w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
article
Synergistic
antifungal
activity
of
the
lipophilic
fraction
of
Hypericum
carinatum
and
fluconazole
Gabriela
C.
Meirelles
a,
Bruna
Pippi
b,
Camila
Hatwig
a,
Francisco
M.C.
de
Barros
a,
Luis
F.S.
de
Oliveira
c,
Gilsane
L.
von
Poser
a,∗,
Alexandre
M.
Fuentefria
a,baProgramadePós-graduac¸ãoemCiênciasFarmacêuticas,UniversidadeFederaldoRioGrandedoSul,PortoAlegre,RS,Brazil
bProgramadePós-graduac¸ãoemMicrobiologiaAgrícolaedoAmbiente,UniversidadeFederaldoRioGrandedoSul,PortoAlegre,RS,Brazil cProgramadePós-graduac¸ãoemCiênciasFarmacêuticas,UniversidadeFederaldoPampa,Uruguaiana,RS,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received9June2016 Accepted29August2016 Availableonline28September2016
Keywords: Candida
Checkerboard Fluconazole
Hypericumcarinatum
Isobologram Synergisticactivity
a
b
s
t
r
a
c
t
Hypericumspecies,Hypericaceae,arerecognizedasasourceoftherapeuticalagents.Purifiedfractions
andisolatedcompoundshavebeenshownantimicrobialactivity.Astheindiscriminateuseofantifungals
andtheincreaseofinfectionscausedbyemergingspeciesareleadingtothesearchofnew
alterna-tivetreatments,theaimofthisstudywastocontinuethestudywithHypericum carinatumGriseb.
lipophilicfraction,richinphloroglucinolderivatives,investigatingtheeffectofitsassociationwith
flu-conazoleagainstemergingyeasts(Candidakrusei,C.famata,C.parapsilosisandCryptococcusneoformans).
ThesynergisticactivitybetweenH.carinatumlipophilicfractionandfluconazolewasassessedbytwo
methodologiesformultipledose–responseanalysis:checkerboardandisobologram.Regarding
syner-gisticexperiments,theeffectoftheassociationwashigherthantheeffectoffluconazolealoneagainst
CandidakruseiandC.famataisolates(MICfluconazoledecreasedabouteightandfourfolds,respectively),
suggestingthat,somehow,H.carinatumlipophilicfractioncompoundsarefacilitatingtheactionofthis
drug.Ontheotherhand,whentestedagainstCryptococcusneoformansandC.parapsilosis,fluconazole
showedbetterresultsthantheassociation.Thus,againstCandidakruseiandC.famata,thelipophilic
fractionofH.carinatumwasabletoreducetheMICvaluesoffluconazoleandcouldbeconsideredasa
potentialalternativetobeusedagainstemergingyeastspecies.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen
accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Fungalinfectionsareassociatedwithhighmorbidityand mor-talityrates.Inthelastdecades,emergingfungalinfections,also calledopportunisticinfections,havedrawnattentionduetothe high number of immunocompromised patients affected (Silva etal.,2012).SomespeciesofCandidaandCryptococcus,previously considerednonpathogenic,arenow recognizedasopportunistic pathogensresponsiblefordeep-seatedmycoses(Vandeputteetal., 2012;Alcazar-FuoliandMellado,2014).
ThehighincidenceofinfectionbyCandidaspeciesisduetomany factorssuchasimunossupressivetherapies,invasivesurgical pro-ceduresanduseofbroad-spectrumantibiotics(Pfalleretal.,2012).
Candidaalbicansisstillthemostprevalentspeciesbutinfections
causedbynon-Candidaalbicans(NCA)havesignificantlyincreased, bringingevenmoreworryingscenarioduetohighresistanceto
∗ Correspondingauthor.
E-mail:gilsane@farmacia.ufrgs.br(G.L.vonPoser).
antifungalexhibitedbythesemicroorganisms(Pfalleretal.,2010, 2012).Sincetheepidemiologyofthesefungalinfectionsiscurrently changing,newalternativesareneededincaseofantifungaltherapy failure(Alcazar-FuoliandMellado,2014).
Becauseofyeastsinconstantsusceptibilityprofilesandlackof differentmoleculartargets,drugcombinationsappearasastrategy fortherapyduetothemultiplicityoftargets(Musioletal.,2014). Themainadvantageofthesecombinationsisthesynergistic inter-action,inwhichtheantifungalactivityisbetterthantheindividual effectsofeachcompound.
Plants from genus Hypericum, Hypericaceae, are an impor-tantsourceoftherapeuticagents.Purifiedfractionsandisolated compounds have shown antibacterial and antifungal activities (Barrosetal.,2013;DulgerandDulger,2014).Barrosetal.(2013) havereportedtheantifungalactivityoflipophilicextractsoffive
Hypericumspecies (H.carinatum,H.caprifoliatum,H.linoides,H.
myriathumand H.polyanthemum)againstseveralemerging
fun-galstrains,withbetterresultsforH.carinatum.Accordingtothese authors,dimericphloroglucinolderivatives(uliginosinB, hyper-brasilolBand japonicinA),present inlipophilicfractions could
http://dx.doi.org/10.1016/j.bjp.2016.08.001
beresponsiblefor theantifungalactivityshowed byHypericum
species.Othercompoundswithphloroglucinolpatternsuchas ben-zopyransandbenzophenonesalsoshowedantifungalactivity.
Duetotheindiscriminateuseofantifungalsandtheincrease ofinfections causedbyemerging species newalternative treat-mentsarenecessary.Thus,theaimofthisworkwastocontinue the study with Hypericum carinatum Griseb. lipophilic fraction (LF),investigating the effect of its associationwithfluconazole againsttheemergingyeastsCandidakrusei,C.famata,C.parapsilosis
andCryptococcusneoformans.ThesynergisticactivitybetweenLF
andfluconazolewasassessedbytwomethodologiesformultiple dose–responseanalysis:checkerboardandisobologram.
Materialsandmethods
Plantmaterial
AerialpartsofHypericumcarinatumGriseb.,Hypericaceae,were collectedinRioGrandedoSul,Brazil,inDecemberof2009.Voucher specimens are deposited in the herbarium of Federal Univer-sityRioGrandedoSul(ICN).Plantscollectionwasauthorizedby IBAMA(BrazilianInstituteofAmbientMediaandRenewable Nat-uralResources)(n◦003/2008,protocol:02000.001717/1008-60).
Lipophilicfractionpreparation
Thedriedandpowderedplantmaterial(ca.500g)wasextracted withhexaneatroomtemperature.Theextractwaspooled, evap-oratedtodryness under reduced pressure,and theepicuticular waxeswereremovedbyacetonetreatment.Thelipophilicfraction (LF)wasstoredat−20◦Cuntilbiologicalandchemicalevaluation. LFwasanalyzed byHPLC using a Shimadzu600pump (LC-6AD) and a Shimadzu SPD-10A dual absorbance detector. The separations were carried out with an isocratic solvent system (60% acetonitrile:40% water) to benzophenones determination and (95% acetonitrile, 5% water, 0.01% trifluoroacetic acid) to phloroglucinolderivativesusingaWatersNova-PackC18column (4m,3.9mm×150mm)adaptedtoaWatersNova-PackC1860 ˚A (3.9mm×20mm)guardcolumn.Theflowratewas1ml/min,the detectorsensitivitywas1.0Aufs,andthedetectionwasperformed at270/220nmatroomtemperature.
Constituentswereidentifiedbycomparisonwiththeretention timesoftheauthenticsamplesandco-injectionofisolated com-pounds.The yieldswereexpressedin %(weightcompound per weightdryextract)asmeanoftwoinjections.
LFtoxicity
The experimental protocol was approved by Local Ethical Committee(Protocol23081,UNIPAMPA).Thetoxicity ofLFwas evaluatedbycellviabilitytestandcometassay,accordingtoGüez etal.(2012),analyzingthreedifferentfractionconcentrations:500, 250and100g/ml.
Fungalstrains
Fourresistantstrainstofluconazolewereusedinthisstudy. Interpretativecriteriaofresistancewereusedaccordingto break-points from M27-S4 document (CLSI, 2012) to Candida and accordingtoEspinel-Ingroffetal.(2012)toCryptococcus neofor-mans.AllstrainsaredepositedintheMycologyCollectionofFederal UniversityofRioGrandedoSul,Brazil:Candidafamata(RL23) ori-ginatesfromhemoculture,C.krusei(CK03)fromNationalProgram ofQualityControl, C.parapsilosis(RL11) fromurineand
Crypto-coccusneoformans(HCCRY01)fromenvironment(environmental
pathogenic).C.kruseiATCC6258wasincludedascontrolinthe susceptibilitytesting.
Antifungalactivity
The screeningfor antifungal activity wascarried out witha concentration of 500g/ml. In order to achieve the test con-centration,samplesweresolubilizedwithdimethylsulfoxide2% (DMSO)andsabourauddextrosebroth(SDB).Further,the mini-malinhibitoryconcentration(MIC)wasdeterminedbythebroth microdilutionmethodaccordingtoM27-A3protocol(CLSI,2008). TheMICwasdefinedasthelowestconcentrationofLFinwhich themicroorganismtesteddidnotdemonstratevisiblegrowth.In microdilutionexperiments,samplesweresolubilizedwithDMSO 2%andRPMI-MOPSmedium(RPMI1640medium)containingl
-glutamine,withoutsodiumbicarbonatebufferedtopH7.0with 0.165mol/lofMOPSbuffer.TheconcentrationsofLFrangedfrom 1.9to500g/mlandallexperimentswerecarriedoutinduplicate. ControlwithDMSO2%waspreviouslyperformed.
Associationstudies
Checkerboardassay
TheeffectoffluconazolecombinedwithLFwasevaluatedin quadruplicateusingthecheckerboardmethod(Johnsonetal.,2004) withslightlymodifications.Thefluconazolefinalconcentrations ranged from 0.5 to 32g/ml for C. famata and C. neoformans,
and 4to64g/ml forC.krusei andC.parapsilosis.On theother
hand, theconcentration of LFranged from 31.25to 250g/ml
for C.famata and C.neoformansand 4 to250g/ml for C.
kru-sei and C. parapsilosis. Plates were incubated at 37◦C for 48h
and then, thetetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide(MTT)wasusedtoassessthefungal cellviability.Interactionwasevaluatedalgebraicallyby determin-ingthefractionalinhibitoryconcentrationindex(FICI)definedas thesumoftheMICofeachdrugincombination,dividedbythe MICofthedrugusedalone.AnFICI≤0.5isconsideredsynergistic; >0.5and≤1additive;>1and≤4indifferent,and>4antagonistic (KontoyiannisandLewis,2003).
Isobologram
TheisobologramwasperformedwiththeassociationofLFand fluconazoleagainstC.krusei(CK03)andC.parapsilosis(RL11).
A curveconcentration-effectof LFor fluconazolewas deter-minedwithlogarithmicconcentrations,inordertoobtaintheIC50 (inhibitoryconcentration50%)bynon-linearregression.Then,with theseresults,curvesconcentration-effectofassociationwerealso performedbynon-linear regression(Tallarida,2006,2007).The proportionofcombinationsisdemonstratedinTable1.
Theoreticaladditivecurves(IC50add)werecalculatedtoeach combinationaccordingtheequation:
Conc.add=f×Conc.fluconazole+(1−f)×Conc.Fraction
where, Conc.fluconazole and Conc.Fraction represent the equi-effectiveconcentrationofeachtreatmentaloneandfisthefraction ofeachsamplethatcomposestheactiveconcentrationof associ-ation(inthisstudytwofvalues0.5(50:50)and0.7(70:30)were used).Conc.addisthetotalconcentrationanditsvariancewas cal-culatedbythisequation:
VarIC50add=f∗2× VarIC50fluconazole+(1−f)∗2
×VarIC50fraction
Table1
Proportionofcombinationsusedinisobologramstudies.
Yeastsstrains Fluconazoleconcentration (%IC50)
LFconcentration (%IC50)
Candidakrusei
CK03
50 50
25 25
12.5 12.5
6.25 6.25
3.125 3.125
70 30
35 15
17.5 7.5
8.75 3.75
4.38 1.875
2.19 0.938
1.095 0.496
Candida parapsilosisRL11
50 50
25 25
12.5 12.5
6.25 6.25
3.125 3.125
70 30
35 15
17.5 7.5
8.75 3.75
4.38 1.875
2.19 0.938
1.095 0.496
CandidaparapsilosisRL11:IC50fluconazole:26.55g/mlandIC50LF:174.7g/ml; CandidakruseiCK03:IC50fluconazole:35.58g/mlandIC50LF:35.76g/ml.
Besides,theinteractionmagnitudewascalculatedthrough inter-actionindex(),followingtheformula:
␥b=dosefluconazoleIC50mixture+dosefractionIC50mixture
Theinteractionindexisanindicatorofthepotencyofthe asso-ciation.Valuesnextto1indicateadditiveinteraction;valueshigher than1,antagonisticinteraction,andvalueslowerthan1,synergistic interaction(GrabovskyandTallarida,2004).
Statisticalanalysis
Checkerboardandtoxicitydatawereevaluatedusingone-way analysis of variance (ANOVA) followed by Tukey’s test (Sigma Stat3.2software,JandelScientificCorporation®).Incheckerboard, thedifference between antifungal activity of fluconazole alone andincombinationwithLFwasevaluated.Differenceswere con-sideredstatisticallysignificantatp<0.05.The isobologramdata wereperformedwithStudentt test, whereIC50 mixtureis sig-nificantlyshorterthanIC50calculated(IC50add)toadetermined combination,thereisasynergisticinteraction(Coddetal.,2008).
Thenon-linearregressionanalysiswasperformedusingGraphPad Prism®version4.02.
Resultsanddiscussion
Chemicalanalysis
HPLC analysis were carried out to quantify the major con-stituents of LF. As demonstrated by Barros et al. (2013), the main constituents of H. carinatum lipophilic fraction are the phloroglucinolderivativeuliginosinB(1)(1.65±0.08%)andthe benzophenonescariphenoneA(2)(0.08±0.001%)andcariphenone B(3)(0.58±0.009%),confirmingthepreviousresults.
LFtoxicity
Theinvestigatedfraction(LF)didnotshowtoxiceffectsatthe concentrationused(250g/ml)inassociationstudiesas demon-stratedinFig.1.Accordingtotheseresults,theconcentrationof 500g/mlshowedDNAdamage(Fig.1A)aswellasreduced cellu-larviability(Fig.1B).Therefore,thehigherLFconcentrationused atthisstudy(250g/ml)isconsideredsafebythesetwotoxicity methodologies.
50
A
B
10080
60
40
20
0 40
30
20
% DNA damage Cell viability
, %
a
a a a
a a b
b b b
10
0
Negativ e control
Negativ e control
Positiv e control
Positiv e control LF 50
µg/ml
LF 50 µg/ml
LF 250 µg/ml
LF 250 µg/ml
LF 100 µg/ml
LF 100 µg/ml
Fig.1.(A)DNAdamageindexdeterminedbycometassayand(B)CellviabilityinleucocytesforHypericumcarinatumlipophilicfraction(LF)inthreedifferentconcentrations. Phosphatebufferedsaline(PBS)wasusedasnegativecontrolandhydrogenperoxide(10M)(H2O2)aspositivecontrolinbothexperiments.DMSO2%wasusedasdiluent
Table2
Minimalinhibitoryconcentration(MIC)ofHypericumcarinatumlipophilicfraction (LF)againstemergingyeastsstrains.
Species Strains MICaLF
Candidafamata RL23 250
Candidakrusei CK03 >1000
Candidaparapsilosis RL11 250
Cryptococcusneoformans HCCRY01 125
aMIC(
g/ml):minimalinhibitoryconcentration.
Antifungalactivity
Concerningtheantifungal capacity,LFwascapableofinhibit thefungalgrowthinamoderateway(Table2).Thiscapacitymay beattributedto thepresence ofdimeric phloroglucinol deriva-tivesasuliginosinB(1)andthebenzophenonescariphenoneA(2) andcariphenoneB(3).Theseresultsareinaccordancewiththose describedbyBarrosetal.(2013).
Associationstudies
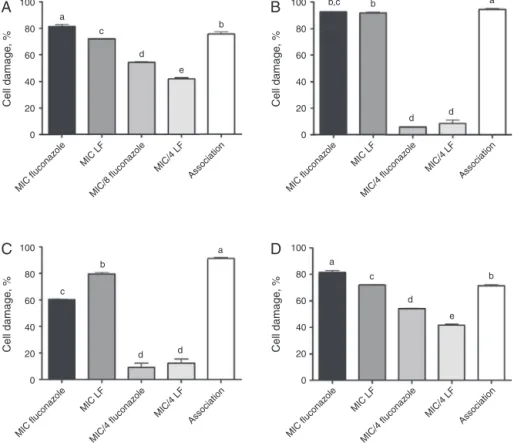
Theresultsobtainedinthecheckerboardanalysis(Fig.2)are interesting,sinceLFwascapableofreducingthefluconazoleMIC values for allspecies tested. For C.neoformans,C. kruseiand C.
parapsilosisthefluconazoleMICdecreasedabouteightfold(%Cell
damage=75.6%,ICIF=0.375;%Celldamage=91.2%,ICIF=0.25and %Celldamage=71.3%,ICIF=0.5,respectively),whileforC.famata
thisvaluewasaboutfourfold(%Celldamage=94.4%,ICIF=0.5). Nevertheless,forC.neoformansandC.parapsilosis,thefluconazole MICwascapableofachievingahighercelldamageincomparision withassociation.Therefore,theuseofthecombinationsisonly jus-tifiedwhendecreaseofdrugdoseisneeded,especiallyincases wherethemicroorganismsareresistanttothisazole.
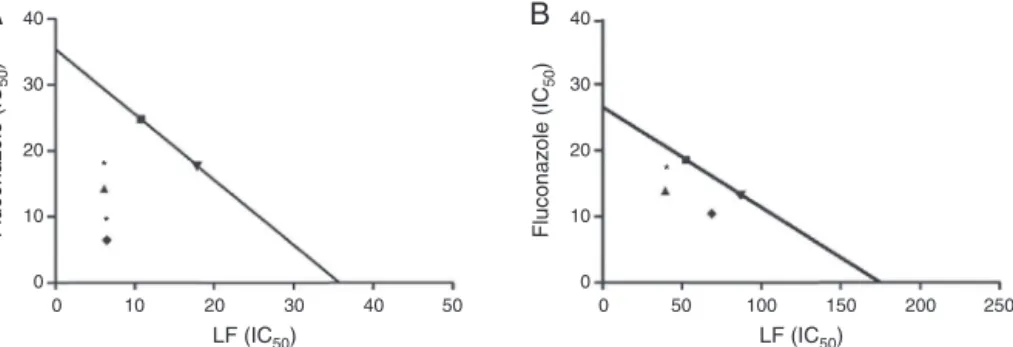
Concerningtheisobologramanalysisthecurvesconcentration effectofeachcompoundtested(fluconazoleandLF)showedIC50 valuesof35.58g/mland35.76g/mlforC.kruseiand26.55g/ml and174.7g/mlforC.parapsilosis,respectively.Itisimportantto notethatthismethodologywasnotappliedtoC.famataand
Cryp-tococcusneoformansduetotheimpossibilityoftoconstructdose
responsecurveswithfluconazolealone.
Theresultsobtainedintheisobologram(Fig.3),areinagreement withthoseobtainedbythecheckerboardanalysis,where synergis-ticeffectwasfoundtobothspeciestested(C.kruseiCK03andC.
parapsilosisRL11).Theinteractionindex()wasless than1for
allproportionstestedforC.krusei(50:50=0.36;70:30=0.57)and forC.parapsilosis(50:50=0.79;70:30=0.75).However,thisindex againstC.parapsilosiswascloserto1,indicatingaprobable pres-enceofadditiveeffectinsteadofsynergistic,corroboratingwiththe ICIF(0.5)foundforthisassociationinthecheckerboardanalysis.
TheincreasedincidenceofsystemicinfectionscausedbyNCA species andthehighmortalityrates due toacquiredresistance againstdrugs currentutilized isworrisome,aswellasthehigh incidenceofpolymicrobialfungalinfections(Ruhnke,2014;Trifilio et al.,2015).Therefore, theassociationbetween different com-poundscouldbeanexcellentstrategytoreducethedrugdoses, andthus,achievetheresistancereversion.
TherearetwohypothesestolipophilicfractionofH.carinatum
decreasestheMICoffluconazole.Thefirstcouldberelatedtothe generalactionmechanismofphenoliccompounds,changethe fun-galdimorphism(Zhangetal.,2011)and/oropeningofmembrane ionicchannels(Raoetal.,2010)bothfoundforC.albicans.The sec-ondhypothesisliesinthefactthatsomebenzophenonesareable toblockthecytochromeP-450(Podustetal.,2007).Nevertheless, sinceitisafraction,thesynergisticeffectsofthebioactive com-poundsmixturecouldberesponsiblebyincreasingtheeffectivity
100 a
c
d e
b
b,c
a c
d e
b
b a
d d
80
60
40
Cell damage
, %
20
0
100
80
60
40
Cell damage
, %
A
C
B
D
20
0
MIC fluconaz ole
MIC/8 fluconaz ole
MIC LF MIC/4 LF
Association
100
c b
d d
a
80
60
40
Cell damage
, %
20
0
MIC fluconaz ole
MIC/4 fluconaz ole
MIC LF MIC/4 LF
Association
MIC fluconaz ole
MIC/4 fluconaz ole
MIC LF MIC/4 LF
Association
100
80
60
40
Cell damage
, %
20
0
MIC fluconaz ole
MIC/4 fluconaz ole
MIC LF MIC/4 LF
Association
Fig. 2.Interaction between the lipophilic fractionof Hypericum carinatum (LF) and fluconazoleagainst Cryptococcus neoformans HCCRY01 (MICFluco=32g/ml,
MICLF=250g/ml,Association=MIC/8Fluco:MIC/4LF)(A),CandidafamataRL23(MICFluco=8g/ml,MICLF=250g/ml,Association=MIC/4Fluco:MIC/4LF)(B),Candidakrusei
CK03 (MICFluco=32g/ml, MICLF>250g/ml, Association=MIC/4Fluco:MIC/4LF) (C) and Candida parapsilosis RL 11(MICFluco=32g/ml, MICLF=250g/ml,
40
30
20 *
* 10
Fluconaz
ole (IC
50
)
A
B
0
0 10 20 30 40
LF (IC50)
50
40
30
20
10
Fluconaz
ole (IC
50
)
0
0 50 100 150 200
LF (IC50)
250 *
Fig.3.InteractionanalysisoffluconazolewiththelipophilicfractionofHypericumcarinatum(LF)(IC50)againstCandidakrusei(CK03)(A)andCandidaparapsilosis(RL11)
(B).Thecontinuouslinerepresentstheadditivitylineandthepointstheexperimentalcombinationsatdifferentlevels.*representssignificantdifferencesbetweenDeqADD (calculated)andDeqmix(experimental)withp<0.05.()Additiveequieffectiveconcentration(70:30),()Concentrationequi-effectiveoftheassociation(70:30),() Additiveequi-effectiveConcentration(50:50)and()concentrationequi-effectiveoftheassociation.
ofitself,andthen,theantifungaleffectisachievedbyasumof mechanisms(Wagner,2011).
Somestudiesreportassociationbetweenextractsand antifun-galdrugssuchasessentialoilsinassociationwithketoconazole againstseveralfungalspecies(Giordanietal.,2004)and benzophe-noneenrichedfractionfromBrazilianredpropoliswithfluconazole andanidulafunginagainstC.parapsilosisandC.glabrata(Pippietal., 2015).Ontheotherhand,manystudieshavedemonstratedthe associationbetweenplantmetabolitesandantifungaldrugsagainst
Candidaspecies.Forexample,theassociationofthetannin
puni-calaginandfluconazoleagainstC.albicansandC.parapsilosis(Endo etal.,2010)andflavonoids(catechin,quercetinand epigallocat-echingallate)associatewithfluconazoleagainstC.tropicalis(Da Silvaetal.,2014).
TherearenodoubtsthatcombinedtherapybetweenLFand flu-conazoleisbenefic,butfurtherstudiesmustbeperformedinorder todeterminethenatureofthisinteraction.Theanalysisofisolated compoundsofthisfractionsaloneand/orcombinedwith flucona-zoleisneededaimingtostandardizethisassociationincaseswhere themonotherapywithfluconazoleisineffective.
Conclusion
TheresultsofthisstudyreinforcetheuseofHypericumspecies as source of products with biological importance. Association studiesareverysignificant,especiallyinemerging fungi,which are worldwide distributed and frequent causesof infections in immunocompromisedpatients. ThelipophilicfractionofH.
car-inatumwasabletoreduce theMICof fluconazole,probablyby
facilitating theaccess ofthe drugwithinthe fungalcell. These resultsareimportantduetotheincreasingresistanceof emerg-ingyeastspeciestoavailabledrugsused foravariety offungal infectionsandtheexplorationofpotentialalternativetherapeutic sourcesformultidrugtherapy.
Authors’contributions
GCM(PhDstudent)contributedinfractionpreparation, chem-ical characterization, biological studies (antifungal activity and associationstudies), analysis ofdata and draftedthe paper.BP contributedtobiologicalstudies(antifungalactivityand associa-tionstudies–checkerboard)andcriticalreadingofmanuscript,CH contributedtobiologicalstudies(antifungalactivity),FMCB con-tributedtochemicalcharacterization,LFSOcontributedtotoxicity studies,GLVPandAMFsupervisedthelaboratoryworkand con-tributedtocriticalreadingofthemanuscript.Alltheauthorshave readthefinalmanuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
TheauthorsaregratefultotheBrazilianagencies(CAPES,CNPq andFAPERGS)forfinancialsupportandbyfellowships.Theauthors are also grateful toDr. Sérgio Bordignon (UNILASALLE, RS) for botanicalspeciesidentification.
References
Alcazar-Fuoli,L.,Mellado,E.,2014.Currentstatusofantifungalresistanceandits impactonclinicalpractice.Br.J.Haematol.166,471–484.
Barros,F.M.C.,Pippi,B.,Dresch,R.R.,Dauber,B.,Luciano,S.C.,Apel,M.A.,Fuentefria, A.M.,vonPoser,G.L.,2013.Antifungalandantichemotacticactivitiesand quan-tificationofphenoliccompoundsinlipophilicextractsofHypericumspp.native toSouthBrazil.Ind.CropsProd.44,294–299.
CLSIM27–A3,2008.ReferenceMethodforBrothDilutionAntifungal Susceptibil-ityTestingofYeasts:ThirdInformationalSupplement.ClinicalandLaboratory StandardsInstitute,Wayne,PA,USA.
CLSIM27-S4,2012.ReferenceMethodforBrothDilutionAntifungal Susceptibil-ityTestingofYeasts:FourthInformationalSupplement.ClinicalandLaboratory StandardsInstitute,Wayne,PA,USA.
Codd,E.E.,Martinez,R.P.,Molino,L.,Rogers,K.E.,Stone,D.J.,Tallarida,R.J.,2008.
Tramadolandseveralanticonvulsantssynergizeinattenuatingnerve injury-inducedallodynia.Pain134,254–262.
DaSilva,C.R.,DeAndradeNeto,J.B.,DeSousaCampos,R.,Figueiredo,N.S.,Sampaio, L.S.,Magalhães,H.I.F.,Cavalcanti,B.C.,Gaspar,D.M.,DeAndrade,G.M.,Lima, I.S.P.,DeBarrosViana,G.S.,DeMoraes,M.O.,Lobo,M.D.P.,Grangeiro,T.B.,Nobre, H.V.,2014.Synergisticeffectoftheflavonoidcatechin,quercetin,or epigallocat-echingallatewithfluconazoleinducesapoptosisinCandidatropicalisresistant tofluconazole.Antimicrob.AgentsChemother.58,1468–1478.
Dulger,G.,Dulger,B.,2014.AntifungalactivityofHypericumhavvaeagainstsome medicalCandidayeastandCryptococcusspecies.Trop.J.Pharm.Res.13,405–408.
Endo,E.H.,GarciaCortez,D.A.,Ueda-Nakamura,T.,Nakamura,C.V.,DiasFilho,B.P., 2010.Potentantifungalactivityofextractsandpurecompoundisolatedfrom pomegranatepeelsandsynergismwithfluconazoleagainstCandidaalbicans. Res.Microbiol.161,534–540.
Giordani,R.,Regli,P.,Kaloustian,J.,Mikaïl,C.,Abou,L.,Portugal,H.,2004.Antifungal effectofvariousessentialoilsagainstCandidaalbicans.Potentiationofantifungal actionofamphotericinBbyessentialoilfromThymusvulgaris.Phyther.Res.18, 990–995.
Grabovsky,Y.,Tallarida,R.J.,2004.Isobolographicanalysisforcombinationsofafull andpartialagonist:curvedisoboles.J.Pharmacol.Exp.Ther.310,981–986.
Güez,C.M.,Waczuk,E.P.,Pereira,K.B.,Querol,M.V.M.,daRocha,J.B.T.,deOliveira, L.F.S.,2012.Invivoandinvitrogenotoxicitystudiesofaqueousextractof Xan-thiumspinosum.Braz.J.Pharm.Sci.48,461–467.
Johnson,M.D.,Macdougall,C.,Ostrosky-zeichner,L.,Perfect,J.R.,Rex,J.H.,2004.
Minireviewcombinationantifungaltherapy.Antimicrob.AgentsChemother.48, 693–715.
Kontoyiannis,D.P.,Lewis,R.E.,2003.Combinationchemotherapyforinvasivefungal infections:whatlaboratoryandclinicalstudiestellussofar.DrugResist.Updat. 6,257–269.
Musiol,R., Mrozek-Wilczkiewicz,a, Polanski,J.,2014. Synergy againstfungal pathogens:workingtogetherisbetterthanworkingalone.Curr.Med.Chem. 21,870–893.
clinicalbreakpointsforfluconazoleandCandida:timeforharmonizationofCLSI andEUCASTbrothmicrodilutionmethods.DrugResist.Updat.13,180–195.
Pfaller,M.,Neofytos,D.,Diekema,D.,Azie,N.,Meier-Kriesche,H.U.,Quan,S.P.,Horn, D.,2012.Epidemiologyandoutcomesofcandidemiain3648patients:data fromtheprospectiveantifungaltherapy(PATHAlliance®)registry,2004–2008. Diagn.Microbiol.Infect.Dis.74,323–331.
Pippi,B.,Lana,A.J.D.,Moraes,R.C.,Güez,C.M.,Machado,M.,deOliveira,L.F.S.,Lino vonPoser,G.,Fuentefria,A.M.,2015.Invitroevaluationoftheacquisitionof resis-tance,antifungalactivityandsynergismofBrazilianredpropoliswithantifungal drugsonCandidaspp.J.Appl.Microbiol.118,839–850.
Podust,L.M.,vonKries,J.P.,Eddine,A.N.,Kim,Y.,Yermalitskaya,L.V.,Kuehne,R., Ouellet,H.,Warrier,T.,Alteköster,M.,Lee,J.S.,Rademann,J.,Oschkinat,H., Kaufmann,S.H.E.,Waterman,M.R.,2007.Small-moleculescaffoldsforCYP51 inhibitorsidentifiedbyhigh-throughputscreeninganddefinedbyX-ray crys-tallography.Antimicrob.AgentsChemother.51,3915–3923.
Rao,A.,Zhang,Y.,Muend,S.,Rao,R.,2010.Mechanismofantifungalactivityof ter-penoidphenolsresemblescalciumstressandinhibitionoftheTORpathway. Antimicrob.AgentsChemother.54,5062–5069.
Ruhnke,M.,2014.AntifungalstewardshipininvasiveCandidainfections.Clin. Micro-biol.Infect.20Suppl.6,11–18.
Silva,S.,Negri,M.,Henriques,M.,Oliveira,R.,Williams,D.W.,Azeredo,J.,2012. Can-didaglabrata,CandidaparapsilosisandCandidatropicalis:biology,epidemiology, pathogenicityandantifungalresistance.FEMSMicrobiol.Rev.36,288–305.
Tallarida,R.J.,2007.Interactionsbetweendrugsandoccupiedreceptors.Pharmacol. Ther.113,197–209.
Tallarida,R.J.,2006.Anoverviewofdrugcombinationanalysiswithisobolograms. J.Pharmacol.Exp.Ther.319,1–7.
Trifilio,S.,Zhou,Z.,Fong,J.L.,Zomas,A.,Liu,D.,Zhao,C.,Zhang,J.,Mehta,J.,2015.
Polymicrobialbacterialorfungalinfections:incidence,spectrumofinfection, riskfactors,andclinicaloutcomesfromalargehematopoieticstemcell trans-plantcenter.Transpl.Infect.Dis.17,267–274.
Vandeputte,P.,Ferrari,S.,Coste,A.T.,2012.Antifungalresistanceandnewstrategies tocontrolfungalinfections.Int.J.Microbiol.2012,1–27.
Wagner,H.,2011.Synergyresearch:approachinganewgenerationof phytophar-maceuticals.Fitoterapia82,34–37.
Zhang,L.,Chang,W.,Sun,B.,Groh,M.,Speicher,A.,Lou,H.,2011.Bisbibenzyls,a newtypeofantifungalagent,inhibitmorphogenesisswitchandbiofilm for-mationthroughupregulationofDPP3inCandidaalbicans.PLoSOne6,1–8,