DOI: http://dx.doi.org/10.18363/rbo.v76.2019.e1754 Literature Review / Epidemiology
Tutorial to plan and perform
randomized clinical trials
Olavo Barbosa de Oliveira-Neto,1 Raul Ribeiro de Andrade,2 Isabelle Oliveira Santos,3 Luciano Timbó Barbosa,4 Célio Fernando de Sousa-Rodrigues,5,6
Fabiano Timbó Barbosa7
1Department of Anatomy, Biosciences Center, Federal University of Pernambuco (UFPE), Recife, PE, Brazil 2Medical School, CESMAC University Center, Maceió, AL, Brazil
3Medical School, Tiradentes University Center (UNIT), Maceió, AL, Brazil 4Cardiology Service, General Hospital of Alagoas, Maceió, AL, Brazil
5Human Anatomy Division, Institute of Health and Biological Sciences, Federal University of Alagoas (UFAL), Maceió, AL, Brazil
6Human Anatomy Division, Center for Integrative Sciences, Governador Lamenha Filho Campus, State University of Health Sciences of Alagoas (UNCISAL), Maceió, AL, Brazil
7Medical School, Federal University of Alagoas (UFAL), Maceió, AL, Brazil • Conflicts of interest: none declared.
AbstrAct
Objective: the aim of the present study was to present a tutorial for planning and performing randomized clinical trials. Materials and methods: an online search was performed on MEDLINE via PubMed. The following search strategy was used: “randomized controlled trial”[Publication Type] OR “randomized controlled trials as topic”[MeSH Terms] OR “randomized controlled trial”[All Fields]. A hand search was also performed. Results: the present tutorial presents and discusses topics of utmost importance for randomized clinical trials studies, as follows: importance of randomized clinical trials, methodological items (focused question, registration of the study protocol, eligibility criteria, outcomes of interest, data collection and data analysis, risk of bias), and methodological quality. The study design of randomized clinical trials features the lowest risk of bias to assess healthcare interventions. Conclusions: this tutorial offers a concise and practical description of important items regarding randomized clinical trials, which can be used by clinicians and researchers to better understand, plan, and perform randomized clinical trials.
Keywords: randomized controlled trial; bias; review; intention to treat analysis.
Introduction
R
andomized Clinical Trial (RCT) is considered as the gold standard for analysis of interventions in Dentistry and Medicine. It has been arousing interest of researchers since the 19th century, mainly in search for causes of infectious diseases.13,22 Two RCTpublished in 1931 and 1948 were paramount to improve clinical researches to a new standard of higher interest.1,14
The randomization strategy is the methodological fea-ture that sets apart the RCT from other types of study, and makes it less prone to research bias.13
Randomiza-tion does not guarantee the absence of all types of bias and, when unproperly performed, may lead to untruthful results.2,10 In addition, an unproperly written report of
methodological items in RCT may reduce the reliability of results.2,17
Thus, it becomes important to create a tutorial to help au-thors to reduce the risk of bias of future randomized clinical trials.
The aim of the present narrative review was to present a tutorial to plan and perform randomized clinical trials.
M
aterials and methods
A cross-sectional search was performed to seek for scientific papers available at MEDLINE via PubMed. The following search string was used: “randomized
controlled trial”[Publication Type] OR “randomized con-trolled trials as topic”[MeSH Terms] OR “randomized controlled trial”[All Fields]. Authors also performed a hand search to seek for possible relevant articles.
Results
The importance of randomized clinical trials
The assessment and incorporation of evidences from clinical trials with low risk of bias is of utmost importance.13
The World Health Organization (WHO) defines the clinical trial as any scientific research that prospectively assesses the effects of interventions to the health of human patients. 25
The results from clinical trials may be distorted by the presence of bias and psychics effects.11 The effects that can
lead to untruthful results are the Hawthorne effect and the placebo effect.11 The Hawthorne effect is described as
a change in study results due to changes in the healthcare behavior of assessor or participants being researched.11 The
placebo effect is a change in study results due to expecta-tions created by participants about the benefits of interven-tion.11 The RCT is considered as the gold standard in
inter-ventions because uses methodological strategies to reduce the possibility of bias and psychic effects as well as to allow an equally distribution of known and unknown factors be-tween researched groups, which prevents results from being distorted.13
Methodological items
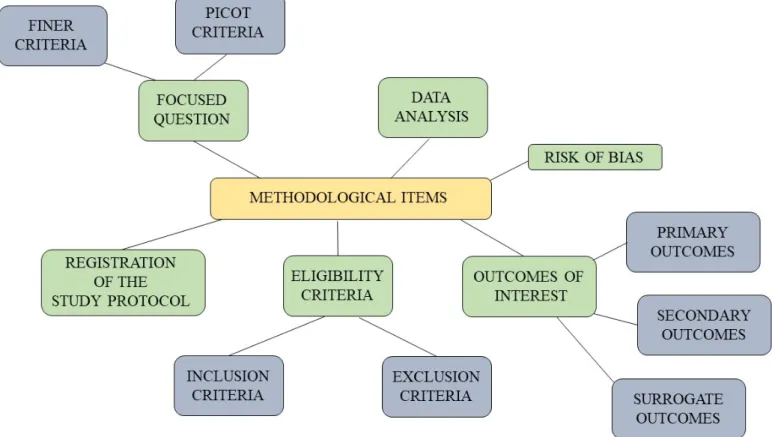
Randomized clinical trials require that a series of methodological demands are fulfilled in order to avoid bias of several origins. These items are the following: the focused question, the registration of study proto-col, the eligibility criteria, the outcomes of interest, the risk of bias, and the data analysis. Figure 1 summarizes these items and a detailed explanation of each item is shown below. Figure 2 shows main sources of bias in randomized clinical trials and how to avoid it.
Focused question
The FINER criteria must aid in the creation of focused questions that involves interventions.4 The acronym FINER
comes from: feasible, interesting, new, ethical, and relevant.4
A good focused question (i.e. research question) must indi-cate the population to whom results may interest, it must arouse interest by the academical community, it must be sci-entifically relevant, and it must provide current knowledge.4
The PICOT criteria provide a higher specificity to the focused question. The acronym PICOT comes from:
Figure 1. Summary of methodological items to be addressed on a randomized clinical trial.
Figure 2. Decision tree showing sources of bias in randomized clinical trials and how to avoid it. ITTA = intention to treat analysis; PPA = per protocol analysis.
participants, intervention, comparison, outcomes, and time.4 The following questions must be answered: which
is the researcher’s population of interest? Which interven-tion will be assessed? To which comparator the interveninterven-tion will be compared? What will be measured, performed, en-hanced, or renewed? What is the necessary time to evaluate all outcomes?4
The focused question is created when all these answers are united. An example of focused question that fulfill these criteria may be: what is the effectiveness of dental implants placed in smokers compared to non-smokers during at least one year of follow-up?
The PICOT items on the aforementioned question are the following: smokers (population), dental implant placement in smokers (intervention), dental implant placement in non-smokers (comparison), effectiveness of dental implants (outcome) and at least one year of follow-up (time).
Registration of the study protocol
The registration of the study protocol is demanded by editors of scientific journals and it was proposed by WHO with ethical, moral, and scientific purposes.25 It is a way
of patients, professionals, and researchers to obtain access to information about studies that are being currently conducted as well as its phases of conduction.25
The access to these information prevent unnecessary efforts from happening in new researches and also facili-tates the identification of scientific papers that are being published or that will be published in a near future.
WHO suggests that the following set of information are reported in each clinical trial: number of identification, date of registration, secondary identity, source of founding, sponsors, contacts for queries, published title, public title, scientific title, countries of recruitment, clinical background of the research, tested interventions, inclusion criteria, ex-clusion criteria, type of study, date of recruitment of the first patient, sample size, primary outcome, secondary outcomes, and phases of recruitment.15
There are also national databases around the world. For example, Brazil has a virtual site for registration of clinical trials (ReBEC), that can be accessed at www.ensaiosclinicos. gov.br. ReBEC is a cooperative project between the Brazilian Health Ministry, the Pan American Health Organization, and the Oswaldo Cruz Foundation.
Eligibility criteria
Inclusion and exclusion criteria identify a population from whom will be extracted the participants that will form the sample, and to whom the results will be destined to.3
Inclusion criteria must allow participants to be enrolled to a research in a sufficient number, so events can occur;
they also must not be too specific to not reduce generaliza-tion of results.3
Exclusion criteria must be gentle to avoid unnecessary exclusions. A few general examples are: harmful research intervention, low probability of adherence to research pro-tocol, high probability of research abandonment, and issues of practical nature to fully perform research protocol.3 A
proper and pre-calculated number of patients associated to a higher rigor on internal validity items provide a higher re-liability in study results.2,21
Participants may be included, but they still may not ad-here to study protocol or abandon during the follow-up peri-od.3 There are two ways to reduce the lack of adherence and
research abandonment: the screening consultation and the entrance test.3
The screening consultation is a consult that occur before the beginning of the research and aims to identify patients that are fit to not follow study protocol and thus exclude them to avoid issues by lack of adherence.3
The entrance test may be performed using a placebo for several weeks before the beginning of research, excluding all participants that did not adhere to the intervention.3 The use
of an active drug instead of a placebo may also be used, but it can reduce adherence if the patient is allocated to the place-bo group due to comparison that the patient makes between the entrance test and the actual research.
Data extraction must be performed after the beginning of the research, but it can be a source a bias. Selection bias and gauging bias may lead to biased results. Randomization may avoid selection bias.24 Allocation concealment and blinding
reduce gauging bias.9
Randomization is a technique used in research that allows the participant to have the same chance of entering in either research group.9 Randomization guarantees that resear-
ches cannot foresee to which groups each participant will be allocated. 24 There are several types of randomization, such
as: simple, which may be performed by coin tossing, dice rolling, or list of randomization generated by computer9;
blocking randomization, which is a random sequence of blocks of participants with a pre-specified size24; stratified,
which is performed after the classification of all types of participants in subgroups according to baseline features and forming a list that will be used for randomization5; and the
adaptative, which uses computer algorithms to consider risk factors and the previous allocation of participants.5
Allocation concealment is a technique used in research that prevents the researcher from foreseen to which group the participant will be allocated and thus it prevents him from modifying the allocation after the randomization.14
Studies that assess drugs use sealed and opaque envelopes to guarantee the allocation concealment.15 Blinding is a
caretaker, the person who is in charge of data extraction, the statistician and, if possible, the researcher from knowing to which group the participant was randomized to, until the research had ended.9
While the allocation concealment occurs on the re-searcher until the patient receives the intervention, the placebo, or the comparison therapy, the blinding is di-rected to the persons that oversee the research until after the participant had received the intervention, placebo, or comparison therapy.9
Outcomes of interest
Outcomes are features that vary for each participant in a research and can answer the focused question. The outcomes can be classified as primary or secondary out-comes.23 The primary outcomes are the ones the matter
the most to the study; they are generally related to the effectiveness and are used for the sample size calcula-tion.23 The other outcomes are considered as secondary
and regard efficacy and safety as well.23 The researcher
must considerer as primary outcome the one that an-swers the focused question directly and as secondary outcome the one that answers it undirectedly.
Outcomes of convenience of short-term outcomes re-fers to extracted data that may not be directly relevant to the research and not have validity to investigate the ef-fect of a treatment, and thus, are also known as surrogate outcomes.23 The outcomes of convenience may be easily
acquired for being of low cost and less invasive; They are also important for prognostic factors assessment.23
The decision about which outcome should be included for each study must consider three factors: the type of study and its goals, the relevance of the outcome from a major set of outcomes, and the likelihood of bias for each outcome considering all conditions in which the study will be per-formed.12
Data analysis
Data analysis must be performed with data from all participants, however, a subset of patients may not par-ticipate in the research exactly like it was planned and described on the protocol.16 The violations of protocol
may occur for several reasons, such as: the allocation occur for one group and the patient receives the treat-ment given to another group; when patients receive another therapy, different from the therapy given in the study, that interferes on data analysis; or when the par-ticipant is not available on the moment of assessment.16
Is possible that the researcher excludes from the analysis patients’ data for motives related to the research.16 This
problem, violation of protocol, may be reduced by means of intention to treat analysis (ITTA).
ITTA is the analysis that includes all patients regardless of their adherence, of the received treatment and its partici-pation or not until the end of the experiment.16,20 The ITTA
is the comparison with all participants regarding its ran-domization.16,20 The advantages of this analysis are: to keep
the principle of randomization and the balance between known and unknown confounding factors; to avoid that the violation of protocol leads to an untruthful conclusion that one intervention was more effective than the other; and to maintain the statistical power to avoid that effect estimative is overstimated.6
Per-protocol analysis is the data analysis of participants that complied the research protocol that was planned by the researcher.19 The analysis disregards the deviations
of protocols and is often used in exploratory studies that assess the effects of a given intervention under ideal cir-cumstances.19 The violations of protocol may lead to
dif-ferences in treatment due to difdif-ferences between groups and not the effect of the analyzed intervention.19
Risk of bias
Bias is the presence of a factor that interferes on the research generating false results, i.e., overestimated or understimated.7,8 Bias can result in the implementation
of therapies or interventions that are not effective.7
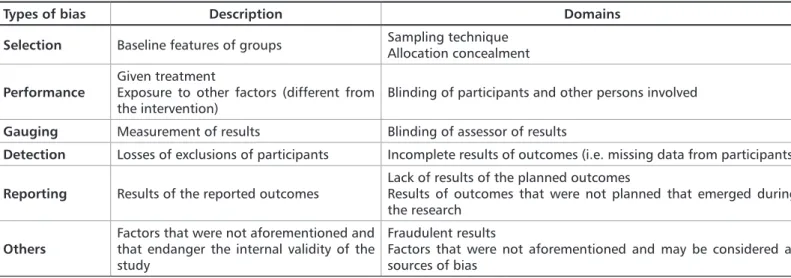
Ta-ble 1 shows the classification of bias and the domains for approaching the steps were bias occur. The researcher should take into consideration these items in the moment of project writing to avoid introduction of bias when the study is performed.
Quality of a randomized clinical trial
RCT are assessed using scales, individual items, and checklists, however, the most used tool nowadays is the risk of bias (RoB) table.8 The items of this tool should be taken
into consideration for planning and performing any RCT to avoid false results or results that do not answer the focused question.
The researcher bias may be transferred to the participant by researcher’s attitudes that may encourage or discourage the participants regarding their permanence on the re-search.18 Blinding protects the research from the researcher
bias as well as from other participants that have contact with the patients.
The CONSORT statement has been continuously indi-cated by journals and used by authors of RCT to report their results more comprehensively, which guarantees a higher transparency on the reporting of original articles.23
It regards a set of items that must be reported in all article sections.23 By using the items of the method of this
docu-ment in a RCT research project, is more likely to reduce the probability of bias and to improve the overall quality of the study.
The main steps for planning and performing ran-domized clinical trials were listed and explained in the present narrative review. One must highlight that we considered the most common design (i.e., the parallel clinical trial). However, there are other clinical trials that require other recommendations.
Conclusions
The study design of randomized clinical trials is con-sidered as the one with the smaller risk of bias to assess in-terventions, and the evidences from theirs results provide
Types of bias Description Domains Selection Baseline features of groups Sampling techniqueAllocation concealment
Performance Given treatmentExposure to other factors (different from
the intervention) Blinding of participants and other persons involved
Gauging Measurement of results Blinding of assessor of results
Detection Losses of exclusions of participants Incomplete results of outcomes (i.e. missing data from participants)
Reporting Results of the reported outcomes Lack of results of the planned outcomesResults of outcomes that were not planned that emerged during the research
Others Factors that were not aforementioned and that endanger the internal validity of the study
Fraudulent results
Factors that were not aforementioned and may be considered as sources of bias
Table 1. Types of bias in randomized clinical trials.
more tools for decision making of clinicians;
The present tutorial offers a concise and practical de-scription regarding important items of randomized clinical trials, which may be used by researchers and clinicians to better understand, plan, and performed this type of study.
Acknowledgements
The authors thank the members of the research group “Applied Morphology and Health” (CNPq – Brazil) for valu-able scientific contributions for the present tutorial.
2014; 399: 263-72.
13.Manchikanti L, Hirsch JA, Smith HS, et al. Evidence-based medicine, sys-tematic reviews, and guidelines in interventional pain management: Part 2: Randomized controlled trials. Pain Physician. 2008; 11: 717-73.
14.Marshall G, Blacklock JWS, Cameron C, et al. Streptomycin treatment of pulmonary tuberculosis: A Medical Research Council investigation. BMJ. 1948; 2: 769-82.
15.Peccin MS. Registro de ensaios clínicos: quando e por que fazer? Rev Bras Fisioter. 2007; 11: v-vi.
16.Ranganathan P, Pramesh CS, Aggarwal R. Common pitfalls in statistical analysis: Intention-to-treat versus per-protocol analysis. Perspect Clin Res. 2016; 7: 144-46.
17.Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. BMJ. 2010; 340: c332. 18.Schulz KF, Chalmers I, Altman DG. The landscape and lexicon of blinding in randomized trials. Ann Intern Med. 2002; 136: 254-59.
19.Sedgwick P. Intention to treat analysis versus per protocol analysis of trial data. BMJ. 2015; 350: h681
20.Shah PB. Intention-to-treat and per-protocol analysis. CMAJ. 2011;183: 696. 21.Sousa-Rodrigues CF, Lima FLC, Barbosa FT. Importância do uso adequa-do da estatística básica nas pesquisas clínicas. Rev Bras Anestesiol. 2017;67: 619-25.
22.Stolberg HO, Norman G, Trop I. Randomized Controlled Trials. AJR. 2004;183: 1539-44.
23.Vaz-Carneiro A, Luz R, Borges M, et al. Primary and secondary outcomes in oncology clinical trials: definitions and uses. Acta Med Port. 2014;27: 498-02. 24.Vickers AJ. How to randomize. J Soc Integr Oncol. 2006;4: 194-8.
25.World Health Organization. International Clinical Trials Registry Platform (ICTRP). Available at: <www.who.int/entity/ictrp/en/>.
References
1.Amberson JB, McMahon BT, Pinner MA. Clinical trial of sanocrysin in pul-monary tuberculosis. Am Rev Tuberc. 1931; 24: 401-35.
2.Barbosa FT, Jucá MJ. Assessing the quality of random clinical anesthesiology trials published on the Brazilian Journal of Anesthesiology from 2005 to 2008. Rev Bras Anestesiol. 2009; 59: 223-33.
3.Cummings SR, Grady D, Hulley SB. Delineando um ensaio clínico randomiza-do cego. In: Hulley SB, Cummings SR, Browner WS, et al. Delineanrandomiza-do a Pesquisa Clínica: Uma Abordagem Epidemiológica. Artmed/Porto Alegre, 3ª ed. 2008, 165-79.
4.Farrugia P, Petrosor B, Farrokhyar F, Bhandari M. Research questions, hy-potheses and objectives. Can J Surg. 2010; 53: 278–81.
5.Ferreira JC, Patino CM. Randomização: mais do que o lançamento de uma moeda. J Bras Pneumol. 2016; 42: 310.
6.Gupta SK. Intention-to-treat concept: A review. Perspect Clin Res. 2011; 2: 109–12.
7.Hartling L, Hamm MP, Fernandes RM, et al. Quantifying bias in randomized controlled trials in child health: a meta-epidemiological study. PLoS One. 2014; 9: e88008.
8.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ. 2011; 343: d5928. 9.Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collabora-tion, 2011. Available at: http://handbook.cochrane.org.
10.Jüni P, Altman DG, Egger M: Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001; 323: 42-6.
11.Kao LS, Tyson JE, Blakely ML, et al. Clinical research methodology I: Intro-duction to randomized trials. J Am Coll Surg. 2008; 206: 361-69.
12.Macefield RC, Boulind CE, Blazeby JM. Selecting and measuring optimal outcomes for randomised controlled trials in surgery. Langenbecks Arch Surg.
Submitted: 11/29/2019 / Accepted for publication: 12/27/2019 Corresponding author
Olavo Barbosa de Oliveira-Neto E-mail: olavobarbosa91@gmail.com
Mini Curriculum and Author’s Contribution
1. Olavo Barbosa de Oliveira-Neto – DDS; MSc. Contribution: Formal analysis; Wrote the original draft; Wrote, reviewed, and edited the manuscript. ORCID: 0000-0003-1280-659X
2.Raul Ribeiro de Andrade – Undergraduate medical student. Contribution: Wrote, reviewed, and edited the manuscript. ORCID: 0000-0002-0294-1483
3. Isabelle Oliveira Santos – Undergraduate medical student. Contribution: Wrote the original draft; Wrote and edited the manuscript. ORCID: 0000-0002-3796-2807 4. Luciano Timbó Barbosa – MD. Contribution: Wrote the original draft. ORCID: 0000-0001-5987-5264
5. Célio Fernando de Sousa-Rodrigues – MD; PhD. Contribution: Conceptualization; Data curation; provided insights to the methodology; Supervised the project. ORCID:0000-0002-1361-8139
6. Fabiano Timbó Barbosa – MD; PhD. Contribution: Conceptualization; Data curation; Investigation; created the study design; Supervised the project. ORCID: 0000-0001-6630-0629