w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Comparative
central
effects
of
the
aqueous
leaf
extract
of
two
populations
of
Passiflora
edulis
Adriana
S.F.S.J.
Ayres
a,
Luana
L.S.
de
Araújo
a,
Thaciane
C.
Soares
b,
Geison
M.
Costa
d,
Flávio
H.
Reginatto
c,
Freddy
A.
Ramos
d,
Leonardo
Castellanos
d,
Eloir
P.
Schenkel
c,
Vanessa
P.
Soares-Rachetti
a,
Silvana
M.
Zucolotto
b,
Elaine
C.
Gavioli
a,∗aLaboratoriodeFarmacologiaComportamental,DepartamentodeBiofisicaeFarmacologia,UniversidadeFederaldoRioGrandedoNorte,Natal,RN,Brazil bLaboratóriodeFarmacognosia,DepartamentodeFarmacia,UniversidadeFederaldoRioGrandedoNorte,Natal,RN,Brazil
cProgramadePós-graduac¸ãoemFarmácia,UniversidadeFederaldeSantaCatarina,Florianópolis,SC,Brazil dDepartamentodeQuímica,FacultaddeCiencias,UniversidadNacionaldeColombia,Bogotá,Colombia
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received6March2015
Accepted25June2015
Availableonline26July2015
Keywords:
Passifloraedulisedulis Passifloraedulisflavicarpa
Anxiolytic Antidepressant Sedation
Animalmodels
a
b
s
t
r
a
c
t
PassifloraedulisSims,Passifloraceae,hasbeenusedinBraziliantraditionalfolkmedicinetothetreatment
ofanxietyandinsomnia.P.edulisiscommonlyknownforitseconomicinterestsinBrazil.Thisspecies
exhibitssignificantvariabilityinthefruitrindcolor,thentwosubpopulationshasbeendescribed(P.
edulisfo.flavicarpaO.Deg.(PEF);P.edulisfo.edulis(PEE)).Thisstudycomparedphytochemicalprofile
andbiologicalactionsofaqueousleafextractofPEEandPEF.HPLCanalysisshowedmarkeddistinct
chromatogramstotheP.edulisvarieties.However,inbothextractsthemajorcompoundsobserved
wereflavonoidsC-glycosides.BehavioralstudiesshowedthatPEE(300mg/kg,p.o.)andPEF(100and
300mg/kg,p.o.)reducedanxietyintheelevatedplusmazetest.PEE(300and1000mg/kg,p.o.)andPEF
(1000mg/kg,p.o.)alsoinducedantidepressant-likeactionsintheforcedswimmingtest.PEE1000mg/kg
significantlyreduceddistancemoved,thussuggestingsedation.Noalterationsinsleepingtimewere
observedwithPEEandPEFextracts.Inconclusion,despitethesimilaritiesbetweenthebiologicalactions
observedforbothP.edulisvarieties,quitedifferentphytochemicalprofilewashereinreported.Thesedata
suggestthattheanxiolyticandantidepressantactionsarenotduetoaspecificphytochemicalcomponent.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
ThegenusPassiflora,Passifloraceae,comprisesabout500plant species,mostlydistributedintropicalandsubtropicalregionsofthe world.Manyspeciesofthisgenushavelongbeenusedintraditional folkmedicinesworldwidetothetreatmentofanxiety,insomnia, epilepsy,spasm,andaches(forreviewsee:Dhawanetal.,2004; Guzman-Gutiérrezetal.,2014).PassifloraedulisSimsisavariety commonlyknownforitstastyfruitbutalsousedinBrazilian tra-ditionalfolkmedicine(Bernaccietal.,2008).Asfirstlynoticedby Degener(1932),P.edulisspeciesexhibitsconsiderable morpholog-icalvariability.Thisplantproducestwotypesoffruit:thepurple (Passiflora edulis fo.edulis) andyellow fruit(Passifloraedulis fo. flavicarpaO.Deg.)(Bernaccietal.,2008).Thesedistinctionsgo fur-ther,theP.edulis fo.edulisspecies presentsflowerswithwhite petalsandpurplefilamentsonthelowerhalf,andwhiteonthe
∗ Correspondingauthor.
E-mail:ecg@pq.cnpq.br(E.C.Gavioli).
tophalfandbrownish-purplefruit,withalengthof6–7cm.The flowersofP.edulisfo.flavicarpaexhibithighlypurplecoronaatthe baseandlargeryellowfruits(i.e.,6–12cmlong)(Bernaccietal., 2008).Indeed,theirgeneticconstitutionswerefoundhavinglow similarity(Aukaretal.,2002),andtheirvolatilecompoundswere significantlydifferent(Pontesetal.,2009).Despitethese dissimi-larities,P.edulisfo.flavicarpaisusuallyconsideredjustavarietyof theP.edulisspecies,thustheinfraspecifictaxonomyofP.edulisis contradictoryandworthfurtherdiscussion(forreviewseeBernacci etal.,2008).
In2010,theofficialmonographofP.edulisspecies(no descrip-tionofthevariety)wasincludedintheBrazilianPharmacopoeia (2010)aswellastheofficialmonographofP.alataCurtis.An impor-tantfeatureofPassifloraspeciesisthat,duetothefolkmedicinal uses,P.edulis,P.alataandP.incarnatawereincludedintheNational ListofMedicinalPlantsofInteresttoBrazilianPublicHealth Sys-tem(MinistériodaSaúdedo Brasil,2009).Thislistincludes71 species ofmedicinalplants withpotentialtogenerate pharma-ceuticalproductsofinteresttoBrazilianpublichealth(Ministério daSaúdedo Brasil,2009).Regardingtherelevance ofPassiflora
http://dx.doi.org/10.1016/j.bjp.2015.06.007
speciesas food,Brazilis considered thetop worldproducer of yellowfruits(P.edulisfo.flavicarpa).Theseobservationsreinforce thevalueof pharmacologically validating thetraditional useof Passifloraspeciesabundantlycultivatedand medicinallyusedin Brazil.
Invivostudieshavelongbeenreportingthebiologicalactions ofP.edulisSimsinrodents(Petryetal.,2001;Coletaetal.,2006; Reginattoetal.,2006).However,noformaldistinctionbetweenP. edulisvarietieshasbeenregistered.Anexceptionwasfoundina studypublishedin2011byaChinesegroup,whichcomparedthe chemicalconstitutionandbiologicaleffectsoftheethanolextract oftwopopulationsofP.edulis(‘edulis’and‘flavicarpa’)cultivated inChina(Lietal.,2011).In2010,thesameChineseresearchgroup hasreportedthebehavioraleffectsofethanolextractoftheaerial partofP.edulisfo.flavicarpaanditsfractionsinmice(Dengetal., 2010). Considering theimportance of validating the traditional folkusesofP.edulisvarieties,whichhashugeeconomicrelevance inBrazil,thepresentstudyaimedtocomparethephytochemical profileandthecentraleffectsoftheaqueousleafextractofthe twopopulationsofP.edulis(‘edulis’and‘flavicarpa’)cultivatedin SouthAmerica.
Materialsandmethods
Chemicalandreagents
AllsolventsusedwereHPLCgradeandMilliQwater,filtered in 0.45m membranes (MILEX®) and degassed by ultrasound bath.Thereferencestandardsusedwerevitexin-2′′-O-rhaminoside (Fluka,98.2%),isoorientin(Sigma,>98%), orientin(Sigma,>97%), isovitexin(Fluka,>95%), vitexin(Fluka, >95%),and luteolin-7-O -glucoside(Fluka, >98%).The compound6,8-di-C-glycosylchrysin wasisolatedfromLychnophoraericoidesleavesandwasprovidedby Dr.NorbertoPeporineLopes.Spinosinandswertisinwereisolated fromWilbrandea ebracteataroots (Santos etal., 1996). Vicenin-2wasisolatedfromP.edulisfo. flavicarpaleavesand identified byNMRspectraldata(1Hand 2D-NMRtechniques-COSY, HSQC andHMBC)andthepuritywasconfirmedbyHPLC/DAD(Zucolotto etal.,2009).Thereferencestandardswereanalyzedat50g/ml concentration.Thesolutionswerefilteredin0.45mmembranes (MILEX®)anda20
laliquotofthefiltratewasinjectedforHPLC analysis.
Plantmaterial
Theleavesof Passifloraedulisfo.flavicarpa O.Deg.were col-lectedinAntônioCarlos,SantaCatarina,Brazilandidentifiedby thebotanistProf.Dr.DanielFalkenberg(DepartmentofBotanyof theFederalUniversityofSantaCatarina,Florianópolis,SC,Brazil). AvoucherspecimenwasdepositedintheHerbariumatthesame university(FLOR33886).TheleavesofP.edulisSimsfo.eduliswere collectedin SantaSofia,Boyacá,Colombiaand identifiedbythe botanistLuisCarlosJimenez(InstitutoNacionaldeCiencias, Univer-sidadNacionaldeColombia).Avoucherspecimenwasdepositedin theherbariumatthesameuniversity(COL530661).
Preparationofextracts
The leaves of P. edulis fo. edulis and P. edulis fo. flavicarpa wereair-driedat40◦C,powderedandextractedusinghotwater (90◦C) by infusion(plant: solvent,1:10, w/v) for 10min.After that,theextractswerefilteredandfreeze-dried.ForHPLCanalysis, eachextractwaspreviouslyfilteredthrougha0.45mmembrane (MILEX®).Theextractsweresolubilizedinmethanol:water(1:1,
v/v)at7.5mg/mland20lwasusedforHPLCanalysis.
Phytochemicalanalysis
HPLC analysis was performed in a Varian® chromatograph
pumpProStar240,ProStar410autoinjector,coupledtoProStar 335 DAD detector and Phenomenex-Luna 5m C18 (2) 100A (250mm×4.6mm)(column).Themobilephasewas(A) acetoni-trileand(B)aceticacid0.3%,usingthefollowinggradient0–10min 10:90%(A) in (B),10–40min 20:80%(A) in (B),and 40–90min 20:80%(A)in(B)ataflowrateof1ml/minandUV345nm.Peaks foundin thechromatogramswere identified bycomparingthe retentiontime(RT)andUVspectraofthereferencestandardsand byco-injectionofreferencestandardsplusextractstocomparethe increaseofpeakarea.
Animals
Male Swiss mice (weighing 25–40g) from our breeding colony were used. Animals were housed in plastic cages (41cm×34cm×16cm),12percage,undera12-hlight/darkcycle (lightson6a.m.) atroom temperatureof 23±1◦C, withwater andfoodadlibitum.Allexperimentalprocedureswereconducted between2 and 5p.m.and were performedin accordance with theBrazilianlawn◦11.794/2008forexperimentaluseofanimals. ThisstudywasapprovedbylocalEthicCommitteeforAnimalUse (LicenseN◦032/2010).
Drugsandtreatments
Diazepam(CristáliaProd.Quím.FarmacêuticosLtda.,SãoPaulo, Brazil), an anxiolyticdrug, was solubilized in tween 80 (0.5%) andsaline,anditwasinjectedintraperitoneally(i.p.)at1mg/kg (10ml/kg),30minbeforetesting.Nortriptyline(Novartis Biociên-ciasS.A.,SãoPaulo,Brazil),anantidepressantdrug,wassolubilized insalineandi.p.administeredat30mg/kg(10ml/kg),30minprior theforcedswimmingtest.Salinewasusedascontrol.
Aqueousextracts of Passiflora weresolubilized in tap water andadministered orally(p.o.), byusinga syringecoupledtoan oral cannula (0.1cm×4cm), in a volume of 10ml/kg, 60min beforetesting.Tapwaterwasusedascontrol.Thiopentalsodium 50mg/kg (Cristália Prod. Quím. FarmacêuticosLtda., São Paulo, Brazil),abarbituricacid,wasinjectedi.p.60minpriorthePassiflora extracts.
Behavioraltests
Elevatedplus-maze(EPM)test
Openfieldtest
Spontaneouslocomotoractivityofmicewasmeasuredusingthe openfieldtest.Theapparatus,madeofwoodcoveredwith imper-meableformica,hadablackfloorof40cm×40cmandblackwalls of40cmhigh.Thetestroomhadacontrolledillumination (dimly-lightcondition).Eachmousewasplacedinthecenteroftheopen fieldandthedistancetraveledevery5minwereregistered dur-ing30minthroughautomaticobservation(Anymaze,StoeltingCo., WoodDale,IL,USA).Afterthebehavioralevaluationofeachmouse, thearenawascleanedwith10%ethanolsolution.
Forcedswimmingtest
ThistestwasperformedaccordingtoPorsoltetal.(1977).Mouse wasindividuallyforced toswim ina transparentglasscylinder (24cminheight,18cmindiameter)filledwith18cmofwaterat 23±1◦C.Thetimespentimmobility(ins)wasmeasuredduring thelast4minofasingle6-mintestsession.Micewereconsidered immobilewhentheymadenofurtherattemptstoescapeexcept themovementsnecessarytokeeptheirheadsabovethewater.
Thiopental-inducedsleepingtimetest
Thiopentalwasusedtoinducesleepaspreviouslydescribedby Vogel andVogel (1997).Thiopental50mg/kg wasadministered i.p. 60minafter administrationof Passiflora extracts.The inter-val time betweeninjection of thiopental and time that animal lossrightingreflexwasrecorded(ins)aslatencytime.The inter-val,inseconds,betweenlossandrecoveryofrightingreflexwas
registeredassleepingduration,anditwasusedasindexofhypnotic effect.
Statisticalanalysis
Thedatahereinpresentedwerereportedasmean±SEM.All datawereanalyzedusingGraphPadInStat3.06(GraphPadSoftware Inc.,SanDiego,USA).AllresultswereinitiallysubmittedtoLevene’s testforhomogeneityofvarianceandtoKolmogorov–Sminorv’stest fornormality. Comparisonsbetweentreatedandcontrolgroups wereperformedusingone-wayANOVAfollowedbyDunnett’stest orStudent’st-test,asdetailedin thefigurelegends.Differences wereconsideredsignificantwhenp<0.05.
Resultsanddiscussion
Phytochemicalanalysis
TheaqueousextractsfromP.edulisfo.edulisandP.edulisfo. flavi-carpawereanalyzedbyHPLC/PAD.Thechemicalprofileshowedin chromatogramsismarkedlydifferenttotheP.edulisvarieties.P. edulisfo.edulispresentspeaksbetween30and80min,themajor peakswereobservedinRT=32.4and58min(Fig.1A).Incontrast,P. edulisfo.flavicarpashowspeaksbetween20and50minandmajor peakswithRT=29.3and36min(Fig.1B).Accordingtothe max-imaabsorptionfoundintheUVspectra,themainpeaksobserved in thechromatograms of both extracts seem to be flavonoids.
70
60
50
40
30
20
10
0
70 80
Absorbance Units (mA
U)
5
0 10 20
OH
OH
OH
OH
OH HO
mAU
mAU rham-O-glc
glc
glc
glc glc
glc
glc glc
HO
HO
HO
HO O
O O
O
O O OH
OH
OH
OH
OH
OH
OH
OH O
O O O
O O
O
(2)
(2)
(4)
(5)
(7)
1
2
2
3
5
6
7
4
1
3
4
5
6
7
(3)
A
B
2515 30 35 40 45 50 55 60 65 70 75 80 85 90
0 5 10 15 20 25 30 40 45 50 55 60
Time (min)
65 70 75 80 85 90
35 60
50
40
30
20
10
0
Fig.1.HPLCchromatogramsofaqueousextractsofPassifloraedulisfo.edulis(A)andPassifloraedulisfo.flavicarpa(B)leaves7.5mg/ml.UV=345nm.(A)1,4,5and
7=luteolinderivatives,2=vitexin-2′′-O-rhamnoside,3=luteolina-7-O-glucoside,6=apigeninderivative.(B)1and6=luteolinderivatives,2=vicenin-2,3=apigenin
Table1
FlavonoidsidentifiedinPassifloraedulisfo.edulisandP.edulisfo.flavicarpa.
Flavonoid Isoorientin Orientin Vitexin Isovitexin Vitexin-2′′-O
-rhamnoside
Luteolin-7-O -glucoside
Vicenin-2 Spinosin 6,8-di-C-glycosyl chrysin
Swertisin
P.edulisfo.flavicarpa + − − ++ − − ++ − ++ −
P.edulisfo.edulis − − − − + + − − − −
Notes:+,Compoundidentified;++majorcompound;−compoundnotidentified.
Co-injection of extracts plus reference standards showed an
increasein thepeakareaofsomeflavonoids assummarized in
Table1.
RegardingtheP.edulisfo.edulisextract,onlytwominoritypeaks were identified: vitexin-2′′-O-rhamnoside (peak 2, RT=36min) and luteolin-7-O-glucoside (peak 3, RT=42min) (Fig. 1A). In general, peaks showed the typical UV absorption of luteolin derivatives:peak1(max=261,349nm),4(max=264,348nm),5 (max=264,348nm),and 7(max=297,348nm);exceptfor peak 6 (max=267,342nm) that presented UV absorption similar to flavonoidapigenin (Fig. 1A) (Mabry et al., 1970).As regardsP. edulisfo.flavicarpaextract,vicenin-2(peak2,RT=28.2min), isoori-entin (peak 4, RT=30.2min), 6,8-di-C-glycosylchrysin (peak 5, RT=35.5min)andisovitexin(peak7,RT=38.5min)wereidentified asthemajorpeaks(Fig.1B).Thepeaks1(max=266,347nm)and6 (max=266,345nm)displayedanUVabsorptionsimilartoluteolin derivatives,whilepeak3(max=268,339nm)wascharacterizedas anapigeninderivative(Fig.1B)(Mabryetal.,1970).
Briefly,thechromatogramsofP.edulisfo.flavicarpawerequite differentfromthoseofP.edulisfo.edulis.Sixmajorpeaks(between 55and80min)observedintheP.edulis fo.edulishadnotbeen detectedin P.edulis fo. flavicarpa. Theseobservations reinforce theviewthatthesetwoformsofP.edulisaredifferentfromeach other.Zucolottoandcolleagues(2011) havepreviously demon-stratedthepresenceofC-glycosyl flavonoidsinSouthAmerican Passiflora species, includingthe P.edulis fo. edulisand P.edulis fo.flavicarpa.ConcerningtheP.edulisfo.flavicarpa,inthisstudy, vicenin-2,isoorientin, isovitexin, orientin, vitexin, spinosin and 6,8-di-C-glycosylchrysin were identified, while to the P. edulis fo.edulisextractnoreferencestandardsusedinthis workwere identified(seeTable1).Previously,Lietal.(2011)showedthe pres-enceofthefollowingflavonoidsinP.edulisfo.flavicarpaethanol extract: lucenin-2, vicenin-2, isoorientin, isovitexin, luteolin-6-C-chinovoside,andluteolin-6-C-fucoside.Inagreementwithour data,Lietal.(2011)alsodemonstratedthatnoneoftheseflavonoids
weredetectedinP.edulisfo.edulis.Theseobservationsreinforce theviewthatthesetwovarietiesofP.edulisaredifferentfromeach other.However,distinctlocaland collectiontimesoftheherein studiedP.edulisvarietiesshouldbetakenintoaccounttoexplain differencesinthephytochemicalprofile.
Behavioraltests
Elevatedplus-mazetest
BeforestartingthebehavioralevaluationofPassifloraextracts, theeffectsofthestandardanxiolyticdrug,diazepam,wasassessed inourexperimentalconditions.Micei.p.injectedwithdiazepamat 1mg/kgdisplayedasignificantincreaseinthepercentageoftime spentin (vehicle: 12.3±7.0%; diazepam: 42.0±4.5%*; *p<0.05, Student’st-test)andentriesinto(vehicle:19.3±5.0%;diazepam: 51.0±4.5*%;*p<0.05,Student’st-test)openarms.Asshowedin Fig.2,P.edulisfo.edulis300mg/kgsignificantlyincreasedthe per-centageofentriesintoopenarms(Fig.2A;F(3,42)=3.096;p<0.05, ANOVA,Dunnett’stest).Similarly,P.edulisfo.flavicarpa100and 300mg/kgevokedasignificantincreaseinthepercentageofentries intoopenarms(Fig.2D;F(4,51)=4.206;p<0.05,ANOVA,Dunnett’s test).Nosignificanteffectsinthepercentageoftimespentintoopen armsandfrequencyofentriesintoclosedarmswereobservedin micetreatedwiththeextractsofthesetwopopulationsofPassiflora (Fig.2B,C,E,F;p>0.05).
Open-fieldtest
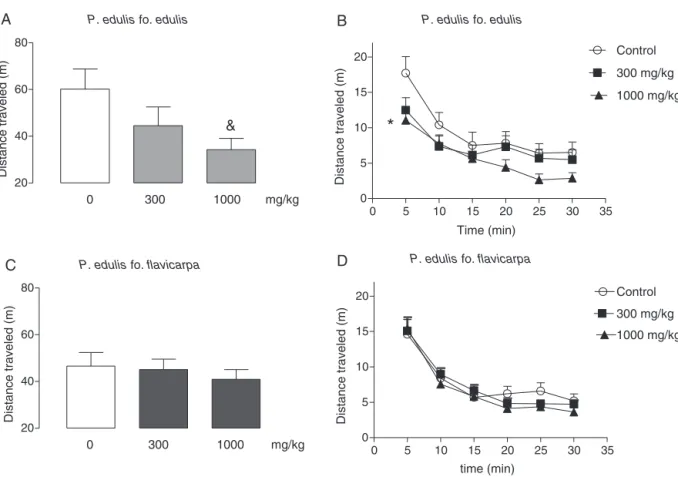
Consideringthecumulativedistancetraveled,atrendtoreduce this behavioral parameter was observed in P. edulis fo. edulis (1000mg/kg)-treated mice (Fig. 3A; F(2,25)=2.965; #p=0.07, ANOVA,Dunnett’stest).TheadministrationofP.edulisfo.edulisat 1000mg/kgsignificantlyreducedthedistancetraveledatthefirst 5minofobservationcomparedtocontrol(Fig.3B;F(2,25)=3.233; *p<0.05, ANOVA,Dunnett’stest).P.edulis fo.flavicarpa didnot
P. edulis fo. edulis
10 20 30 40 50
A
*
0 100 300 1000 mg/kg
0 30 100 300 1000
% Entries into open arms
P. edulis fo. edulis
10 20 30 40 50
B
% Time in open arms
P. edulis fo. edulis
0 5 10 15 20
C
Entries into closed arms
P. edulis fo. flavicarpa
10 20 30 40 50
D
*
*
mg/kg
0 100 300 1000 mg/kg 0 100 300 1000 mg/kg
0 30 100 300 1000 mg/kg 0 30 100 300 1000 mg/kg
% Entries into open arms
P. edulis fo. flavicarpa
0 10 20 30 40
E
% Time in open arms
P. edulis fo. flavicarpa
0 5 10 15 20
F
Entries into closed arms
Fig.2.EffectsoftheacuteadministrationofP.edulisfo.edulis(100,300and1000mg/kg,p.o.)andP.edulisfo.flavicarpa(30,100,300and1000mg/kg,p.o.)onthepercentage
ofentriesinto(A,D)andtimespentinopenarms(B,E),besidesonthefrequencyofentriesintoenclosedarms(C,F)intheelevatedplusmazetest.Resultsarerepresented
P. edulis fo. edulis
20 40 60 80
A
&
0 300 1000 mg/kg
Distance traveled (m)
P. edulis fo. edulis
0 5 10 15 20 25 30 35
0 5 10 15
20 Control
300 mg/kg
1000 mg/kg
B
*
Time (min)
Distance traveled (m)
P. edulis fo. flavicarpa
20 40 60 80
C
0 300 1000 mg/kg
Distance traveled (m)
P. edulis fo. flavicarpa
0 5 10 15 20 25 30 35
0 5 10 15
20 Control
300 mg/kg
1000 mg/kg
D
time (min)
Distance traveled (m)
Fig.3.EffectsoftheacuteadministrationofP.edulisfo.edulis(300and1000mg/kg,p.o.)andP.edulisfo.flavicarpa(300and1000mg/kg,p.o.)onthecumulativedistance
traveledduring30min(A,C)andontheaccumulateddistancetraveledoverconsecutive5mintime(B,D)intheopenfieldtest.Resultsareexpressedasmean±SEMof8–10
animalspergroup.*p<0.05vs.control;&p=0.07vs.control,ANOVAfollowedbyDunnett’stest.
affectthedistancetraveledbymiceduringthe30minof obser-vation(Fig.3CandD;p>0.05).
SeveralstudiesinvestigatedtheeffectsofP.edulisinanxiety. However,experimentalresultshave broughtsomecontroversy. Petryetal.(2001)showedthatP.edulisSimsreducedanxietyin rodents,aswellasColetaetal.(2006)andBarbosaetal.(2008). However,Dhawanetal. (2001)showedthat P.edulis Simswas devoidof anxiolyticactivity.These differences couldbedue to the fact that distinct subpopulations of P. edulis were used. In favorof thisview,Lietal. (2011)comparedtheanxietyeffects ethanolleafextractsof twovarietiesof Passifloraedulis(‘edulis’ and ‘flavicarpa’). They showed that P. edulis fo. flavicarpa dis-playedanxiolytic-likeactivityonlyatthehigherdosetested(i.e., 400mg/kg). However, P. edulis fo. edulis wasinactive at lower doses,whileat400mg/kgevokedsedation.Basedonthese obser-vations,Liet al.(2011) suggestedthat thebiological actionsof thesesubpopulationsofP.edulisaredistinct.IncontrastwithLi etal.,weobservedthat bothvarietiesofP.edulisreduces anxi-etybehaviors;beingP.edulisfo.flavicarpamorepotentthanthe variety‘edulis’.Indeed, thehypolocomotor/sedativeeffects of P. edulisfo.edulis,firstlyreportedbyLietal.(2011),wasalsoherein observed.DifferencesobservedbetweenthepresentstudyandLi etal.(2011)canbeexplainedbythedistinctionsintechnicalextract preparations(i.e.,aqueousandethanol,respectively),besidesthe differences in local and collection times of the used P. edulis varieties.
Forcedswimmingtest
Student’st-testrevealedasignificanteffectfortheacute admin-istration of nortriptyline in mice in the forced swimming test. Nortriptylinereducedthetimethatanimalsspentimmobileinthe watercomparedtocontrols(saline:100.16±15.11s;nortriptyline:
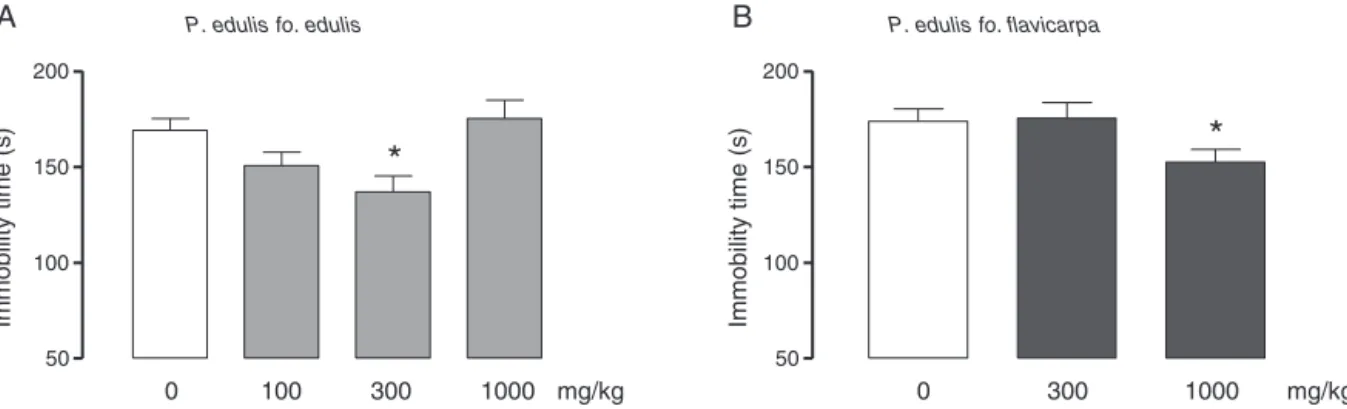
49.28±7.31s*;*p<0.05,Student’st-test).AsillustratedinFig.4, theadministration of theaqueous extract of P.edulis fo.edulis 300mg/kg(Fig.4A;F(3,81)=5.028;p<0.05,ANOVA,Dunnett’stest) and P.edulis fo. flavicarpa 1000mg/kg (Fig. 4B; F(2,56)=3.489; p<0.05,ANOVA,Dunnett’stest)significantlyreducedimmobility timeofmiceintheforcedswimmingtest.
Verylittleliteratureinformation supportstheantidepressant actionsof Passifloraspecies. A studysuggestedthat the antide-pressive effects of Hypericum perforatum are potentiatedwhen it iscombinedwithP.incarnata(Fiebichetal., 2011).Recently, forthefirsttimeitwasshowedthataPassifloraextractinduces antidepressant-likeeffects inmice.Anethanolextract ofleaves andstemsofP.edulis(thevarietyofP.eduliswasnotdetailed), 400–500mg/kg,givenorallyduringsevendays,reduced immobil-itytimeinthetailsuspensionandforcedswimmingtests(Wang etal.,2013).Inthesamestudy,cyclopassiflosidesIXandXI, com-pounds observed in largeamountin theethanol extract, were isolated, andthese cycloartanetriterpenoids weresuggested to bemediatingtheseeffects(Wangetal.,2013).Inourstudy,both extractsevokedantidepressantactions.However,P.edulisfo.edulis seemed tobemore potentcompared tothevariety‘flavicarpa’. Thesefindingsarepromisingandtheysupportaninnovative bio-logicalactioninducedbyP.edulisvarieties.
Thiopental-inducedsleepingtimetest
Atthedosestested,thetreatmentwiththeaqueousextractofP. edulisfo.edulisandP.edulisfo.flavicarpadidnotsignificantlyaffect neitherthelatencytosleepnorthesleepingtimedurationinduced bythiopental(Table2).
P. edulis fo. edulis
50 100 150 200
*
A
0 100 300 1000 mg/kg
Immobility time (s)
P. edulis fo. flavicarpa
50 100 150 200
B
*
0 300 1000 mg/kg
Immobility time (s)
Fig.4.EffectsoftheacuteadministrationofP.edulisfo.edulis(100,300and1000mg/kg,p.o.)(A),andP.edulisfo.flavicarpa(300and1000mg/kg,p.o.)(B)ontheimmobility
timeofmiceintheforcedswimmingtest.Resultsarerepresentedmean±SEMof16–18animalspergroup.*p<0.05,ANOVAfollowedbyDunnett’stest.
Table2
EffectsofacuteadministrationofPassifloraedulisfo.edulis(PEE;300and1000mg/kg,
p.o.)andP.edulisfo.flavicarpa(PEF;300and1000mg/kg,p.o.)onthelatencytosleep
andsleepingtimedurationinducedbythiopental.
Extract Dose Latencytosleep(s) Sleepingtimeduration(s)
Vehicle – 155.2±8.0 403.5±66.9 PEE 300mg/kg 163.3±17.3 599.8±167.2 PEE 1000mg/kg 146.2±27.8 584.2±87.3
Vehicle – 223.1±17.0 647.7±187.1 PEF 300mg/kg 174.7±15.3 513.2±117.1 PEF 1000mg/kg 181.3±14.0 686.0±267.4
Resultsareexpressedasmean±SEMof10–12animalspergroup.
morphine-inducedsleeptimeinmice,andthisextractalsopartially blockedtheamphetamine-induced stimulatedeffects (Doetal., 1983).FewstudiesshowedthatP.edulisaqueousextractsreduce spontaneouslocomotoractivityandprolongsleepinmice(Maluf etal.,1991;Meier,1995).Senaetal.(2009)showedthatthe aque-ousextractfromthepericarpofP.edulisfo.flavicarpapotentiated thehypnoticeffectsofethyletherinmice.Inourstudy,the treat-mentwiththeaqueousextractofP.edulisfo.edulisandP.edulis fo.flavicarpadidnotsignificantlyaffectthiopental-induced sleep-ingtime.Ascommentedabove,apotentialsedativeeffectwasonly detectedfortheaqueousextractofP.edulisfo.edulisinmiceat 1000mg/kg.Possibly,thelowsedativeeffectofourextractscould beduetothepartofplantusedinourstudy(whichwasnotstated inthoseoldreports),methodsofextractpreparationordifferences betweenbioassays;additionally,aspectsrelatedtoseasonal varia-tionandgeographicaloriginofplantsshouldbetakenintoaccount.
Conclusion
Thepresentfindingssupportthatbothextractsshare anxiolytic-andantidepressant-likeactivities.Bycontrast,quitedistinct phy-tochemicalprofilewasreportedfortheaqueousextractofP.edulis varieties.Inboth extractsthemajorcompoundsobservedwere flavonoidsC-glycosides,suggestingthatthesebiologicalactionsare notduetoaspecificglycoside.Apossibleexplanationtothe biolog-icalsimilaritiesbetweenP.edulisfo.edulisandP.edulisfo.flavicarpa couldbeonthefactthatflavonoidsC-glycosidesorothersimilar productofthemetabolismofP.edulisvarietiesarepromotingthese sharedbiologicalactions.Finally,bothvarietiesofP.eduliscouldbe usedasaremedyforanxietyanddepression.
Authors’contributions
SMZandECG definedtheexperimentaldesignof thisstudy. ASFSJA,LLSA,TCSandGMCcontributedbyperformingthe biolog-icalandphytochemicalassays.FHR,FAR,LCandEPScontributed
topreparetheextracts.ASFSJA,SMZandECGdraftedthepaper, whileEPS,VPSRcontributedtocriticalreadingofthemanuscript. Allauthorsreadandapprovedthefinalmanuscriptsubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
Thiswork wassupportedby funds fromCNPq/Brazil (Grant No. 304453/2012-9toECG)and fromthe FederalUniversity of RioGrandedo Norte.ASFSJAhelda PhDfellowship foundedby CAPES/Brazil.
References
Aukar,A.P.A.,Lemos,E.G.M.,Oliveira,J.C.,2002.Geneticvariationsamongpassion
fruitspeciesusingRAPDmarkers.Rev.Bras.Fruticultura24,738–740.
Barbosa,P.R.,Valvassori,S.S.,BordignonJr.,C.L.,Kappel,V.D.,Martins,M.R.,Gavioli,
E.C.,Quevedo,J.,Reginatto,F.H.,2008.TheaqueousextractsofPassifloraalata
andPassifloraedulisreduceanxiety-relatedbehaviorswithoutaffectingmemory processinrats.J.Med.Food.11,282–288.
Bernacci,L.C.,Soares-Scott,M.D.,Junqueira,N.T.V.,Passos,I.R.S.,Meletti,L.M.M.,
2008.PassifloraedulisSims:thecorrecttaxonomicwaytocitetheyellowpassion
fruit(andofotherscolors).Rev.Bras.Fruticultura30,566–576.
2010.BrazilianPharmacopoeia,5thed.AgênciaNacionaldeVigilânciaSanitária,
Brasília,pp.1111–1119.
Coleta,M.,Batista,M.T.,Campos,M.G.,Carvalho,R.,Cotrim,M.D.,Lima,T.C.,Cunha,
A.P.,2006.Neuropharmacologicalevaluationoftheputativeanxiolyticeffects
ofPassifloraedulisSims,itssub-fractionsandflavonoidconstituents.Phytother. Res.20,1067–1073.
Degener,O.,1932.Passifloraedulis.In:Degener,O.(Ed.),FloraHawaiiensis.Honolulu:
family250.
Deng,J.,Zhou,Y.,Bai,M.,Li,H.,Li,L.,2010.Anxiolyticandsedativeactivitiesof
Passifloraedulisf.flavicarpa.J.Ethnopharmacol.128,148–153.
Dhawan,K.,Dhawan,S.,Sharma,A.,2004.Passiflora:areviewupdate.J.
Ethnophar-macol.94,1–23.
Dhawan,K.,Kumar,S.,Sharma,A.,2001.Comparativebiologicalactivitystudyon
PassifloraincarnataandP.edulis.Fitoterapia72,698–702.
Do,V.,Nitton,B.,Leite,J.R.,1983.Psychopharmacologicaleffectsofpreparationsof
Passifloraedulis(Passionflower).CiênciaeCultura35,11–24.
Fiebich,B.L.,Knörle,R.,Appel,K.,Kammler,T.,Weiss,G.,2011.Pharmacological
studiesinanherbaldrugcombinationofSt.John’sWort(Hypericumperforatum) andpassionflower(Passifloraincarnata):invitroandinvivoevidenceofsynergy betweenHypericumandPassiflorainantidepressantpharmacologicalmodels. Fitoterapia82,474–480.
GuzmanGutiérrez,S.L.,Chilpa,R.R.,Jaime,H.B.,2014.Medicinalplantsforthe
treat-mentofnervios,anxiety,anddepressioninMexicantraditionalmedicine.Rev. Bras.Farmacogn.24,591–608.
Li,H.,Zhou,P.,Yang,Q.,Shen,Y.,Deng,J.,Lic,L.,Zhao,D.,2011.Comparativestudies
onanxiolyticactivitiesandflavonoidcompositionsofPassifloraedulis‘edulis’ andPassifloraedulis‘flavicarpa’.J.Ethnopharmacol.133,1085–1090.
Lister,R.G.,1987.Theuseofaplus-mazetomeasureanxietyinthemouse.
Psy-chopharmacology(Berl.)92,180–185.
Mabry,T.J.,Markham,K.R.,Thomas,M.B.,1970.TheSystematicIdentificationof
Maluf,E.,Barros,H.M.T.,Prochtengarten,M.L.,Benti,R.,Leite,J.R.,1991.Assessment ofthehypnotic/sedativeeffectsandtoxicityofPassifloraedulisaqueousextract inrodentsandhumans.Phytother.Res.5,262–265.
Meier,B.,1995.Passifloraeherba-pharmazeutischequalitat.Z.Phytother.,90–99.
Ministérioda SaúdedoBrasil,2009. Fitoterapia:plantasde interesseaoSUS,
http://bvsms.saude.gov.br/bvs/sus/pdf/marco/msrelacaoplantasmedicinais sus060.pdf(accessedAugust2014).
Petry, R.D., Reginatto, F., de-Paris, F., Gosmann, G., Salgueiro, J.B., Quevedo,
J., Kapczinski, F., Ortega, G.G., Schenkel, E.P., 2001. Comparative
pharmacological study of hydroethanol extracts of Passiflora alata and Passifloraedulisleaves.Phytother.Res.15,162–164.
Pontes,M.,Marques,J.C.,Câmara,J.S.,2009.Headspacesolid-phase
microextrac-tiongaschromatography–quadrupolemassspectrometricmethodologyforthe establishmentofthevolatilecompositionofPassiflorafruitspecies.Microchem. J.93,1–11.
Porsolt,R.D.,LePichon,M.,Jalfre,M.,1977.Depression:anewanimalmodelsensitive
toantidepressanttreatments.Nature266,730–732.
Reginatto,F.H.,De-Paris,F.,Petry,R.D.,Quevedo,J.,Ortega,G.G.,Gosmann,G.,
Schenkel,E.P.,2006.Evaluationofanxiolyticactivityofspraydriedpowders
oftwoSouthBrazilianPassifloraspecies.Phytother.Res.20,348–351.
Rodgers,R.J.,Dalvi,A.,1997.Anxiety,defenceandtheelevatedplus-maze.Neurosci.
Biobehav.Rev.21,801–810.
Santos,R.I.,Marlise,A.,Schenkel,E.P.,1996.AnalysisoftheplantdrugWilbrandea
ebracteata.Int.J.Pharmacogn.34,300–302.
Sena,L.M., Zucolotto,S.M.,Reginatto, F.H.,Schenkel, E.P.,De Lima,T.C.,2009.
NeuropharmacologicalactivityofthepericarpofPassifloraedulisflavicarpa Degener:putativeinvolvementofC-glycosylflavonoids.Exp.Biol.Med.234, 967–975.
Vogel,H.G.,Vogel,W.H.,1997.Psychotropicandneurotropicactivity.In:Franz,H.
(Ed.),DrugDiscoveryandEvaluation–PharmacologicalAssays.Springer-Verlag, Berlin-Heidelberg,pp.267–269.
Wang,C.,Xu,F.,Shang,J.,Xiao,H.,Fan,W.,Dong,F.,Hu,J.,Zhou,J.,2013.
Cycloar-tanetriterpenoidsaponinsfromwatersolubleofPassifloraedulisSimsandtheir antidepressant-likeeffects.J.Ethnopharmacol.148,812–817.
Zucolotto,S.M.,Fagundes,C.,Reginatto,F.H.,Ramos,F.A.,Castellanos,L.,Duque,
C.,Schenkel,E.P.,2011.AnalysisofC-glycosylflavonoidsfromSouthAmerican
PassifloraspeciesbyHPLC-DADandHPLC–MS.Phytochem.Anal.3,112–116.
Zucolotto,S.M.,Goulart,S.,Montanher,A.B.,Reginatto,F.H.,Schenkel,E.P.,Fröde,
T.S.,2009.Bioassay-guidedisolationofanti-inflammatoryC-glucosylflavones