Tânia Sofia Granja Tavares
Dissertation presented to obtain
the Ph.D degree in
Engineering and Technological Sciences
–
Biochemistry
Instituto de Tecnologia Química e Biológica | Universidade Nova de Lisboa
Oeiras,
Tânia Sofia Granja Tavares
Dissertation presented to obtain the Ph.D degree in
Engineering and Technological Sciences
–
Biochemistry
Instituto de Tecnologia Química e Biológica | Universidade Nova de Lisboa
Oeiras, October, 2011
biological activity of peptides obtained via
hydrolysis from whey proteins by cardosins
RESUMO
É cada vez mais importante seguir e manter uma dieta saudável; porém, a maioria das pessoas predispõe-se a fazê-lo desde que não altere demasiado os seus hábitos. Para além do seu elevado valor nutritivo, as proteínas desempenham, em geral, diversas funções fisiológicas. Existe, com efeito, uma forte relação entre a nutrição e a ocorrência de doenças, o que sugere que as proteínas dos alimentos – e em particular os péptidos delas derivados (através da digestão, ou por processos anteriores à ingestão), desempenham funções importantes na prevenção e/ou tratamento de patologias associadas à desnutrição ou a agentes patológicos. Assim sendo, tais compostos podem ser bastante promissores como ingredientes no desenvolvimento de novos alimentos funcionais, susceptíveis de reduzir (ou controlar) certas doenças crónicas, para além de promoverem a saúde e assim reduzirem os custos associados com os cuidados de saúde. Em particular, péptidos bioactivos podem ser produzidos comercialmente para aplicação como ingredientes nutracêuticos.
O leite e os produtos lácteos são comprovadamente alimentos funcionais, o que tem suscitado um grande interesse por parte da indústria de lacticínios; um exemplo é o desenvolvimento de tecnologias destinadas à produção de péptidos bioactivos em grande escala, a partir de proteínas do leite. Especificamente, as proteínas do soro (obtidas como sub-produto da produção do queijo) e os peptidos bioactivos delas derivados podem ser usados como suplementos dietéticos, assim como em preparações farmacêuticas ou ingredientes funcionais – constituindo-se, assim, numa promissora utilização alternativa do soro.
usando extractos aquosos de Cynara cardunculus; e a caracterização in vivo de quatro tipos de actividade biológica (anti-hipertensiva, antiulcerogénica, antinociceptiva e anti-inflamatória) desses concentrados peptídicos.
As condições óptimas de hidrólise de CPS, α-lactoalbumina (α-La) e caseinomacropeptido (CMP), catalisada por aquelas enzimas vegetais, foram determinadas por resposta de superfície, usando como funções objectivo o grau de hidrólise (GH), a actividade inibitória da enzima conversora de angiotensina (ECA) e a actividade antioxidante; o tempo de hidrólise e a razão enzima/substrato foram usados como parâmetros. O modelo sugerido mostrou-se estatisticamente apropriado para descrever a actividade inibitória da ECA exibida pelos hidrolisados de CPS e α-La, mas não de CMP. As condições óptimas encontradas foram: 1.6 %(v/v) de extracto enzimático da planta, durante 7 h, a 55 ºC e pH 5.2, sobre 40 g L-1 de substrato proteico. Tais condições óptimas conduziram a máximos de GH de 18 e 9 % para CPS e α-La, respectivamente; a concentração de proteína que conduziu à inibição da ECA em 50 % (IC50) foi de 105.4 (fracção total) e 25.6 µg mL-1 (fracção <3 kDa) para o CPS, e 47.6 e 22.5
µg mL-1, respectivamente, para a α-La. A actividade antioxidante do CPS e da α-La foi de 0.96 ± 0.08 e 1.12 ± 0.13 µmolequivalentes trolox mg-1proteína
hidrolisada, respectivamente.
apresentaram baixos níveis de lactose e sais, para além de uma carga microbiana desprezável.
Sabendo que a ECA desempenha um importante papel na regulação da função cardiovascular, foi então efectuada uma triagem preliminar da actividade inibidora da ECA associada a PepC e <3PepC – a qual apresentou valores de IC50 de 52.9 ± 2.9 e 23.6 ± 1.1 µg mL-1, respectivamente. O concentrado <3PepC foi submetido a cromatografia liquida de alta eficiência em fase reversa, tendo-se obtido 6 fracções com actividade inibidora da ECA; e a sua composição peptídica foi devidamente determinada. De entre os vários péptidos encontrados, 14 foram identificados através de espectrometria de massa. Destes péptidos, 11 foram sintetizados de novo – por forma a avaliar a sua actividade inibidora da ECA na ausência de qualquer contaminante, e também para averiguar da sua estabilidade quando submetidos a digestão gastrointestinal simulada. De entre eles, foram encontrados 3 novos péptidos bastante potentes – correspondendo à α-La f(16-26) com a sequência KGYGGVSLPEW, α-La f(97-104) com DKVGINYW e β-Lg f(33-42) com DAQSAPLRVY; os seus valores de IC50 foram tão baixos como 0.80 ± 0.1, 25.2 ± 1.0 e 13.0 ± 1.0 µg mL-1, respectivamente. Nenhum deles se manteve estável na presença de enzimas gastrointestinais: de facto, os mesmos foram parcialmente, ou até mesmo totalmente hidrolisados a pequenos péptidos. No entanto, a actividade inibidora da ECA não foi substancialmente afectada em dois destes péptidos.
Por forma a validar in vivo tais estudos, foi investigada a actividade anti-hipertensiva do PepC, da fracção <3PepC e dos supracitados 3 péptidos bastante potentes. Para tal, foram medidas as pressões sanguíneas sistólicas e diastólicas através do método de tail-cuff – antes, e 2, 4, 6, 8, e 24 h após a administração oral, por entubação gástrica, de 400 mg kg-1
peso corporal (pc) dos concentrados, ou 5 mg kg-1pc dos péptidos
de idade. Utilizou-se a água e o zofenopril (5 mg kg-1
pc) – um conhecido inibidor da ECA, como controlo negativo e positivo, respectivamente. A administração pontual de PepC, <3PepC, KGYGGVSLPEW, DKVGINYW e DAQSAPLRVY despoletou efeitos anti-hipertensivos nos REH; o efeito máximo ocorreu 4 h e 6 h após a administração dos referidos concentrados peptídicos e dos correspondentes péptidos sintéticos, respectivamente. No caso de PepC e KGYGGVSLPEW, o efeito sobre a pressão sanguínea foi significativamente mais baixo, quando os ratos foram previamente tratados com angiotensina I; e aumentou após o tratamento prévio com bradiquinina.
Por forma a elucidar se os referidos concentrados peptídicos são capazes de proteger a mucosa estomacal in vivo contra lesões ulcerativas, procedeu-se a experiências com ratos aos quais foi administrado oralmente etanol absoluto. Pôde concluir-se que PepC e <3PepC são capazes de reduzir, de forma significativa, as referidas lesões gástricas: experiências envolvendo doses únicas de 100 mg kg-1
pc de <3PepC ou PepC conduziram a uma protecção de 68.5 % e 37.4 %, respectivamente – a qual se pode comparar com 93.4 % oferecida por 200 mg kg-1
pc de carbenoxolona (controlo positivo). No entanto, não se encontrou uma relação dose/resposta. A citoprotecção gástrica através de <3PepC parece depender do conteúdo em grupos sulfidrilo, enquanto que o PepC provavelmente protege a mucosa gástrica através do ciclo das prostaglandinas e da produção de óxido nítrico.
O efeito antinociceptivo do referido concentrado peptídico foi determinado, usando os testes de contorção abdominal, placa quente e formalina em ratinhos; enquanto a actividade anti-inflamatória foi avaliada através do edema de pata. O PepC foi, para isso, administrado oralmente em doses de 100, 300 e 1000 mg kg-1
pc; dos resultados obtidos pelo teste
carragenina, verificaram-se actividades antinociceptiva e anti-inflamatória significativas quando se comparou com o grupo de controlo negativo – apesar da ausência de relação dose/resposta. O PepC a 300 mg kg-1
pc apresentou um resultado significativo como adjuvante, no teste de contorções abdominais, quando co-administrado a baixas concentrações (1 e 3 mg kg-1
pc) de indometacina – relativamente à administração desta
droga isoladamente. Pelo contrário, não se observaram diferenças estatisticamente significativas quando a mesma concentração de PepC foi co-administrada com dexametasona a 3, 10 e 30 mg kg-1
pc, no teste do edema de pata. No teste da placa quente, o PepC a 1000 mg kg-1
pc não despoletou nenhuma resposta notável.
ABSTRACT
It is increasingly important to follow and maintain a healthy diet; however, most people are not willing to do so unless they can avoid dramatic changes in their habits. In addition to their high nutritional value, proteins in general play various physiological parts; there are indeed relationships between nutrition and occurrence of disease, thus suggesting that food proteins, and the peptides derived therefrom via digestion or processing upstream, may contribute to preventing and/or treating diseases. Hence, they are promising ingredients for the development of functional foods. The reduction in incidence and the treatment of chronic diseases using dietary supplements will obviously promote health, and thus reduce the overall costs of health care.
Several milk and dairy products have proven functional foods, and have consequently aroused considerable interest from the dairy industry. One example is the development of appropriate technologies for large-scale production of bioactive peptides from dairy proteins. In particular, whey proteins, obtained as a byproduct of cheese production, and their biologically active peptides can be used as dietary supplements, as well as in pharmaceutical preparations or functional ingredients – thus providing a promising alternative for whey upgrade.
Therefore, this PhD research program sought to produce, on a pilot scale, a bioactive whey peptide concentrate, via hydrolysis of a bovine whey protein concentrate (WPC) brought about by aqueous extracts of Cynara cardunculus. It then entailed characterization, in vivo, of a number of biological activities of such peptide concentrates (i.e. antihypertensive, antiulcerogenic, antinociceptive and anti-inflammatory).
degree of hydrolysis (DH), angiotensin-converting enzyme (ACE)-inhibitory activity and antioxidant activity as objective functions. The hydrolysis time and enzyme/substrate ratio were chosen as manipulated parameters. The model hypothesized was statistically appropriate to describe the ACE-inhibitory activity of hydrolyzates from WPC and α-La, but not from CMP. Optimum conditions found were 1.6 %(v/v) crude plant enzyme extract, and action for 7 h, at 55 ºC and pH 5.2, on 40 g L-1 substrate protein. The maximum DH was 18 and 9 %, for WPC and α-La, respectively. The concentration leading to 50 % ACE inhibition (IC50) was 105.4 (total fraction) and 25.6 µg mL-1 (<3 kDa fraction) for WPC, and 47.6 and 22.5 µg mL-1, respectively, for α-La. The antioxidant activities of WPC and α-La were 0.96 ± 0.08 and 1.12 ± 0.13 µmoltrolox equivalent mg-1hydrolyzed protein, respectively.
Employing optimal hydrolysis conditions, the pilot scale manufacture of protein and peptide concentrates from bovine whey was achieved using selective filtration techniques. The profiles of said concentrates were assessed by chromatography and electrophoresis; ca. 87 % of α-La was hydrolyzed, but essentially no degradation of β-lactoglobulin (β-Lg) occurred. A peptide concentrate (PepC), its fraction below 3 kDa (<3PepC) and a β-Lg-rich fraction were obtained as final products, containing ca. 73, 43 and 91 % protein (on a total solid mass basis, respectively). All these fractions were low in lactose and salt, and their microbial loads were negligible.
Among the various peptides found, a total of 14 were identified via sequencing with ion-trap mass spectrometry. Eleven of said peptides were synthesized de novo to validate their ACE-inhibitory activity without any contaminant, and also to ascertain their stability when exposed to simulated gastrointestinal digestion. Among them, 3 novel, highly potent peptides were found, corresponding to α-La f(16-26) with the sequence KGYGGVSLPEW, α-La f(97-104) with DKVGINYW and β-Lg f(33-42) with DAQSAPLRVY. Their IC50 values were as low as 0.80 ± 0.1, 25.2 ± 1.0 and 13.0 ± 1.0 µg mL-1, respectively. None of them remained stable in the presence of gastrointestinal enzymes. They were partially, or even totally, hydrolyzed to smaller peptides; however, the observed ACE-inhibitory activity was not substantially affected in two of those peptides.
To validate these results in vivo, the antihypertensive activity of PepC, <3PepC and the aforementioned 3 highly potent peptides found was further investigated. Twelve week-old, spontaneously hypertensive rats (SHR), received 400 mg kg-1
body weight (bw) of said concentrates orally, by
gastric intubation. Their systolic and diastolic blood pressures were monitored by the tail-cuff method – before, and by 2, 4, 6, 8 and 24 h afterwards. Water and zofenopril (5 mg kg-1
bw), a known ACE inhibitor, were used as negative and positive controls, respectively. Acute administration of PepC, <3PepC, KGYGGVSLPEW, DKVGINYW and DAQSAPLRVY triggered antihypertensive effects in SHR. The maximum effect occurred at 4 h and 6 h after the administration of said peptide concentrates and synthetic peptides, respectively. In the case of PepC and KGYGGVSLPEW, the pressor effect of angiotensin I was significantly lower, and the response to bradykinin increased when the rats were pre-treated with either product.
PepC and <3PepC were able to reduce said gastric injuries to significant levels. Single dose experiments, using 100 mg kg-1
bw of either <3PepC or PepC, led to 68.5 % and 37.4 % protection, respectively; these figures compare well with 93.4 % protection by 200 mg kg-1
bw carbenoxolone (a
positive control). However, no dose-response correlation was demonstrated. Gastric cytoprotection by <3PepC appears to depend on sulfhydryl-containing moieties, whereas PepC most likely protects the gastric mucosa via the prostaglandin cycle and production of nitric oxide.
The antinociceptive effect of these peptide concentrates was further assessed using writhing, hot plate and formalin tests in mice. The anti-inflammatory effect was also evaluated using the paw edema test. PepC was thus orally administered at 100, 300 and 1000 mg kg-1
bw; despite the lack of a dose-response correlation, significant antinociceptive and anti-inflammatory activities were obtained using acetic acid-induced abdominal writhing, (late phase) formalin test and carrageenan-induced paw edema test, as compared with the negative control group. PepC at 300 mg kg-1
bw conveyed a significant performance as adjuvant when co-administered with low concentrations (1 and 3 mg kg-1
bw) of indomethacin in the writhing test, as compared to administering said drug alone. Conversely, no statistically significant differences were observed when the same PepC concentration was co-administered with dexamethasone at 3, 10 and 30 mg kg-1
bw in the paw edema test. In the hot plate test, PepC at 1000 mg kg-1
bw did not cause any remarkable response.
commercial β-Lg and 3 %(w/v) PepC, at different pH values (3.0, 4 and 5.5), were submitted to gelation at 80 °C for 30 min. Firmer gels resulted at pH 5.5 and 4.0 than at 3.0. The gelled systems were then subjected to HIUS at 0-4 ºC, and the effect of processing time (2.5-20 min) was ascertained. The size distribution of particles, prior to and immediately after HIUS treatment was measured by dynamic laser light scattering and confocal microscopy. The ultrasound led to an important reduction in particle mean diameter for gels at pH 5.5, with monomodal dispersion, whereas it was not so marked for gels at pH 4 – which even exhibited a bimodal distribution. The peptide encapsulation efficacy was confirmed by chromatographic techniques after precipitating the encapsulated material, and the analysis of soluble peptides.
ACKNOWLEDGEMENTS
Completion of a PhD thesis would hardly be possible without the contribution of several people and institutions. Hence, I would like to formally express my sincere gratitude to:
Instituto de Tecnologia Química e Biológica of Universidade Nova de Lisboa, ITQB-UNL, for acceptance as PhD student in the final stage of my research program.
Escola Superior de Biotecnologia of Universidade Católica Portuguesa, ESB-UCP, for acceptance as PhD student in the first stage of said program.
Fundação para a Ciência e a Tecnologia, for financial support in the form of a PhD grant (ref. SFRH/BD/31604/2006), supported by POPH-QREN, under the supervision of Prof. F. Xavier Malcata.
professional engagements and solicitations during all these years never disturbed my needs, and I never felt as a second priority – on the contrary, so many times he took my needs as the basis for his agenda.
Dr. Tiago Bandeiras, my co-supervisor, for his suggestions throughout the course of the preparation of this written thesis, and for his prompt willingness to help me finalize it.
Dr. Ana Pilosof, Dr. Carlos Dias Pereira, Dr. Isidra Recio, Dr. João Ernesto de Carvalho and Dr. Rosalía Carrón I would like to thank their topical and direct laboratory supervision, for precise guidelines and scientific accuracy; and for the excellent working conditions and great hospitality they made available to me during my stays at Industrial Department of Universidad de Buenos Aires, UBA (Buenos Aires, Argentina), Escola Superior Agrária de Coimbra, ESAC (Coimbra, Portugal), Instituto de Fermentaciones Industriales del Consejo Superior de Investigaciones Científicas, IFI-CSIC (Madrid, Spain), Pharmacology and Toxicology Department of Centro Pluridisciplinar de Pesquisas Químicas Biológicas e Agrícolas da Universidade de Campinas, CPQBA-UNICAMP (Campinas, Brazil) and Physiology and Pharmacology Department of Facultad de Farmacia de la Universidad de Salamanca, FF-US (Salamanca, Spain) respectively. Their knowledge and experience were rather important for the timely and successful completion of my work, and for my evolution as a researcher.
Rizzo, Sirlene Tinti, Vanessa Souza, Dr. María-Ángeles Sevilla and Dr. María-José Montero – thanks for their help and support during the periods spent in the aforementioned research facilities.
Finally, Dr. Manuela Pintado of ESB, for guidance during my research career, and for her trust and dedication to my work – together with the valuable discussions and suggestions throughout the course of the early stages of my PhD program.
I also thank Manuela Amorim of ESB for her constant support – made available during my entire PhD program, for her help in the experimental work, for always being available when I needed (even if that implied working late or after hours), and for her unconditional sympathy and friendship during these 6 years – with so many moments of happiness, and so many experiences shared.
To my family, my husband’s family and my friends. Thanks for all the optimistic incentives throughout these years, as no words can fully express my gratitude.
My dad and mother, for their support and trust, since the beginning of this PhD quest – and for their everlasting encouragement throughout all these years and long before. They were, together with my brothers and husband, the main support to overcome the difficult moments of my life – but thus were also the first to rejoice with my moments of happiness. They always made me believe I could reach my goals, and attain any task I engaged in – and they consistently convinced me that I should live my life but never forgetting the principles of character, fellowship, honesty, respect, truth, honor, justice and perseverance.
My brother and sister, just for being there (for the better and the worse) – always with such an energy and big smile, thus allowing me believe that all my gray days were actually minor problems that I would eventually solve.
At last, but not least, my husband Pedro – for his unconditional patience, understanding, caring support, smile and love, during all these years; for his help when submerged in difficulties, and for his help toward the huge challenges to be overcome – that made (and constantly make) me stronger and stronger.
For all that the above five people have meant and mean to me, and which words fall totally short to describe. I hope they can feel proud of me.
LIST OF ABBREVIATIONS
ACE Angiotensin-converting enzyme
AAPH 2,2′-azobis(2-methylpropionamidine) dihydrochloride Ach Acetylcholine
ANOVA Analysis of variance ATP Adenosine triphosphate AUC Area under the curve BOD Biological oxygen demand BSA Bovine serum albumin bw Body weight
cfu Colony forming units
CGRP Calcitonin gene-related peptide CID Collision-induced dissociation CMP Caseinomacropeptide
CN Casein
CNS Central nervous system COX Cyclooxygenase
CPS Concentrado proteico de soro D32 Means diameter
DBP Diastolic blood pressure
DE50 Dose required to lower ULI by 50 %
DH Degree of hydrolysis DLS Dynamic light scattering DRT Dorsal reticular nucleus DTT Dithiothreitol
E/S Enzyme/substrate EAA Excitatory amino acid
ECA Enzima conversora de angiotensina EDTA Ethylenediamine tetracetic acid EIN Excitatory interneuron
eNOS Endothelial nitric oxide synthase ESI Electrospray ionization
FAB Fast atom bombardment FAPGG Furanocryloyl tripeptide FDM Fat in dry matter FL Fluorescein
GH Grau de hidrólise GSH Glutathione
HHL Hippuryl-histidine-leucine HIUS High-intensity ultrasound
HPLC High performance liquid cromatography
HPLC-MS/MS Liquid cromatography – tandem mass spectroscopy HR Hydrolysis rate
5-HT Serotonin
IC50 Protein concentration required to inhibit ACE activity by 50 %
Ig Immunoglobulin INI Inhibitory interneuron
IT Ion-trap
L-NAME N-ω-L-arginine methyl ester LAB Lactic acid bacteria LDM Lactose in dry matter LF Lactoferrin
LP Lactoperoxidase
MALDI Adsorption by laser-induced matrix MBP Mean blood pressure
MS Mass spectrometry MW Molecular weight MWHEY Microfiltrate skim whey
MWPC Microfiltrate whey protein concentrate NA Noradrenaline
NEM N-ethylmaleimide NO Nitric oxide
NOS Nitric oxide synthase
NSAID Nonsteroidal anti-inflammatory drug NTS Nucleus tractus solitarius
NYPC Non-hydrolyzed protein concentrate ORAC Oxygen radical absorbance capacity PAF Primary afferent fibers
PAG Periaqueductal gray PBN Parabranquial nucleus PDM Protein in dry matter PepC Peptide concentrate
<3PepC Peptide concentrate (<3 kDa) >3PepC Peptide concentrate (>3 kDa) PG Prostaglandins
PN Projection neuron PP Proteose-peptone
QSAR Quantitative structure activity relationship R Enzyme/substrate ratio
R2 Coefficient of determination
REH Ratos espontaneamente hipertensivos
RP-HPLC Reverse-phase high performance liquid cromatography RRSD Relative residual standard deviation
RSD Residual standard deviation RVM Rostroventral medulla SBP Systolic blood pressure SD Standard deviation SDS Sodium dodecyl sulphate
SDS-PAGE Sodium dodecyl sulphate – polyacrylamide gel electrophoresis SEM Standard error of the mean
SH Sulphydryl
SHR Spontaneously hypertensive rats
SP Substance P
T Hydrolysis time
TFF Tangential flow filtration Tgel Gelation temperature TNBS Picrylsulfonic acid TPP Total proteose-peptone
Tris Tris(hydroxymethyl)aminomethane
TX Thromboxane
ULI Ulcerative lesions index USAI Ultrasons de alta intensidade VDF Via descending facilitators VDI Via descending inhibitory WPC Whey protein concentrate WP Whey permeate concentrate WPH Whey protein hydrolyzate
WPHF Whey protein hydrolyzate fraction WPI Whey protein Isolate
α-La α-Lactalbumin
Amino acids nomenclature (ordered by increasing hydrofobicity)
Name Chemical
formula
1 letter - code
3 letter - code
Molecular weight (Da)
Structural formula
Arginine C6H14N4O2 R Arg 174.2
Aspartic acid C4H7NO4 D Asp 133.1
Glutamic acid C5H9NO4 E Glu 147.1
Histidine C6H9N3O2 H His 155.2
Asparagine C4H8N2O3 N Asn 132.1
Glutamine C5H10N2O3 Q Gln 146.2
Lysine C6H14N2O2 K Lys 146.2
Serine C3H7NO3 S Ser 105.1
Treonine C4H9NO3 T Thr 119.1
Alanine C3H7NO2 A Ala 89.1
Cysteine C3H7NO2S C Cys 121.2
Proline C5H9NO2 P Pro 115.1
Methionine C5H11NO2
S M Met 149.2
Valine C5H11NO2 V Val 117.2
Tryptophan C11H12N2O
2
W Trp 204.2
Tyrosine C9H11NO3 Y Tyr 181.2
Isoleucine C6H13NO2 I Ile 131.2
Leucine C6H13NO2 L Leu 131.2
SCOPE AND OUTLINE
Food markets are becoming more and more competitive. In search for better added-value, food companies are eager to innovate – at the expense of scientific and technological achievements that bring e.g. more pleasant texture and appearance, more nutritional benefits or health-promoting features to foods. The possibility of adding bioactive peptides to food products targeted at particular consumer groups – e.g. specific health risk groups, older people or athletes, has brought about a strong market potential. On the other hand, development of health-promoting foods comprises a range of processes that need to be integrated – including development of easier and less expensive technologies to obtain peptides, optimization of protein hydrolysis, peptide characterization in terms of physico-chemical properties and interactions with other food components (e.g. lipids, polysaccharides and salts), and finally rational establishment of health claims including in vivo experimentation. Note that use of whey obtained from cheese making to produce such added-value products has also the advantage of reducing its polluting load, thus helping alleviate an environmental problem.
This thesis is accordingly divided in five parts (see page XVII), which closely mimic development of the research program: Part I, Part II, Part III,
Part IV and Part V. Chapters are related to each other, and the approach
chosen in each one was typically dependent on the conclusions attained in previous one(s). The text is overall organized in 10 chapters; each nuclear chapter (Chapters 2 to 8) contains experimental results, a specific introduction and a specific list of references.
Part I includes Chapter 1, and presents a short overview pertaining
to whey proteins, their properties and applications. The hydrolysis of whey proteins and the bioactivities of the resulting concentrates and peptides, as well as membrane techniques of separation and analysis of proteins, are also described therein. The principles underlying the antihypertensive activity of peptides – with a special focus on angiotensin-converting enzyme (ACE)-inhibitory activity, and the importance of gastrointestinal digestion upon bioavailability of those peptides are addressed; the antiulcerogenic, antinociceptive and anti-inflammatory activities are addressed as well, but to a lesser extent. Finally, some basic concepts of microencapsulation are put forward.
Preliminary tests, aimed at optimizing hydrolysis and other technological conditions, are considered in Part II. Hydrolysis of whey
proteins is presented in Chapter 2, focused on aqueous extracts of Cynara
cardunculus as source of proteolytic enzymes. The degree of hydrolysis, and the antioxidant and ACE-inhibitory activities were monitored, using different source proteins and hydrolysis conditions, and optimal operational conditions were found. Liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) was used to elucidate the peptide profiles of said hydrolyzates produced under optimal conditions. Chapter 3 aimed at
under the optimized conditions found in Chapter 2. All fractions were chemically characterized.
Since some whey hydrolyzates have shown to contain peptides with biological activity, studies of their specific biological activities were reported
in Part III. In Chapter 4, ACE-inhibitory activity was sought; the peptide
profile of the most active fractions was ascertained via HPLC MS/MS. To exert their ACE-inhibitory effect, peptides have to overcome the aggressive conditions prevailing in the gastrointestinal tract, so simulated gastrointestinal digestion of the most promising peptides was carried out – and their ACE-inhibitory activity was measured, before and after. The antihypertensive activity of those whey peptide concentrates and of the most promising pure peptides was ascertained in Chapter 5, using the
tail-cuff method in animal models. An in vivo study of the relationship of antihypertensive activity to the ACE-inhibitory activity was also developed.
Chapter 6 assessed the protection of the stomach mucosa of rats
against ulcerative lesions brought about by the whey peptide concentrates obtained in Chapter 3. All tests resorted to acute and consecutive oral administration, and protection mechanisms were hypothesized, experimentally tested and duly discussed. In attempts to find new analgesic and anti-inflammatory agents, as well as preventive adjuvants to decrease the therapeutical doses required, the antinociceptive and anti-inflammatory effects of our peptide concentrate were analyzed in Chapter 7. The in vivo
effects were ascertained, following oral administration to experimental mice, via writhing, hot plate, formalin and paw edema tests.
intensity ultrasonic processing, so as to obtain a homogeneous particle size distribution. The presence of biopeptides in said microcapsules was also discussed.
Finally, overall conclusions were presented in Chapter 9, and
Most information presented in the ten chapters that constitute this dissertation have been already submitted to international peer reviewing, toward publication in international scientific journals – as detailed next:
Chapter 1:
Madureira, A. R., Tavares, T. G., Gomes, A. M. P., Pintado, M. E., and Malcata, F. X. (2010). Physiological properties of bioactive peptides obtained from whey proteins. Journal of Dairy Science, 93, 437-455.
Chapter 2:
Tavares, T. G., Contreras, M. M., Amorim, M., Martín-Álvarez, P. J., Pintado, M. E., Recio, I., and Malcata, F. X. (2011). Optimization, by response surface methodology, of degree of hydrolysis, antioxidant and ACE-inhibitory activities of whey protein hydrolyzates obtained with cardoon extract. Accepted in International Dairy Journal. DOI 10.1016/j.idairyj.2011.05.013.
Chapter 3:
Tavares, T. G., Amorim, M., Gomes, D., Pintado, M. E., Pereira, C. D., and Malcata, F. X. (2011). Bioactive peptide-rich concentrates from whey: pilot process characterization. Submitted to Journal of Food Engineering.
Chapter 4:
Tavares, T. G., Contreras, M. M., Amorim, M., Pintado, M. E., Recio, I., and Malcata, F. X. (2011). Novel whey-derived peptides with inhibitory activity against angiotensin-converting enzyme: in vitro activity and stability to gastrointestinal enzymes. Peptides, 32, 1013-1019.
Chapter 5:
Tavares, T. G., Sevilla, M.-A., Montero, M.-J., Carrón, R., and Malcata, F. X. (2011). Acute effect of whey peptides upon blood pressure of hypertensive rats, and relationship with their angiotensin-converting enzyme inhibitory activity. Accepted by Molecular Nutrition of Food Research.
Chapter 6:
Tavares, T. G., Monteiro, K. M., Possenti, A., Pintado, M. E., Carvalho, J. E. and Malcata, F. X. (2011). Antiulcerogenic activity of peptide concentrates obtained from hydrolysis of whey proteins brought about by proteases from Cynara cardunculus. International Dairy Journal, 21, 934-939.
Chapter 7:
Tavares, T. G., Spindola, H., Longato, G., Pintado, M. E., Carvalho, J. E. and Malcata, F. X. (2011). Antinociceptive and anti-inflammatory effects of whey protein hydrolyzate brought about by proteases from Cynara cardunculus. Submitted to Journal of Dairy Science.
Chapter 8:
TABLE OF CONTENTS
RESUMO ... v
ABSTRACT ... xi
ACKNOWLEDGEMENTS ... xvi
LIST OF ABBREVIATIONS ... xx
SCOPE AND OUTLINE... xxv
TABLE OF CONTENTS ... xxxiii
PART I - Bibliographic survey
CHAPTER 1 State of the art
1.1. CHEESE WHEY ... 6 1.1.1. Physicochemical composition ... 7 1.1.2. Whey protein ... 8 1.1.2.1. β-Lactoglobulin ... 9 1.1.2.2. α-Lactalbumin ... 10 1.1.2.3. Caseinomacropeptide ... 12 1.1.2.4. Bovine serum albumin ... 14 1.1.2.5. Immunoglobulins ... 15 1.1.2.6. Lactoferrin ... 15 1.1.2.7. Lactoperoxidase ... 16 1.1.2.8. Proteose-peptones ... 16 1.1.3. Whey proteins as source of functional ingredients ... 20
1.2. ENZYME COAGULANTS ... 22 1.2.1. Coagulating systems of animal/vegetable nature ... 22 1.2.2. Cynara cardunculus ... 23
1.3. MEMBRANE PROTEIN SEPARATION ... 25
1.4. PROTEIN ANALYSIS TECHNIQUES ... 27 1.4.1. Mass spectrometry applied to dairy proteins and peptides... 28
1.5.1. Peptide-inhibitors of angiotensin-converting enzyme (ACE) activity ... 31 1.5.2. Structure/activity ... 36 1.5.3. Bioavailability ... 39 1.5.3.1. Gastrointestinal digestion ... 40
1.6. GASTRIC ULCER ... 43 1.6.1. Gastric mucosal cytoprotection ... 44
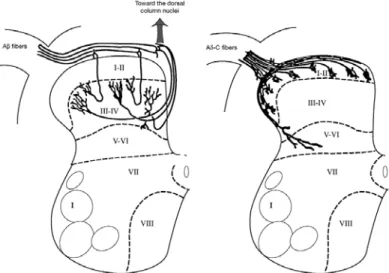
1.7. PHARMACOLOGICAL CHARACTERIZATION OF ANALGESIC AND/OR ANTI-INFLAMMATORY ACTIVITY ... 49 1.7.1. Pain and nociception ... 52
1.8. MICROENCAPSULATION ... 60
1.9. REFERENCES ... 61
PART II - Whey concentrate production
CHAPTER 2
Optimization of degree of hydrolysis, antioxidant and ACE-inhibitory activities of whey protein hydrolyzates obtained with cardoon extract
2.1. INTRODUCTION ... 100
2.2. MATERIALS AND METHODS ... 102 2.2.1. Experimental design, modelling and optimization ... 102 2.2.2. Performance of enzymatic hydrolyzes... 104 2.2.3. Assessment of degree of hydrolysis ... 105 2.2.4. Assessment of ACE-inhibitory activity ... 106 2.2.5. Assessment of antioxidant activity... 107 2.2.6. Statistical analyses ... 108 2.2.7. Identification of potentially bioactive peptides ... 109
2.3. RESULTS AND DISCUSSION ... 110 2.3.1. Experimental design, modelling and optimization ... 110 2.3.2. ACE-inhibitory activity and antioxidant activity ... 117 2.3.3. Identification of potentially bioactive peptides ... 119
2.4. CONCLUSIONS ... 122
CHAPTER 3
Bioactive peptide-rich concentrates from whey: pilot process characterization
3.1. INTRODUCTION ... 134
3.2. MATERIALS AND METHODS ... 136 3.2.1. Source of whey proteins ... 136 3.2.2. Production of whey protein and whey peptide concentrates ... 137 3.2.3. Characterization of whey protein and whey peptide concentrates ... 140 3.2.3.1. Gross chemical composition ... 140 3.2.3.2. Microbiological analyses ... 140 3.2.3.3. Quantification of enzymatic hydrolysis ... 141 3.2.3.4. Electrophoretic characterization of hydrolysis ... 142
3.3. RESULTS AND DISCUSSION ... 142 3.3.1. Production and characterization of whey protein and whey peptide
concentrates ... 142 3.3.2. Quantitative characterization of enzymatic hydrolysis ... 145
3.4. CONCLUSIONS ... 147
3.5. REFERENCES ... 148
PART III - Bioactivity characterization
CHAPTER 4
Novel whey-derived peptides with inhibitory effect against angiotensin-converting enzyme: in vitro effect and stability to gastrointestinal enzymes
4.1. INTRODUCTION ... 158
4.3. RESULTS AND DISCUSSION ... 165 4.3.1. Identification of ACE-inhibitory peptides ... 165 4.3.2. Stability of ACE-inhibitory peptides to simulated gastrointestinal digestion ... 173
4.4. CONCLUSIONS ... 175
4.5. REFERENCES ... 177
CHAPTER 5
Acute effect of whey peptides upon blood pressure of hypertensive rats, and relationship with their angiotensin-converting enzyme inhibitory activity
5.1. INTRODUCTION ... 188
5.2. MATERIALS AND METHODS ... 191 5.2.1. Feedstocks ... 191 5.2.2. Animals and feed ... 192 5.2.3. Measurement of in vivo blood pressure... 192 5.2.4. Measurement of in vivo ACE-inhibitory activity ... 193 5.2.5. Statistical analysis ... 194
5.3. RESULTS AND DISCUSSION ... 194 5.3.1. In vivo blood pressure ... 194 5.3.2. In vivo ACE-inhibitory activity ... 201
5.4. CONCLUSIONS ... 206
5.5. REFERENCES ... 207
CHAPTER 6
Antiulcerogenic activity of peptide concentrates obtained from hydrolysis of whey proteins by proteases from Cynara cardunculus
6.1. INTRODUCTION ... 218
6.2.7. Protection from ulcerative lesions by nitric oxide ... 224 6.2.8. Statistical analyses ... 225
6.3. RESULTS AND DISCUSSION ... 225 6.3.1. Assessment of antiulcerogenic activity of whey protein hydrolyzates ... 225 6.3.2. Postulation of cytoprotection mechanisms of whey protein hydrolyzates . 228
6.4. CONCLUSIONS ... 233
6.5. REFERENCES ... 234
CHAPTER 7
Antinociceptive and anti-inflammatory effects of whey protein hydrolyzate brought about by proteases from Cynara cardunculus
7.1. INTRODUCTION ... 244
7.2. MATERIALS AND METHODS ... 247 7.2.1. Feedstock ... 247 7.2.2. Animals and feed ... 247 7.2.3. Evaluation of locomotion activity... 248 7.2.4. Antinociceptive testing ... 248 7.2.4.1. Writhing test ... 248 7.2.4.2. Hot plate test ... 249 7.2.4.3. Formalin test ... 249 7.2.5. Anti-inflammatory testing ... 250 7.2.5.1. Paw edema test ... 250 7.2.6. Statistical analyses ... 251
7.3. RESULTS AND DISCUSSION ... 251 7.3.1. Evaluation of locomotor activity ... 251 7.3.2. Antinociceptive testing ... 252 7.3.2.1. Writhing test ... 252 7.3.2.2. Hot plate test ... 255 7.3.2.3. Formalin test ... 257 7.3.3. Anti-inflammatory testing ... 260 7.3.3.1. Paw edema test ... 260
7.4. CONCLUSIONS ... 265
PART IV - Peptide protection
CHAPTER 8
Microencapsulation of peptide concentrate using high-intensity ultrasound
8.1. INTRODUCTION ... 280
8.2. MATERIALS AND METHODS ... 282 8.2.1. Substrates ... 282 8.2.2. Sample preparation ... 282 8.2.3. Sol-gel transition ... 283 8.2.4. High-intensity ultrasound (HIUS) treatment ... 283 8.2.5. Dynamic light scattering (DLS) measurement ... 284 8.2.6. Confocal microscopy analysis ... 285 8.2.7. HPLC chromatography resolution ... 285
8.3. RESULTS AND DISCUSSION ... 286 8.3.1. Size distribution before HIUS treatment ... 286 8.3.2. Size distribution upon HIUS treatment ... 288 8.3.3. Morphological characterization after HIUS treatment ... 290 8.3.4. Containment mode after HIUS treatment ... 291
8.4. CONCLUSIONS ... 292
8.5. REFERENCES ... 293
PART V - Conclusions and future prospects
CHAPTER 9 General conclusions
9.1. GENERAL CONCLUSIONS ... 303
CHAPTER 10 Future prospects
PART I
CHAPTER 1
Health is one of the major reasons that determine consumers’s choices of food; follow and maintain a healthy diet is crucial. However, the difficulty in having consumers change radically their eating habits has led to the emergence of a number of foods that have the same appearance of conventional ones, but which contain certain functional ingredients able to bring health benefits in addition to responding to the nutrition issue (Korhonen and Pihlanto, 2003).
A food can be considered as "functional” if, beyond its nutritional effect, it provides benefits upon one or more functions of the body, thus improving health or welfare, or reduce the risk of illness (Diplock et al., 1999). This definition proposed by FUFOSE (Functional Food Science in Europe), should be refined in that: (i) the functional effect is different from the nutritional one; (ii) the benefit provided shall be reasoned; and (iii) it may bring about an improvement in physiological functions, or reduce the risk of developing a pathological condition. The concept of functional food emerged in Japan during the 80's, because of the need to improve the quality of life of a growing elderly population – that was about to incur in much higher health costs (Arai, 1996). Currently, due also to a growing consumer awareness of the relationship between nutrition and health, the market of functional foods is booming.
Bioactive peptides can be commercially sold as nutraceuticals. A nutraceutical is an edible substance possessing health benefits – which can be used to prevent a disease treat. However, it is advisable to make a distinction between nutraceuticals that are taken to prevent diseases – and which are present as natural ingredients of functional foods that can (and should) be consumed as part of the daily diet, from those used as adjuvants in the treatment of diseases requiring pharmacologically active compounds.
play a role in several metabolic processes, so a considerable interest has arisen from the dairy industry – towards large-scale production of bioactive peptides. This production is usually carried out through hydrolysis with digestive, microbial, plant or animal enzymes, or by fermentation with lactic starter cultures. In some cases, it was found that a combination of these processes is crucial to obtain functional peptides of small size (Korhonen and Pihlanto 2003, 2006). Specifically, whey proteins from cheese manufacture are already current ingredients on an industrial scale. Use of these proteins (concentrated or isolated), or biologically active peptides derived therefrom, as dietary supplements, pharmaceutical preparations or functional ingredients is of the utmost interest to the pharmaceutical and food industries, while helping relieve the pollution problems of whey.
1.1. CHEESE WHEY
Despite having been labeled over the years as a waste and pollutant due to its high lactose and protein content (Pintado et al., 1999), whey is a popular protein supplement in various functional foods (Recio and López-Fandiño, 2005). In fact, whey compounds have a number of functional, physiological and nutritional properties that make them potentially useful in a wide range of applications (Table 1.1).
Table 1.1. Major features of using whey (adapted from Alais, 1984).
Advantageous features Disadvantageous features
Protein fractioning: with high nutritional
value (Lys, Thr, Leu, Ser) High dilution – dehydration necessary – high energy costs Major milk components recovery High salt content (ca. 10 % of dry matter)
Lactose production High protein/sugar content – delactozation needed
Reduce pollution Highly putrescible raw material (protection;
celerity)
Highly dispersed cheese production facilities
Today, whey can be converted into lactose-free whey powder, condensed whey, whey protein concentrates and whey protein isolates (Hambreus, 1992; Mulvihill, 1992) – all of which are commercially available.
In the case of bovine milk, ca. 9 L of whey is produced from 10 L of milk during cheese making. Portugal has a long tradition in cheese production, but most dairies are small, and tendentially present in specific regions with a tradition in cheese production. In 2008, they produced ca. 68000 tons of cheese, of which 56000 tons were obtained from bovine milk - thus leading to 35000 ton of whey (INE, Agricultural Statistics, 2008). About 500 tons of whey is dried and used as feed; in addition, most small industries produce a traditional whey cheese – Requeijão, via a heat treatment of whey; in 2008, ca. 7000 tons were manufactured in Portugal (INE, Agricultural Statistics, 2008). Worldwide, a large part of whey is not utilized yet cheese manufacture has a tendency to increase worldwide; hence, upgrade of whey (to avoid its disposal) as become a growing problem. For environmental reasons, whey cannot be rejected into rivers due to its high chemical and biological oxygen demand. On the other hand, it is difficult to use whey as animal feed or fertilizer for economic reasons.
1.1.1. Physicochemical composition
Despite containing ca. 93 % water, whey is a reservoir of milk components of high value: it contains ca. 50 % of the nutrients found in whole milk. This composition depends obviously on how the cheese is produced, and the milk source; the residue found to higher level is lactose (4.5-5 %, w/v), as well as soluble proteins (0.6-0.8 %, w/v), lipids (0.4-0.5 %, w/v) and minerals (8-10 %, w/wdry extract) – particularly calcium, and vitamins such as thiamine, riboflavin and pyridoxine (Barth and Behnke, 1997; Blenford, 1996; Walzem et al., 2002). In fact, whey is now considered as a co-product rather than a by-product of cheese production, due to its wide amount of possible applications (Balagtas et al., 2003; McIntosh et al. 1998; Walzem et al., 2002).
1.1.2. Whey protein
Milk has been recognized as one of the main sources of protein (Miller et al., 2000) in feed for young animals and food for humans of all ages (Sgarbieri, 1996). Bovine milk contains ca. 3 % protein (Fox and McSweeney, 1998) – of which 80 % is caseins and 20 % whey proteins (Pihlanto and Korhonen, 2003). Whey is comprised by a heterogeneous group of proteins that remain in the supernatant after separation from casein; and as the latter, they are characterized by a genetic polymorphism that usually translates into replacement of one or more amino acid residues in the peptide sequence.
very different amino acid profile from caseins: they have a smaller amount of Glu and Pro, but a greater amount of sulfur-containing amino groups (i.e. Cys and Met). These proteins are desphosphorylated, easily denatured by heat, insensitive to Ca2+, and suffer intramolecular bond formation via disulfide bridges between Cys sulfhydryl groups. Some physicochemical parameters typical of whey proteins are tabulated in Table 1.2.
Table 1.2. Characteristics of major whey proteins (adapted from Zydney, 1998).
Proteins Concentration (g L-1) MW (kDa) Isoelectric Point (pI)
β-lactoglobulin 3 – 4 18.4 5.2
α-lactalbumin 1.5 14.2 4.7 – 5.1
Bovine serum albumin 0.3 – 0.6 69 4.7 – 4.9
IgG, IgA, IgM 0.6 – 0.9 150 – 1000 5.5 – 8.3
Lactoperoxidase 0.006 89 9.6
Lactoferrin 0.05 78 8.0
Protease-peptone 0.5 4 – 20
Caseinomacropeptide 7
1.1.2.1. β-Lactoglobulin
The major protein in whey of ruminants is β-Lg, which represents ca. 50 % of total whey proteins from cow's milk and 12 % of total milk proteins (Fox and McSweeney, 1998; Law et al., 1993). This is a globular protein, with 162 amino acid residues in its primary structure and a MW of 18.4 kDa. There are at least twelve genetic variants for β-Lg (A, B, C, D, DR, DYAK/E, F, G, H, I, W and X) of which A is the most common.
kDa (Nakai and Modler, 1996). At low pH, association leads to octamer formation; and, at high temperatures the dimer dissociates into its monomers. Its solubility depends on pH and ionic charge – but it does not precipitate during milk acidification (Walstra et al., 1999).
It is a rather interesting protein in whey because of its great capacity of gelling – which can be taken advantage of as structuring and stabilizer agent in such dairy products as yogurt and cheese spread. This protein is resistant to gastric digestion, as is stable in the presence of acids and proteolytic enzymes; hence, it tends to remain intact through passage in the stomach. This protein has the ability to bind small hydrophobic molecules – e.g. fatty acids or lipids, thus assuming their transport. This is a rich source of Cys – an amino acid with a key role in stimulating synthesis of glutathione (GSH), which is composed by three amino acids – Glu, Cys and Gly, forming the tripeptide γ-glutamilcisteinilglicine (Anderson, 1998). Glutathione is a molecule widely distributed in cells throughout the body, where it performs several functions – including cellular defense against oxidative stress and detoxification (Anderson, 1998; Baruchel et al., 1995; Bounous, 2000; Bounous et al., 1991; Regester et al., 1996). Besides that, its being rich in sulfur guarantees a high nutritional value.
1.1.2.2. α-Lactalbumin
rates of irreversible denaturation/aggregation of those proteins. The results showed that α-La is more heat resistant than β-Lg – due in part to the fact that its denaturation is reversible below 75 ºC (Law et al., 1994). Owing to a relatively high thermal stability, it holds a poor capacity to form a gel; however, it can be used in fluid or viscous products, in order to increase their nutritional value. This protein is used commercially in supplements for infant formulae, because of its similarity in structure and composition to the proteins of human milk – coupled with its higher content of Cys and Trp; it is also used in sport supplements (Heine et al., 1991; Tolkach and Kulozik, 2005). Recall that Cys is regarded as the limiting substrate for synthesis of GSH, is an important element of the antioxidant system (Heine et al., 1991) – and is involved in transport of amino acids (Anderson, 1998). On the other hand, Trp is the precursor of niacin (Miller et al., 2000), serotonin (5-HT) neurotransmitter and melatonin hormone; the latter two regulate many neurobehavioral effects, e.g. appetite, satiety, mood modulator, airway sensory perception, pain sensation, and rhythm of sleep and wake (Heine et al. 1991; Lien, 2003; Yogman et al., 1982).
α-Lactalbumin is one of the most studied proteins with regard to understanding the mechanism of protein stability and folding/unfolding (Chang et al., 2000); at low pH (Dolgikh et al., 1985), high temperature (Vanderheeren and Hanssens, 1994) or moderate concentrations of denaturants – e.g. guanidine hydrochloride (Kuwajima, 1989), α-La adopts a conformational structure called "molten globule". A partially unfolded state, the "apo" state, is formed at neutral pH when Ca2+ is removed by EDTA (ethylenediamine tetracetic acid) (Kuwajima, 1996; Kuwajima et al., 1985); this "apo" state essentially preserves the secondary structure, but not the tertiary structure (Dolgikh et al., 1981).
The "molten globule" state of α-La is characterized by a high degree of its native secondary structure, and a flexible tertiary structure (Dolgikh et al., 1981; Kuwajima, 1989; Ptitsyn, 1995); it thus appears as an intermediate in the balance between native and unfolded state (Arai and Kuwajima, 2000; Leandro and Gomes, 2008). This structure of α-La is highly heterogeneous, and has a predominant structure of α-helix formed mainly via weak hydrophobic interactions – while the β-sheet domain is significantly unfolded (Kuwjima, 1996).
1.1.2.3. Caseinomacropeptide
post-transcriptional modifications of κ-CN occur exclusively in the CMP portion of the molecule.
The amino acid sequence of CMP is well-known; it is characterized by absence of aromatic amino acid residues (Phe, Trp, and Tyr) and Arg, but several acidic and hydroxyl amino acids (Manso and Lopéz-Fandiño 2004). CMP from cow is soluble at pH in the range 1-10, with a minimum solubility (88 %) between 1 and 5 (Chobert et al., 1989; Moreno et al., 2002). CMP appears to remain essentially soluble following heat treatments at 80-95 °C for 15 min at pH 4 and 7 (Moreno et al., 2002). Its emulsifying activity undergoes a minimum near its isoelectric point (Chobert et al., 1989). Dziuba and Minkiewicz (1996) showed that a decrease in pH leads to a decrease in CMP volume, owing to reduction of internal electrostatic forces and steric repulsion – which has a significant influence upon its emulsifying capacity.
Intake of CMP supplements increases absorption of Zn2+ (Kelleher et al., 2003). The sialic acid content of CMP is also interesting in terms of bioactivity; large amounts of that carbohydrate are found in the brain and central nervous system, in the form of gangliosides and glycoproteins, which contribute to the regular functioning of cell membranes and membrane receptors, as well as to normal brain development (Heine et al., 1993). Since the human brain is predominantly developed in the third trimester and during the early postnatal period, newborns and children can benefit from supplementation of infant formulae with CMP.
1.1.2.4. Bovine serum albumin
Bovine serum albumin is derived directly from the blood, and represents 0.7-1.3% of all whey proteins (Nakai and Modler, 1996). Its molecule has 582 amino acid residues, and a MW of 69 kDa. The molecule contains 17 disulfide bonds, and one free sulphydryl group (Fox and McSweeney, 1998). Because of its size and higher levels of structure, BSA can bind to free fatty acids and other lipids, as well as flavor compounds (Kinsella and Whitehead, 1989) – a feature that is severely hampered upon denaturation.
1.1.2.5. Immunoglobulins
Immunoglobulins represent 1.9-3.3 % of the total milk proteins, and are derived from blood serum (Nakai and Modler, 1996). Igs constitute a complex group, the elements of which are produced by B-lymphocytes. Igs belong to three distinct classes: IgM, IgA and IgG (IgG1 and IgG2) – being IgG1 the major Ig present in bovine milk and colostrum (Nakai and Modler, 1996), whereas in human milk it is IgA. The physiological function of Igs is to provide various types of immunity to the body. The Igs consist of two heavy (53 kDa) and two light (23 kDa) polypeptide chains, linked by disulfide bridges (Fox and McSweeney, 1998).
The complete Ig, or ‘antibody’ molecule has a MW of about 180 kDa (Korhonen et al., 2000). Igs bind to the “invasor agent” (antigen) – and activate bacteriolytic reactions, increase recognition and phagocytosis of bacteria by leucocytes, prevent adhesion of microbes to surfaces, inhibit bacterial metabolism, agglutinate bacteria and neutralize toxins and viruses. Igs are partially resistant to proteolytic enzymes, and are not in particular inactivated by gastric acids (Korhonen et al., 2000).
1.1.2.6. Lactoferrin
inflammatory response, and in inhibiting both positive and Gram-negative bacteria (Expósito and Recio, 2006; Nakai and Modler, 1996; Simpson and Nicholas, 2002; Wakabayashi et al., 2006) - which that is caused by removal of iron cations. It can also act as an antioxidant.
1.1.2.7. Lactoperoxidase
Lactoperoxidase (LP) is a glycoprotein of 608 amino acid residues, and a MW of ca. 78 kDa. It has a broad biocidal and biostatic activity; in the presence of H2O2, it catalyses oxidation of thiocyanate (SCN-) and produces an intermediate product with antimicrobial properties (Boots and Floris, 2006; Shah, 2000).
1.1.2.8. Proteose-peptones
Table 1.3. Biological functions of whey proteins/peptides (adapted from Madureira et al., 2007, 2010).
Protein/Peptide Treatment Biological function Reference
Whole whey protein
Prevention of cancer Gill et al. (2000)
Breast and intestinal cancer; Badger et al. (2001); McIntosh et al. (1995) Chemical by-induced cancer Hakkak et al. (2000); Rowlands et al. (2001)
Increment of gluthatione levels Parodi (1998)
Increase of tumour cell vulnerability Micke et al. (2001), Micke et al. (2002) Treatment of HIV patients
Antimicrobial activities Clare et al. (2003)
Increment of satiety response
Increment in plasma amino acids, cholecystokinin and
glucagon-like peptide Hall et al. (2003)
β-Lactoglobulin
Transporter
Retinol Fox and Mc. Sweeney (1998); Puyol (2005) et al. (1995); Tolkack and Kulozic Palmitate Wu et al. (1999)
Fatty acids Puyol et al. (1991) Vitamin D and cholesterol Wang et al. (1997)
Enhancement of pregrastic esterase activity Perez et al. (1992)
Transfer of passive immunity Warme et al. (1974)
Regulation of mammary gland phosphorus metabolism Farrel et al. (1987) Enzyme hydrolysis;
Fermentation ACEa-inhibitory
Chen et al. (2007); Hernández-Ledesma et al. (2006); FitzGerald and Murray (2006); Ijäs et al. (2004); Meisel and Schlimme (1996); Mullally et al
(1996); Pihlanto-Leppälä et al. (1997); Pihlanto-Leppälä et al. (1998); Pihlanto (2000); Roufik et al. (2007)
Enzyme hydrolysis Antimicrobial against several gram-positive bacteria Bruck et al. (2003); Expósito and Recio (2006); Pellegrini et al. (1999);
Pihlanto-Leppälä et al. (1999);
Enzyme hydrolysis Antimicrobial (bactericidal) Groleau et al. (2003); Pellegrini et al. (2001) Enzyme hydrolysis Hypocholesterolemic Groleau et al. (2003); Nagaoka et al. (2001)
Enzyme hydrolysis Opioid agonist Antila (2007) et al. (1991); Meisel and Schlimme (1996); Hartmann and Meisel
Enzyme hydrolysis Antihypertensive Murakami et al. (2004); Pihlanto-Leppälä et al. (1997) Enzyme hydrolysis Ileum contracting Meisel and Schlimme (1996); Pihlanto-Leppälä et al. (1997) Enzyme hydrolysis Antinociceptive Yamauchi et al. (2003)
Prevention of cancer
α-Lactalbumin
Prevention of cancer de Wit (1998)
Apoptosis of tumoral cells Hakansson et al. (1995); Svensson et al. (1999, 2000)
Lactose synthesis Markus et al. (2002)
Treatment of chronic stress-induced disease Ganjam et al. (1997) Antimicrobial (bactericidal)
Against Streptococcus pneumoniae Hakansson et al. (2000)
Stress reduction Markus et al. (2000); Markus et al. (2002)
Immunomodulation Montagne et al. (2000)
Enzyme hydrolysis Antimicrobial against several gram-positive bacteria Expósito and Recio (2006); Pellegrini (1999); et al. (1999); Pihlanto-Leppälä et al.
Enzyme hydrolysis Opioid agonist Antila (1996) et al. (1991); Meisel and FitzGerald (2000); Meisel and Schlimme
Enzyme hydrolysis ACEa-inhibitory Chatterton et al. (2006); Meisel and Schlimme (1996); Mullally et al. (1996);
Pihlanto (2000);
Enzyme hydrolysis Antihypertensive Nurminen et al. (2000)
Enzyme hydrolysis Ileum contracting Meisel and Schlimme (1996)
Antiulcerative
Prostaglandins production Matsumoto et al. (2001); Uchida et al. (2003)
Bovine serum albumin
Fatty acid binding Walzem et al. (2002)
Prevention of cancer Laursen et al. (1990)
Enzyme hydrolysis ACEa-inhibitory Abubakar et al. (1998); FitzGerald et al. (2004)
Enzyme hydrolysis Ileum contracting Yamauchi et al. (1992)
Enzyme hydrolysis Opioid agonist Tani (2000) et al. (1993); Meisel and Schlimme (1996); Meisel and FitzGerald
Immunoglobulins
Immunomodulation Ormrod and Miller (1991)
Disease protection through passive immunity Mitra et al. (1995); Tomita et al. (1995)
Antibacterial Freedman et al. (1998); Loimaranta et al. (1999); Oona et al. (1997)
Antifungal Okhuysen et al. (1998)
Opioid agonist Sharpe et al. (1994)
Caseinomacropeptide
Antithombotic
Chabance et al. (1998); Jollès and Fiat (1979); Jollès et al. (1986); Manso et al. (2002); Quian et al. (1995); Rutherfurd and Gill (2000); Shebuski et al.
(1989)
ACEa-inhibitory Manso and López (2003); Mizuno et al. (2005); Nakamura et al. (1995b) Antimicrobial Bruck Neeser et al.et al. (2003); Dziuba and Minkiewiez (1996); Kawasaki (1988); Oh et al. (2000); Schupbach et al. (1996) et al. (1992);
Enzyme hydrolysis Prebiotic Bouhallab et al. (1993)
Increment of satiety response
1.1.3. Whey proteins as source of functional ingredients
Research encompassing bioactive peptides has undergone a notable intensification during the past decade (Korhonen and Pihlanto, 2006; Xu, 1998). Advances in nutritional biochemistry and biomedical research have indeed helped unravel the complex relationship between nutrition and disease, thus suggesting that food proteins and peptides originated during the digestive process (or from in vitro proteolysis) may play important roles in preventing and/or treatment of diseases associated with malnutrition, pathogens and injuries (Amaya-Farfán, 2003; Prates and Mateus, 2001).
Although inactive within the primary structure of their source proteins, enzyme-mediated hydrolysis may release peptides with specific amino acid sequences that exhibit biological activity. Several chemical and biological screening methods have thus been developed to aid in search for specific health effects; however, only some of such health effects found in vitro have eventually been confirmed in studies encompassing human volunteers (Hartmann and Meisel, 2007).
enhancement of mineral absorption and/or bioavailability thereof, cyto- or immunomodulatory effects, and opioid features. With regard to the mechanisms by which bioactive peptides can exert their physiological role, some involve actions only upon certain receptors, whereas others are enzyme inhibitors; they may also regulate intestinal absorption, and exhibit antimicrobial or antioxidant activity. In addition, bioactive peptides derived from food proteins differ in general from endogenous bioactive peptides, in that they can provide multifunctional features (Mullally et al., 1996).
Figure 1.1. Main activities of the bioactive peptides in the body, and their production
A "personalized nutrition" is a new concept in nutrition that takes into account the nutritional needs of each individual according to age, physical condition, illness, status, etc. Note that bioactive peptides could not be absorbed though the gastrointestinal tract, so thus may exert a direct role upon the intestinal lumen, or through interaction with receptors in the intestinal wall; some of these receptors have been implicated in such diseases as cancer, diabetes, osteoporosis, stress, obesity or cardiovascular complications. Hence, bioactive peptides derived from whey proteins address search a new concept, and open up a wide range of possibilities within the market for functional foods (Korhonen, 2009; Korhonen and Philanto, 2006).
1.2. ENZYME COAGULANTS
1.2.1. Coagulating systems of animal/vegetable nature
The enzymes used to milk coagulation are usually selected protein preparations that, in general, possess considerable (and selective) proteolytic activity.
With regard to animal rennet substitutes, pig pepsin has enjoyed a remarkable commercial success in manufacture of cheese. Relative to rennet from microbial origin, the proteases from Mucor miehei, Mucor pusillus, and Endothia parasitica are the most used (Ustunol and Zeck, 1996). Recombinant bovine chymosin is, nowadays, one of the proteinases with greater commercial expression, although its use is still prohibited in certain countries (Fox, 1993). Chymosin and all other rennet substitutes are aspartic proteases, with optimal activity at acidic pH, and showing a high degree of homology in primary structure, 3-dimensional structure and catalytic mechanism. The specificity towards the substrate is, however, rather variable. Although they have a greater tendency to break peptide bonds between hydrophobic amino acids having bulky side residues, they hydrolyze a large number of bond types (Simões, 1998).
The use of vegetable rennet dates back to the Roman period (Fox, 1987) – but, with few exceptions, their use today is still fairly limited worldwide. Many enzyme preparations proved indeed to be excessively proteolytic for appropriate manufacture of cheese, causing defects in terms of flavor and texture. These difficulties arise from the presence of non-specific enzymes and/or which belong to complex enzyme systems (and, which as such, are difficult to control). A general exception to the poor suitability of vegetable coagulants for cheese production are the proteinases present in aqueous extracts of plants of the Cynara genus.
1.2.2. Cynara cardunculus
Portugal, and Serena, Torta del Casar, Flor de Guia and Los Pedroches in Spain.
In Portugal, the cardoon of the C. cardunculus species is more often used. This is a large plant that grows wild in arid, rocky and untamed areas in the south of the country – mainly Extremadura, Alentejo and Algarve (Roseiro, 1991). Thistle flowers are picked up and then air-dried, without moisture control and under direct sunlight, and then stored in cloth or plastic bags and subsequently sold in local markets. Although the thistle sold should contain only flowers of C. cardunculus, tests carried out with commercial samples show that these are often mixed with flowers of C. humilis (Pires et al., 1994). This plant – of a smaller size than C. cardunculus, grows spontaneously and abundantly in the central area of the country and in Alentejo (Pires et al., 1994). Since no standard conditions are used for harvesting and drying, the activity of the extracts of cardoon is extremely variable – and depends on variety, degree of ripeness, drying time and part of flower used.
1.3. MEMBRANE PROTEIN SEPARATION
Development of membrane techniques has been essential, especially for the food industry, because of its relatively easy scale-up, as well as being inexpensive when compared with chromatographic techniques (Tolkach and Kulozik, 2005). Furthermore, elimination of heat treatment allows the nutritional properties and bioactive components remain intact (or only slightly affected) when processed. Membrane separation allows differential concentration of a liquid provided that the solute at stake is larger in diameter than membrane pore. The liquid that percolates the membrane (filtrate) has in its composition components smaller than the membrane pore diameter (Saboya and Maubois, 2000).
Figure 1.2. Relationship between membrane filtration techniques (membrane pore size range) and relative size of various components of milk retained
(adapted from Alfa Laval/Tetra Pak, 1995).
Process: Reverse osmosis (RO) Nanofiltration (NF) Ultrafiltration (UF) Microfiltration (MF) Applied pression: 200 – 1.200 psig 150 – 400 psig 30 – 150 psig 20 – 100 psig
Retentate: Total solids Total solids except monovalent ions Proteins and fats fats, large proteins and particles
Filtrate: only water monovalent ions salts, non-protein nitrogen and
lactose Lactose and salts
Separation method: ability organization of the compounds into tetrahedral structures determine the permeation capacity
diffusion and flow through the pores controlled by mass transfer,
characteristics of diffusion and charges
shape, charge, flexibility, MW determines the ability to cross the membrane