ºC-r.t., 75%. c) TBDMSCl, imidazole, DMF, 0ºC-r.t., 74%. d) NaOMe, MeOH, 0ºC, 16% 8, 76% 8a.
CO2Me OH TBDMSO +
8a
Scheme 7. Synthesis of methyl (2R)-O-tert-butyldimethylsilyl-2,3-dihydroxipropanoate 8.
Concerning the influence of protecting groups at the 6-position for most of the cases donor 3 showed a slightly higher α-selectivity than donor 1, which is an evidence of the importance for the anomeric selectivity of a more electron-withdrawing group at this position.
1,2-cis Stereoselective Galactosylations
Aiming to determine the importance of the sugar structure for the protein stabilisation effect, the synthesis of analogues from galactose was attempted. In general, glycosylations of thiogalactosides afford lower 1,2-cis selectivities and are less studied. This lack of selectivity can be explained by a higher reactivity due to unfavorable steric effects of the axially oriented substituent at C-4.
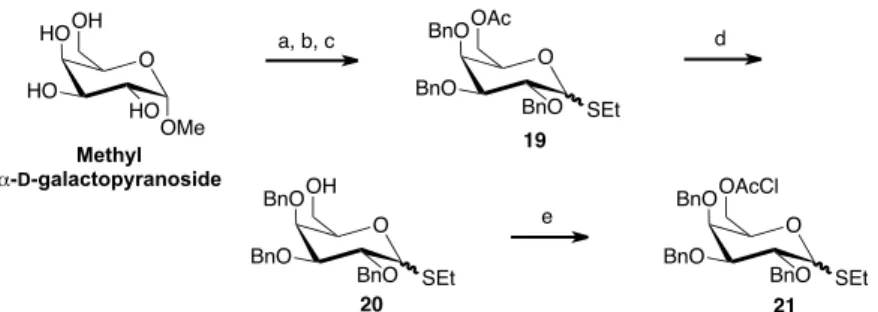
Using the same methodology used for glucose, thiogalactosides 19 and 21
O BnO BnO OAc SEt BnO 19 20 21 O HO HO OH OMe HO d O BnO BnO OH SEt BnO O BnO BnO OAcCl SEt BnO e Methyl α-D-galactopyranoside
a, b, c
a) BnBr, NaH, DMF, 0ºC-r.t., 90%. b) Ac2O/AcOH, H2SO4, 0ºC, 75%, α/β=3.7:1. c) EtSH, BF3.Et2O, CH2Cl2, 0ºC, 91%, α/β=2.4:1. d) NaOMe, MeOH, 0ºC, 81%. e) Chloroacetyl anhydride, Py, 0ºC, 90%.
Scheme 8.Synthesis of thiogalactosyl donors 19 and 21.
In this study not only small and reactive alcohols (Figure 3) were chosen as acceptors but also other molecules with a more complex structure, like lipids, aminoacids, steroids, other sugars and bulkier alcohols (Figure 4).
OH TBDPSO
22
1,3-di-O-tert -butyldimethylsilyl-1,2,3-trihydroxypropyl
OTBDPS
HO
O
H
HO CO2Me 14
OH
Ph CO2Ph OH
27 Benzyl (S)-mandelate
OH
OH MeO2C
NHBoc HO n~12 O OH 23 Methyl 15-hydroxypentadecanoate O BnO BnO O BnO OH
CO2Me OTBDPS 24 25 Epiandrosterone (EPA) 26 1-Adamantanol 28 PEG550 29 L-Menthol 30 Cyclohexanol
Methyl 3-O-tert-butyldiphenylsilyl-(2R)-2-O -[2,3,4-tri-O-benzyl-1-O
-α-D-glucopyranosyl]-2,3-dihydroxypropanoate
31
N-(tert -Butoxycarbonyl)-D-serine methyl ester H
H H
Figure 4. Glycosyl acceptors.
Table 3. Effect of solvent and temperature on the stereoselectivity of the glycosylation of donor 19 and 21.
NIS, TfOH, 4A MS O BnO BnO OR SEt BnO R'OH O BnO BnO OR OR' BnO 19: R=Ac
21: R=AcCl
Entry R’OH T
(ºC) Solvent
Donor 19 Donor 21
Product (Yield,)
t
(mn) α/β
Product (Yield,%)
t
(mn) α/β
1
4
r.t. CH2Cl2 36 (76) 5 3.8:1
2
0
CH2Cl2 32 (87) 5 3:1 36 (95) 5 3.4:1
3 CH2Cl2 32 (68) 30 2.6:1
4 THF 32 (15) a 60 5:1
5 Et2O 32 (70) 40 3:1
6 Tol:Dioxane
(1:3) 32 (54) b
10 2.5:1
7
-60
CH2Cl2 32 (83) 5 1.5:1 36 (81) 15 1.8:1
8 Et2O 32 (40) 86 2.2:1
9
6 0
CH2Cl2 33 (92) 5 2.8:1
10 Et2O 33 (84) 40 2.7:1
11
-60
CH2Cl2 33 (82) 10 1.7:1
12 Et2O 33 (86) 40 4:1
13
7 0
CH2Cl2 34 (87) 5 3:1 37 (80) c 5 3:1
14 Et2O 34 (92) 15 2.2:1
15
-60
CH2Cl2 34 (96) 5 1.2:1
17 9
0 CH2Cl2
35 (70) 5 2.4:1 38 (86) 5 2.6:1
18 22 39 (68) 5 2.4:1
19 23 40 (63) 5 1:2.3
20 24 41 (70) 5 1.4:1
21 25 42 (57) 5 1:1.7
22 26 43 (82) 5 1:1.3
23 27 44 (70) 5 1:0
24 28 45 (97) c 30 1:1
25 29 46 (85) 5 1:1.5
26 30 47 (93) 5 1:1.2
27 31 48 (91) 5 2:1
a 43% of starting material and 38% of the hydrolysis product were recovered. b 18% of the hydrolysis product was recovered. c More equivalents of TfOH were added during the reaction.
Contrary to what happened with the glucose donors, glycosylation with methyl (S)-lactate using dichloromethane (Table 3, entry 2) as solvent gave better results than ethereal solvents. Also when using dichloromethane the reaction times were shorter and by leaving the reaction longer, the anomeric selectivity and the yield were decreased (Table 3, entry 3). Galactosylation in THF afforded the highest selectivity (α/β 5:1, Table 3, entry 4) but unfortunately the reaction time was longer, the yield was only 15% and we recovered 43% of starting material and 38% of the hydrolysis product.
The toluene/dioxane (1:3) mixture, employed by Boons and co-workers19, afforded not only the lowest selectivity but also low yield (α/β 2.5:1, 54%, Table 3, entry 6). When using diethyl ether the same α/β ratio 3:1 was obtained as in dichloromethane but the reaction time was longer and the yield was lower (Table 3, entry 5).
higher α-selectivites were obtained at higher reaction temperatures (Table 3, entries 1-2 and 7). This effect has been attributed to the fact that the addition to the donor at low temperatures (-60ºC) is subjected to kinetic control, and when the temperature is increased the effect of the remote neighbouring group at C-6 is enhanced.9
Experiments with the bulkier benzyl (S)-lactate in dichloromethane did not improve the anomeric selectivity (Table 3, entries 9 and 11), but when using ethyl ether as solvent at -60ºC the α/β ratio increased to 4:1 (Table 3, entry 12). Reaction with the primary hydroxyl group of methyl glycolate in dichloromethane at 0ºC afforded a α/β ratio of3:1 (Table 3, entry 13) and worst results were obtained when other conditions were tested (Table 3, entries 14-16).
Having found that the best solvent for the glycosylation reaction of thiogalactoside donor 19 was dichloromethane, a series of experiments were performed with thiogalactoside donor 21 in order to assess if a more electron-withdrawing group at the C-6 position of the donor would have a dramatic effect on the selectivity of the reaction.
The results of the glycosylation reaction of donor 21 with methyl (S)-lactate in dichloromethane at different temperatures (Table 3, entries 1-2 and 6), confirmed the temperature dependence. The lower the reaction temperature the lower the anomeric selectivity, and the higher the reaction temperature the lower the yield of the reaction.
When comparing the results obtained for the two donors (19 and 21), we found that using the chloroacetyl thiogalactoside 21 with the small and reactive acceptors did not improve the anomeric selectivity and the reaction time remained the same (Table 3, entries 2, 7, 13 and 17). However, in the case of benzyl (S)-mandelate it was obtained exclusively the α-anomer (Table 3, entry 23).
Contrary to what was expected, glycosylation of donor 21 with bulkier and less reactive acceptors (Table 3, entries 18-22 and 24-25) afforded lower anomeric selectivity, with more β anomer being obtained.
the work of Wong and co-workers23 who studied the structural effect of monosaccharides on the reactivity of the glycosylation reaction by comparing reaction rates of several thioglycoside donors with MeOH using NIS/TfOH system. In this study, for example, the perbenzylated tolylthiogalactoside reacts 6.4 times faster than the corresponding thioglucoside, which was explained by the stereo and inductive effects and possible involvement of the axial 4-O lone-pair electrons in the stabilization of the oxocarbenium ion intermediate.
The concept of an axial polar substituent stabilizing a positive charge to a higher degree than the corresponding equatorial equivalent explains these findings, and is the concept behind the idea of very reactive super-armed glycosyl donors developed by Bols and co-workers24. Super-armed donors are synthesised by forcing conformational changes, in this case forcing the oxygen substituents into an axial position by choosing bulky silyl protecting groups.
Since changing the solvent system and temperature were unable to improve the selectivity of the glycosylation reaction for the synthesis of galactosyl derivatives, based on the work of Bols24 a super-armed thiogalactosyl donor
50 (Scheme 9) was designed, synthesised and studied. Super armed donor
50 was synthesised in five steps from D-galactose, which after selective protection of the primary hydroxyl group at C-6 position of the ethyl β-D -thiogalactoside in the form of trityl ether25 49 was silylated with TBDMSOTf according to literature24. When protected with bulky silyl groups, donor 50
O HO HO HO SEt OH O HO HO HO SEt OTr O TBDMSO TBDMSO TBDMSO SEt OTr O OTBDMS SEt TBDMSO TrO 49 50a 50b
a, b, c O HO HO HO OH OH e d
a) Ac2O, Py, DMAP, 0ºC-r.t., quant. b) ZnCl2, EtSH, 0ºC-r.t., 65%. c) NaOMe, MeOH, r.t., 86%. d) TrCl, Py, DMAP, 60ºC, 81%. e) TBDMSOTf, Py, DMAP, 60ºC, 83%.
OTBDMS D-Galactose
Scheme 9. Synthesis of super armed thiogalactosyl donor 50.
Since in solution donor 50 is in slow equilibrium between several conformers the peaks on 1H NMR spectra were broad and complex. The quality of the spectra could be improved by cooling the samples in CDCl3 to -30°C where
the equilibration was slow and the different conformers gave sharper signals. To test the donor, the glycosylation reaction was performed using the NIS/TfOH system as the promoter in dichloromethane at -78ºC, and as acceptors methyl (S)-lactate 4 and methyl glycerate 9 (Scheme 10).
O TBDMSO TBDMSO TBDMSO SEt OTr a O TBDMSO TBDMSO TBDMSO OR OTr 50
a) ROH: 4 or 9, NIS, TfOH, 4A MS, CH2Cl2, -78ºC.
51,R: (S)-CH(CH3)CO2Me, 48%, α/β=1:0. 52, R: (R)-CH(CH2OTBDPS)CO2Me, 22%, α/β=1:0.
Scheme 10. Glycosylation reaction ofsuper armed thiogalactosyl donor 50.