Growth and Optical Properties of Cr
3+Doped GdAlO
3Single Crystals
J.P. Andreeta
a, B.R. Jovanic
baInstituto de Física de São Carlos, Departamento de Física e Ciência dos Materiais, Universidade de São Paulo, C.P. 369, 13560 São Carlos, SP, Brazil;
b
Institute of Physics, Center of Experimental Physics, Lab.Mulltidisc.Res., Belgrade University, P.O. Box 68, 11080 Zemun, Yugoslavia
Received: November 16, 1999; Revised: April 6, 2000
High-Temperature Solution crystal growth experiments on Cr3+ doped GdAlO3, are described where the flux and solute stoichiometry was changed for the process optimization to obtain homogeneous and inclusion free larger crystals. Transparent single crystals up to 3.8 cm3 have been grown. The pressure-induced wavelength shift of laser excited fluorescence in some of these crystals was measured up to 115 kbar at room temperature in relation to the R1 shift of ruby. The wavelength shift fluorescence behaviors of these doped crystals, with low Cr3+ concentrations, indicate that this material could be used in producing a new class of pressure sensors.
Keywords:rare earth, aluminates, oxides, and high-pressure luminescence
1. Introduction
The rare earth aluminates are of considerable interest because of their optical and magnetic properties1-3 and
technological applications. The perovskite structures are strong candidates for solid state lasers as the host material for Cr3+ and Nd3+ in cubic and center symmetric sites, a condition necessary for obtaining long fluorescence life-times4. On the other hand, previous investigation on low-concentration doped Cr3+ GdAlO3 shows that it may be a
promising material for high-pressure sensors5. The factor that restricts the technological application of the GdAlO3:Cr3+ solid solutions in those systems are the
inho-mogeneity and the difficulties in obtaining large and high perfection single crystals. Therefore, the aim of this paper is to describe the optimized conditions to grow Cr3+ doped GdAlO3 crystal using the High-Temperature Solution
method and to investigate the pressure-induced wavelength shift of laser excited fluorescence in some of these crystals with low doping concentrations.
2. Crystal Growth
2.1 The maximum stable growth rate criteria
Due the experimental convenience, in most of the crys-tal growth processes from the solution method it is usual to
adopt a linear temperature program or a constant rate of solvent evaporation. Both criteria provoke a constant flux of solute mass from liquid to solid phase. Since the crystal adsorption area will increase during crystal growth, the growth rate will be faster for small crystals, at the beginning of the crystal growth process, than for the large crystals, at the end of the process. Crystals grown under these condi-tions showed high concentracondi-tions of macroscopic defects near the nucleation regions due the unstable growth condi-tions.
Experimental results6 show that the constant growth rate is also not suitable to grow large crystals due the heat and mass transport mechanism. Based in the Carlson7 sta-bility and hydrodynamics conditions, several authors [6, 8-9] found a new temperature program (Eq. 1) to grow large crystals by High Temperature Solution method known as “maximum stable growth rate”:
T = T0
1 + 2RT0 ∆Hmix
ln[ cosh [ 0.34 βµσ
2
Ce
ρs Vsn P 1⁄3
d (σ+ 1)
]1⁄2 t ]
(1)
where To is the initial temperature, ∆Hmix is the heat of
solution, β is the geometric crystal factor, µ is the fluid velocity, σ is the relative supersaturation, Ce is the initial
a
equilibrium concentration, ρs is the solid density, Vsn is the
initial solution volume and Pd is the Schmidt number.
2.2. Growth of Cr+3 doped GdAlO3
The Cr3+ doped GdAlO3 single crystals were grown by
Czochralski, LHPG (Laser Heated Pedestal Growth) and flux methods. However, high quality large single crystals were obtained only from the flux method due the develop-ment of cracks in all the single crystals grown in the Czochralski method as shown in Fig. 1. These macroscopic defects are attributed to a structural phase transition during the cooling process at temperatures near 2073 K10. Amaz-ingly, using LHPG method, homogeneous single crystal fibers, without cracks, with up to 0.8 mm diameter and several centimeter of length were grown, even when the annealing was performance under high temperature gradi-ent conditions.
The high quality and homogeneous Cr3+ doped GdAl03
single crystals were grown by usual flux (High-Tempera-ture Solution) experiments carried out in sealed platinum crucibles, under the maximum stable growth rate condi-tions. The accelerated crucible rotation technique11 was also used in some experiments in order to homogenize the liquid phase and to control the natural convection. To
optimize the crystal growth experiments, several flux com-positions were tried. These starting materials and the results of the main experiments are published elsewhere12. All flux compositions were based on intrinsic properties previously discussed by several authors13. The major solvent constitu-ents have low eutectic temperature (763 K; 66 mol% of PbF2), high solubility for the crystal constituents and
suit-able viscosity at growth temperature that can contribute to mass transport. The additive compounds (B2O3 , PbO2 and
V2O5) are important as oxidizing agents and to control the
nucleation rate, probably by increasing the width of the metastable regions.
The best results in growing these crystals were obtained using the compositions and conditions of Table 1. From the data of this table we note that an excess of Gd2O3 was added
in the growth experiment (non-stoichiometric composi-tion) to avoid a decrease in the Gd3+ concentration in the solution that might lead to GdOF formation13 during the crystallization process.
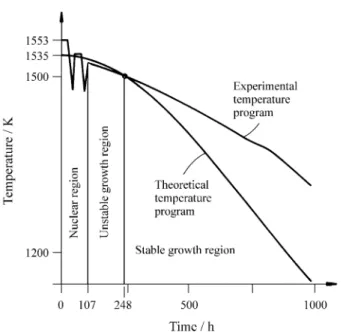
The experimental and theoretical temperature programs (based on the maximum stable growth rate criteria), carried out in most of crystal growth experiments, are shown in Fig. 2. The nucleation control was performed by the slow temperature fluctuation method described else where14.
From the maximum stable growth rate criteria we can distinguish, in this experiment, two growth conditions: a) unstable growth, b) stable growth. The stability conditions transition occurred near 1500 K and 4,0 mm crystal size.
2.3. High temperature solutions crystal growth results
Using this optimized composition, associated with an appropriate temperature program and a suitable hydrody-namic condition, we could grow large and nearly homoge-neous, inclusions free, single crystals. As shown in Fig. 3, the macroscopic defects could be noted only in small regions near the nucleation’s point.
The Cr3+ doping concentration and the Pb solvent in-corporation was determined by electron microprobe in
Figure 1. Macroscopicdefects (cracks) in Cr3+ doped GdAlO3 single
crystal grown by Czochralski method.
Table 1.
Experimental com-position (mol%)
Temperature program
Hydrodynamics conditions
Chromium concentration
(mol%)
Stoichiometric deviation* (mol%)
Results and remarks
Gd2O3 8.7
Al2O3 7.5
Clear Crystals up to 23 x 21 x 8 mm3 -Inclusions free crystals except near in the nucleations regions (unstable growth regions) Cr2O3 0.1
PbO 42.5 PbF2 36.1
B2O3 4.6
PbO2 0.5
Maximum stable growth rate program
(Eq. 1)
Accelerated crucible rotation technique
1.7 16
several samples. As shown in Fig. 4, the profile for the Cr3+ concentration does not obey accurately the Burton, Prim and Slichter equation15 for a keff = 0.3814. The Cr3+
distri-bution is nearly constant in the whole crystal, whereas the Pb incorporation has a maximum in the nucleation point probably due to unstable growth as estimated from the calculated temperature program.
3. Optical Properties
3.1. High-pressures optical measurements
A diamond anvil cell (DAC) of the NBS type, with 1/3 carat stones, was used to generate pressure16. The pressure was increased at room temperature, and the DAC was allowed to equilibrate for at least 12 h after pressure was set up. The pressure used was in the range of 0-115 kbar and at room temperature, 300 K. A small (= 30 µm) chip of ruby (0.l% mol Cr3+) and the GdAlO3:Cr3+ (0.7% mol
Cr3+) crystal were placed in the 300µm hole of a prein-dented stainless steel gasket, together with a methanol-ethanol (4:1) mixture that served as the pressure medium and which remained hydrostatic up to 140 kbar16. A spec-trofluorometer connected with a multiscaler card in a PC was used as a measuring apparatus. The pressure was determined by the shift of the ruby R1 line (0.0365 nm/kbar)1,8. The position of the ruby R1 line and sample R line was determined using a double optical monochromator with a 0.05 nm spectral resolution and a photon counter with a multiscaler. The samples were excited by an argon ion laser at 488 nm. In order to reduce the laser heating of the sample the power of the laser was reduced to 3 mW.
3.2. Results and discussion
T he ro om-te mpera ture emi ssion spec tra of GdAlO3:Cr3+, containing 0.7 mol% of Cr3+, were obtained
at two pressures; these spectra are shown in Fig. 5.
Figure 3. Single crystals of GdAlO3 : Cr3+ grown by HTS. Left - clear
crystal grown after the unstable growth conditions; right - Bulk crystal showing unstable growth conditions near the nucleation regions.
Figure 4. Cr3+ concentration and Pb incorporation profiles in the larger
crystal. The distance (d) was measured from the nucleation point.
Figure 2. The experimental and theoretical temperature programs, based
on the maximum stable growth rate criteria after8.
Figure 5. High-pressure fluorescence spectra of GdAl03: Cr3+ crystal at
The obtained spectrum shows only two well-resolved lines: a) the first, broad line at about 730 nm with the line width about 15.0 nm, and b) the second line is sharp or peaking at about 736 nm, with a line width of about 3.5 nm. This is contrary to the fluorescence spectra in GdAlO3:Cr3+
with a 0.7 mol% concentration of Cr3+ 5 at room tempera-ture. The first line is an R line and appears to be due to transitions between the Cr3+ states 2E and 4A2 16. The sharp
line was denoted in a previous paper as a NPL (Neighboring Pair Line)5,17. NPL is related to the energy transfer between the so-called Cr3+ single ions, which are nearly isolated from each other, and from the exchange coupled Cr3+ pairs formed statistically by Cr3+ ions on neighboring lattice sites.
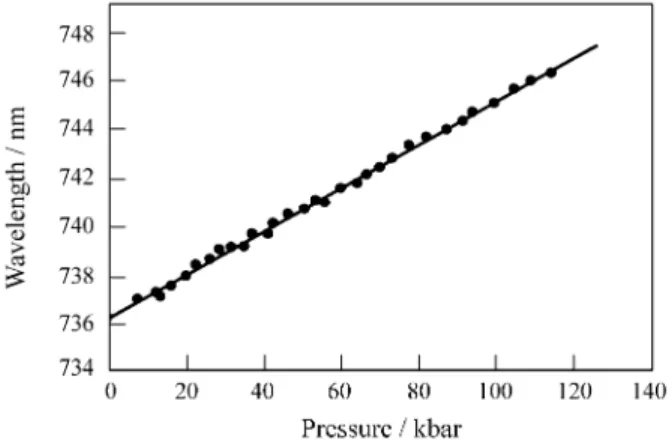
The pressure dependence of the positions for NPL line at room temperature is presented on Fig. 6. The position of NLS line under pressure shifts linearly to the red, similar to the R line of ruby, but with larger rates 0.088 nm/kbar and 0.0364 nm/kbar, respectively. The pressure shifting rate for the NPL line is about 2.42 times larger than ruby’s R line.
Similar to GdAlO3:Cr3+, several materials have
lumi-nescence line peaks far enough away from the solid-state materials broad-emission maximum:YAlO3:Cr3+ with a
pea k at 722.80 nm20, MgO:V2+ a t 870nm22 and YAlO3:Nd3+ at 875.7 nm21. All mentioned materials have
a smaller pressure shift rate than the NPL line pressure shift ra te for GdA lO3:Cr3+. F or YA lO3:Cr3+ i t i s
0.070 nm/kbar20, for MgO:V2+ is 0.055 nm/kbar22; and for YAlO3:Nd3+ it is -0.014 nm/kbar21. On the other hand,
some pressure sensors have a peak far away on the other side of the broad-emission maximum: YAG:Sm2+ a t 617,7 nm23; YAG:Eu3 + at 590 nm25; LaOCI:Eu3+ a t 578,7 nm24. However, their sensitivity to pressure is rela-tively small: for YAG:Sm2+ it is +0.0298 nm/kbar24; for
YAG:Eu3+ it is 0.0197 nm/kbar25; and for LaOCI:Eu3+ it is 0.025 nm/kbar24.
The intensity of the NPL line is about 1.25 time larger than the intensity of the R line. Single crystals with higher Cr3+ ion concentration however, its intensity is approxi-mately 0.35 smaller5. The relative intensity (background ratio) of the NPL line (0.40) is larger than for the R line in ruby (0.01), YAlO3:Cr3+ ( 0. 28 ) a nd R2-Z2 l i n e i n
YAlO3:Nd3+ (22) [20] but smaller than for the R line in
MgO (0.92) and YAG:Cr3+ (0.53) [20]. Also, the NPL line intensity change only slightly with pressure, ≅4%. The linewidth of the NPL line at higher pressures increases by about 20%.
4. Conclusions
It is possible to grow (using an appropriate solvent composition and temperature program, and a suitable hy-drodynamic condition) large and nearly homogeneous Cr3+ doped GdAlO3 single crystals. These doped crystals, with
low chromium concentrations, can be used as active ele-ments in pressure sensors due to the large pressure shift of the NPL line; strong sharp NPL luminescence line; the relatively high fluorescence intensity that is easily detect-able at pressures lower than 115 kbar and the fact the NPL peaks far away from the wavelengths of the region in which most solid-state materials doped with Cr3+ have maximum.
Acknowledgments
The authors would like to acknowledge the MSTS and CNPq - Brazilian agency for financial support.
References
1 Cashion, J.D; Cooke, A.H.; Hawkes, J.F.P.; Leask, M.J.M; Thorp, T.L.; Wells, M.R. J. Appl. Phys., v. 39, p. 1360, 1968.
2. Cashion, J.D; Cooke, A.H.; Leask, M.J.M.; Thorp, T.L.; Wels, M.H. J. Mater. Sci., v. 3, p. 402, 1968. 3. Sivardiere, J.; Quezel-Ambrunas, S. Compt. Rend,
(Paris), v. B 273, p. 619, 1971.
4. Murphy, J.; Olmann, R.L.; Mazelsky, R. Phys. Rev. Letters, v. 13, p. 135, 1964.
5. Jovanic, R. B.; Andreeta, J.P. High Pressure Effect of Fluorescence Spectra of Cr3+ in GdAlO3, J. Phys.
Cond. Matt., v. 10 p. 271, 1998.
6. See, for example, Scheel, H.J.; Elwell, D.J. Crystal Growth, v. 12, p. 153, 1972.
7. Carlson, A.E. Growth and Perfection and Crystal, John Wiley, N.Y., p .421, 1958.
8. Scheel, H.J.; Elwell, D. Crystal Growth from High Temperature Solutions, Academic Press, p. 264, 1975.
Figure 6. Shift in peak of fluorescence of NPL line in GdAlO3 (0.7 mol%
9. Andreeta, J.P. Revista de Fisica Aplicada e Instru-mentação, v. 1, n. 1 p. 97, 1985.
10. Mazelsky, R.; Kramer, W.E.; Hopkins, R.H. J. Crystal Growth v. 2, p. 209, 1968.
11. Elwell, D.; Scheel H.J. Crystal Growth From High Temperature Solutions, Academic Press, London, p. 368, 1975.
12. Andreeta, J.P.; Hernandes, A.C.; Gallo, N.J.H. Mat. Res. Bull., v. 25, p. 51, 1990.
13. Pamplin, B.R. Crystal Growth, Pergamon Press, Ox-ford, p. 217, 1985.
14. Andreeta, J.P.; Hernandes, A.C.; Gallo, N.J.H. Mat. Res. Bull., v. 24, p. 83, 1989.
15. Burton, J.A.; Prim, H.C.; Slichter, W.P. J. Chem. Phys., v. 21, p. 1987, 1953.
16. Jovanic, B.R. Lifetime of Ruby R1 Line under Ultra-high Pressure, Chem. Phys. Letf. v. 190, p. 440, 1992. 17. Jayaraman, A. Ultrahigh pressure Rev. Mod. Phys., v.
55 p. 65, 1983.
18. Ohlmann, C.R.; Murphy, J. Exchange Interaction be-tween Cr3+ and Gd3+ in GdAlO3-. Bull. Amer. Phys.
Soc., v. 11 p. 255, 1966.
19. Jovanic, B.R. and Andreeta, J.P. GdAlO3:Cr3+ as a New Pressure Sensor, Physica Scripta, v. 59, p. 274, 1999.
20. Barnet, J. D.; Block, S.; Piermarini, J.G. An Optical Fluorescence System for Quantitative Pressure Meas-urement in the Diamond, Anvil Cell Rev. Sci. lnstrum., v. 40 p. 1, 1973.
21. Lacam, A.; Cahteau, C. Higher-pressure measure-ments at moderate temperature in a diamond anvil cell with a new optical sensor: SrB4O7:Sm2+. J. Appl.
Phys., v. 66, p. 368, 1989.
22. Chopelas, A.; Boehler, R. Abstract AIRAPT Conf. S’h, SUNY, Alabany, N.Y., 1983.
23. Bi, Q.; Brown, M.J.; Sato-Sorensen, Y. Calibration of Sm:YAG as an alternative high-pressure scale J. Appl. Phys., v. 68 p. 5357, 1990.
24. Chi, Y.; Liu, S.; Shen, W.; Wang, L.; Zou, G. Crystal Field Analysis for Emission Spectra of LaOCI:Eu3+ Under High Pressure, Physics, v. 139-140 p. 555, 1986.
25. Arashi, H.; Ishigama, M. Diamond Anvil Pressure Cell and Pressure Sensor for High-Temperature Use