Vol. 51, n. 6 : pp.1233-1240, November-December 2008
ISSN 1516-8913 Printed in Brazil BRAZILIAN ARCHIVES OF BIOLOGY AND TECHNOLOGY
A N I N T E R N A T I O N A L J O U R N A L
Hydrolysis of Whey Lactose by Immobilized
ββββ
-Galactosidase
Marcela Panaro Mariotti, Hideko Yamanaka, Angela Regina Araujo and Henrique Celso
Trevisan
1∗1
Instituto de Química; Departamento de Bioquímica e Tecnologia Química; Rua Prof. Francisco Degni, s/n; trevisan@iq.unesp.br; 14800-900; Araraquara - SP - Brasil
ABSTRACT
Hydrolysis of whey lactose to glucose and galactose by immobilized galactosidase comes as an alternative to enlarge the possibilities of commercial use of this feedstock. To be applied at industrial scale, the process should be performed continuously .This work aimed to study the hydrolysis of whey lactose by an immobilized enzyme reactor.
β-Galactosidase from Aspergillus oryzae was immobilized on silica and activity and stability were evaluated. The best immobilization results were attained by using glutaraldehyde as support’s activator and enzyme stabilizer. The optimized enzyme proportion for immobilization was 15-20 mg g-1 of support. Treatments of whey were performed (microfiltration, thermal treatment and ultrafiltration), seeking the elimination of sludge, and the effects on operat-ing the fixed bed reactor were evaluated. Ultrafiltration was the best treatment towards a proper substrate solution for feeding the reactor.
Key words: β-galactosidase, immobilization, lactase, whey, lactose, hydrolysis
∗Author for correspondence
INTRODUCTION
It has been estimated that annually as much as 37 million kg of whey are produced in the USA and Canada (Ghaly and Kamal, 2004) and it is a huge waste disposal problem (Revillion et al., 2003). Lactose use in nutritious products is limited be-cause of low solubility, low sweetener power and laxative effect, if consumed in high concentration (Ferreira et al., 2003). The hydrolysis of lactose in glucose and galactose by β-galactosidase is one important process in the food industry, due to the potentially beneficial effects on assimilating the foods containing lactose, as well as the tech-nological and environmental advantages of indus-trial applications (Jurado et al., 2002):
1- Elaboration of milk with sweetened flavor, of good acceptability, that can be consumed by peo-ple with intolerance to this sugar (Jelen and Tos-savainen, 2003);
2- Formation of galacto-oligosaccharides during lactose hydrolysis to favor the growth of intestinal bacterial microflora (Playne et al., 2003);
components, yogurts and ethanol production (Synowiecki and Maciunska, 1999) and
4- In the production of the cheese, the industrial byproducts include whey and permeate that can cause reduced environmental impacts when the lactose is removed (Mukhopadhyay et al., 2003). Hydrolysis of lactose can be considered as a new unit operation, which can be integrated transform-ing several feedstocks containtransform-ing lactose (Pessela et al., 2003). Industries require enzymes having high productivity and stability for repeated use over an extended period. Enzyme immobilization provides many important advantages over the use of soluble enzyme: reusability, continuous opera-tion, controlled product formaopera-tion, and simplified and efficient processing (Turecek et al., 1990). Continuous reactors provide high productivities and minimize downstream time, enzyme cost and capital investment (Abraham et al., 2004), and offer an attractive design for enzyme reactors, especially when dealing with unclarified substrate fluids (Roy and Gupta, 2003).
The present work had as objective to study immo-bilization and staimmo-bilization of β-galactosidase and application on continuous reactor processing whey as substrate, following research on enzyme isola-tion and purificaisola-tion (Monti et al., 2000).
MATERIALS AND METHODS
For immobilization of the enzyme, followings were used as support: aminopropyl silica, granu-lometry 150-300, 106-150 and 63-106 µm and 500Å average pore diameter. The β-galactosidase (E.C.3.2.1.23) from Aspergillus oryzae, product
G5160 and glutaraldehyde (25%) was supplied by Sigma Chemical Co.; lactose was from Synth. Whey was supplied by Só Nata Indústria e Comér-cio de Produtos AlimentíComér-cios Ltda, Votuporanga, SP. For filtering the whey, Pellicon system (from Millipore) was used with ultra or microfiltration cassettes (1 ft2 filtration surface). Analysis of
glu-cose was carried out by spectrophotometry using the enzymatic method (commercial kit, Laborlab). A jacketed chromatographic column was used as reactor (Sigma C5419) with 10cm length and 1cm internal diameter.
Silica Activation and ββββ-Galactosidase
Immobi-lization
Aminoalkyl silica activation was performed by glutaraldehyde (Weetall, 1976). To 1.00 g of sil-ica, glutaraldehyde solution was added (400µL of 25% glutaraldehyde and 3.60 mL of acetate buffer 0.05M, pH 4.5) and it was left to react for 2 h at room temperature. The supernatant was removed and the enzyme was added, dissolved in 3 mL of the same buffer (15mg/mL). It was left in contact for immobilization during 48 h. Supernatant was removed and reserved for determination of enzy-matic activity. Immobilized enzyme was washed and stored at 4°C in the same acetate buffer. As-says were accomplished to optimize the enzyme addition.
Effect of Washings
After activation with glutaraldehyde, the effect of washing the excess of glutaraldehyde with acetate buffer (0.05M, pH 4.5) was studied. Stabilization of the immobilized enzyme by glutaraldehyde after the immobilization (crosslinking) was also evaluated. Tests were accomplished as follows: (1) immobilization and no crosslinking; (2) immo-bilization, following washing and crosslinking, and (3) immobilization without washing or crosslinking.
Determination of the Activity
Lactose solution (25mL of 4.5%) in acetate buffer (0.05 M pH 4.5) was added in the batch reactor and stabilized at 45°C before addition of the en-zyme, solution or immobilized. Samples (0.5mL) were withdrawn at 3 min intervals and inactivated in the boiling water bath for 5min and then 25µL was added to 1mL of the glucose enzymatic re-agent. Absorbances were read after 1 h at 505nm in UV-Visible spectrophotometer (Shimadzu model UV-1203). Glucose concentration was cal-culated by comparing with standard glucose con-centrations.
Operation of fixed bed reactor with lactose so-lution and whey as substrate
Cole-Parmer, 1-100rpm). Operational stability was studied at three temperatures (40, 45 and 55°C), working on two flow rates at each temperature (0.5mL/min and 5.0mL/min). In another assay, the system was fed with processed whey at the same flow rates at 40 and 45°C.
Treatments of the whey to be used as substrate
1- Whey microfiltrated using 0.22µm membrane by the Pellicon system (Millipore);
2- Whey heated for 5min at 90-95°C and microfil-trated through 0.22µm membrane, and,
3- Whey microfiltrated through 0.45µm membrane and ultrafiltrated through 10 kDa MWCO by the Pellicon system.
RESULTS AND DISCUSSION
Immobilization and stabilization of ββββ
-Galactosidase
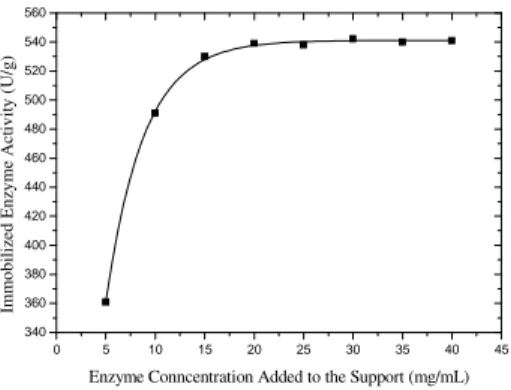
In relation to the concentration of enzyme added for immobilization, it was observed that between 20 and 40 mg/mL, the immobilized activity re-mained practically constant (Fig. 1).
0 5 10 15 20 25 30 35 40 45
340 360 380 400 420 440 460 480 500 520 540 560
Im
m
ob
ili
ze
d
E
nz
ym
e
A
ct
iv
ity
(
U
/g
)
Enzyme Conncentration Added to the Support (mg/mL)
Figure 1 - Effect of enzyme concentration on the immobilization. 2.0 mL of enzyme solution added to 200 mg of silica.
Hence a concentration of β-galactosidase between 15 and 20mg/mL was selected, since above this value, there was only increase of enzyme con-sumption.
Considering that glutaraldehyde addition to the immobilized β-galactosidase resulted in no signifi-cant decay of activity (from 775U/g to 750U/g), stabilization was evaluated by adding the same solution as for activation of the support: 2.5% in sodium acetate buffer (0.05M, pH 4.5). Stability assays were performed at 65°C, taking two sam-ples, after 15 and 30 minutes. Additionally, acti-vation of the support followed by removing the supernatant and addition of enzyme solution, without washing, was considered.
It was observed that the immobilization after washing the glutaraldehyde and without crosslink
ing resulted in higher activity and less stability; using crosslinking the activity decreased, but the stability was better. The immobilization without washing the glutaraldehyde before enzyme addi-tion and followed by the treatment with glutaral-dehyde had the highest activity, initially and after incubation at 65°C (Fig. 2). This immobilized product was the best obtained.
Fixed bed reactor with lactose solution as sub-strate
0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 100
150 200 250 300 350 400 450 500 550 600 650 700 750 800 850
(Washed and crosslinked) (Not washed and crosslinked) (Washed and not crosslinked)
A
c
ti
v
it
y
(
U
/g
)
Time (min)
Figure 2 - Effect of washing and crosslinking with glutaraldehyde on β-galactosidase deactivation at 65°C.
At 45°C, initial activity was 410U/g and the reac-tor operated for 100 days, reduced 53% of the activity. The half-life was about 100 days. At 40°C, the initial activity was slightly lower
(400U/g) and the stability was better; the reactor operated for 130 days, showed reduced activity (32%). The half-life was about 12 months, as shown by the slope of the plotted data (Fig. 3).
0 20 40 60 80 100 120 140
0 100 200 300 400 500
40o
C 450
C 550
C
A
c
ti
v
it
y
(
U
/g
)
Time (day)
Figure 3 - Activity and inactivation of β-galac-tosidase in fixed bed reactors using lactose solution as substrate at 5.0 mL/min flow rate.
During operation at 0.5 mL/min, with high conver-sion, initial activity at 55°C was 112U/g, with decay of 40% after 11 days, and the half-life was above 11 days. At 45°C, the initial activity was 106U/g and the reactor operated for 100 days, decaying 29% of the activity, with half-life above 100 days. At 40°C, the initial activity was 100U/g,
but the stability better; the reactor operated for 130 days, activity was reduced 24%, with half-life of about 12 months (Fig. 4).
0 20 40 60 80 100 120 140 0
20 40 60 80 100 120
55o
C 40o
C 45oC
A
c
ti
v
it
y
(
U
/g
)
Time (day)
Figure 4 - Activity and inactivation of the enzyme in reactors of fixed bed using lactose solution as substrate in the 0.5mL/min flow
Whey treatments and operational stability in fixed bed reactor
The first treatment, to which the crude whey was submitted, consisted of microfiltration through 0.22µm membrane. The purpose was to obtain the clarified whey, free from the fat and insoluble protein that could cause blockage of the reactor. The microfiltrated whey was limpid and appar-ently suitable as substrate. However, during opera-tion at 45°C, precipitation occurred inside the re-actor. This precipitated material consisted of whey proteins subjected to denaturation at the reactor temperature. In an attempt to decrease or to elimi-nate the precipitation, the temperature was reduced to 40°C, but without success. The reactor was operated at 45°C for 40 days and the final activity of the immobilized enzyme was 162U/g (30% of the initial). Analysis of the decrease of the glucose concentration at the exit of the reactor indicated
final activity of 40%, at 5mL/min, with half-life of about 32 days.
In order to increase the half-life of the enzyme, and reduce the precipitation, crude whey was submitted to thermal treatment at 90-95°C, for 5min with subsequent filtration through 0.22µm membrane. The objective was to eliminate the proteins and fats, coagulated by the high tempera-ture, facilitating the microfiltration step. This treatment induced the precipitation of albumins and globulins, allowing the removing colloidal material and fat.
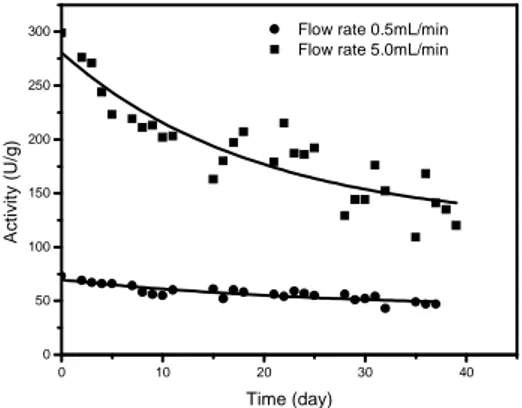
Operation of the reactor at 45°C with thermally treated whey still resulted in precipitate, but to much less extent. The reactor was run for 44 days and the final activity of the immobilized enzyme was 132U/g (20% of the initial). Analysis of the decay of glucose concentration indicated final activity of 44%, at 5mL/min, with half-life around 40 days (Fig. 6).
0 10 20 30 40
0 50 100 150 200 250
300 Flow rate 0.5mL/min
Flow rate 5.0mL/min
A
c
ti
v
it
y
(
U
/g
)
Time (day)
0 10 20 30 40 50 0
50 100 150 200 250 300 350
Flow rate 0.5mL/min Flow rate 5.0mL/min
A
c
ti
v
it
y
(
U
/g
)
Time (day)
Figure 6 - Fixed bed reactor of with thermally treated and microfiltrated whey as substrate, at 45°C.
Despite each treatment to which the whey was submitted, solid accumulation in the reactor still persisted. According to Toledo (1997), a way to remove this from whey would be by ultrafiltration. During ultrafiltration, the components of low mo-lecular weight as lactose, minerals and vitamins leave with the permeate, while proteins become concentrated.
Reactor operation with ultrafiltrated whey was performed for 51 days and the final immobilized enzyme activity was 197U/g (32% of the initial). Analysis on the decay of glucose concentration indicated the final activity of 50% at 5.0mL/min, with half-life of approximately 45 days (Fig. 7).
0 10 20 30 40 50
0 50 100 150 200 250 300 350 400
Flow rate 0.5 mL/min Flow rate 5.0 mL/min
A
ti
v
it
y
(
U
/g
)
Time (day)
Figure 7 - Fixed bed reactor with micro- and ultrafiltrated (10kDa) whey, at 45°C.
Ultrafiltration of the whey allowed the operation of the reactor for a longer period, since there was no obstruction of the reactor as before. However, this didn’t guarantee the stability of the enzyme. Comparing half-life of 45 days at 45°C, with ul-trafiltrated whey, with 100 days with lactose solu-tion, it seemed that additional treatment was nec-essary to optimize the process. It was suggested to remove salts and take special cares as cleaning of the bioreactor, that could help to preserve the in-tegrity of the immobilized enzyme (Vyas and Tong, 2003).
CONCLUSIONS
In relation to the methodology of β-galactosidase immobilization, good results were achieved using glutaraldehyde (2.5% solution in acetate buffer at 0.05 mol.L-1, pH 4.5) as activator and stabilizer.
It was found to be better not to wash the activated silica for the addition of the enzyme, but just to remove the supernatant. Addition of the same glutaraldehyde solution after immobilization re-sulted in increased stability of the enzyme.
enzyme consumption, without significant increase in the activity. The activity of immobilized β -galactosidase was around 650 U/g.
On long-term operation, with lactose solution at 40°C, initial activity of 400U/g was obtained in the reactor and the half-life of the biocatalyst was estimated to be 12 months. This result confirmed the good performance of the immobilized enzyme and allowed to establish the half-life limit for op-eration with whey.
Whey was submitted to three treatments, aiming to increase the operational stability. Microfiltration through 0.22µm membrane resulted only in the clarification of the whey. Heating followed by microfiltration reduced sensibly solid accumula-tion, but did not hinder the blockage. Only the ultrafiltration through 10kDa MWCO membrane eliminated the obstruction, but the half-life of the enzyme did not increase significantly (45 days at 45°C, compared with 40 days for the treatment 2 and 130 days for lactose solution). Then, although the ultrafiltration guaranteed the operational stabil-ity to the system, it was not good enough to in-crease appreciably the lifetime of the biocatalyst. The first treatment to be tested would be the dem-ineralization, preferentially for electrodialysis (Rozhkova et al., 1992).
Another important factor to be considered would be the operation in continuous system where the whey could be ultrafiltrated and pumped straight to the reactor, reducing the risk of microbial con-tamination, as was observed sometimes during the process.
ACKNOWLEDGEMENTS
Authors thank to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the finan-cial support and scholarship to M.P.M.
RESUMO
A hidrólise de lactose de soro de leite, resultando em glicose e galactose, apresenta-se como uma alternativa para ampliar as possibilidades de uso comercial deste insumo. Para ser aplicado em escala industrial, o processo deve ser operado de modo contínuo. Reporta-se o estudo de um siste-ma objetivando hidrólise de lactose de soro de
β-Galactosidase de Aspergillus oryzae foi
imobili-zada em sílica, sendo avaliadas a estabilidade e atividade. Os melhores resultados de imobilização foram obtidos usando glutaraldeído como ativante do suporte e estabilizador da enzima. A proporção otimizada entre enzima e suporte foi 15-20 mg.g-1.
Foram estudadas formas de tratamento do soro (microfiltração, tratamento térmico e ultrafiltra-ção), objetivando eliminação de material suspenso, e avaliando os efeitos na operação de reator de leito fixo. A ultrafiltração foi o melhor tratamen-to, na busca de uma solução de substrato apropria-da para o reator contínuo.
REFERENCES
Abraham, T. E.; Joseph, J. R.; Bindhu, L. B. V. and Jayakumar, K. K. (2004), Crosslinked enzyme crys-tals of glucoamylase as a potent catalyst for biotrans-formations. Carbohydrate Research, 339, 1099-1104. Ferreira, L. S.; Souza, M. B.; Trierweiler, J. O.; Hitz-mann, B. and Folly, R. O. M. (2003), Analysis of ex-perimental biosensor/FIA lactose measurements. Brazilian Journal of Chemical Engineering, 20, 7-13. Ghaly, A. E. and Kamal, M. A. (2004), Submerged
yeast fermentation of acid cheese whey for protein production and pollution potential reduction. Water Research, 38, 631-644.
Jelen, P. and Tossavainen, O. (2003), Low lactose and lactose-free milk and dairy products - prospects, te-chnologies and applications. Australian Journal of Dairy Technology, 58, 161-165.
Jurado, E.; Camacho, F.; Luzon, G. and Vicaria, J. M. (2002), A new kinetic model proposed for enzymatic hydrolysis of lactose by a beta-galactosidase from Kluyveromyces fragilis. Enzyme and Microbial Te-chnology, 31, 300-309.
Monti, R.; Basilio, C. A.; Trevisan, H. C. and Contiero, J. (2000), Purification of papain from fresh latex of
Carica papaya
1. Brazilian Archives of Biology and Technology, 43, 501-507.
Mukhopadhyay, R.; Talukdar, D.; Chatterjee, B. P. and Guha, A. K. (2003), Whey processing with chitosan and isolation of lactose. Process Biochemistry, 39, 381-385.
Playne, M. J.; Bennett, L. E. and Smithers, G. W. (2003), Functional dairy foods and ingredients. Aus-tralian Journal of Dairy Technology, 58, 242-264. Revillion, J. P. D.; Brandelli, A. and Ayub, M. A. Z.
(2003), Production of yeast extract from whey using Kluyveromyces marxianus. Brazilian Archives of Biology and Technology, 46, 121-127.
Roy, I. and Gupta, M. N. (2003), Lactose hydrolysis by Lactozym (TM) immobilized on cellulose beads in batch and fluidized bed modes. Process Biochemis-try, 39, 325-332.
Rozhkova, M. V.; Shaposhnik, V. A.; Mizilina, A. K. and Tyagunova, V. I. (1992), Lactose transport t-hrough ion-exchange membranes in electrodialysis. Journal of Applied Chemistry of the Ussr, 65, 2071-2074.
Synowiecki, J. and Maciunska, J. (1999), Lactose in human and animal nutrition. Medycyna Wetery-naryjna, 55, 497-500.
Toledo, R.T. (1997) Fundamentals of Food Processing Engineering. Chapman and Hall, New York.
Turecek, P. L.; Pittner, F. and Birkner, F. (1990), De-gradation of polysaccharides by immobilized de-polymerizing enzymes. International Journal of Food Science and Technology, 25, 1-8.
Vyas, H. K. and Tong, P. S. (2003), Process for calcium retention during skim milk ultrafiltration. Journal of Dairy Science, 86, 2761-2766.
Weetall, H. H. (1976), Immobilized enzyme techno-logy. Cereal Foods World, 21, 581-587.