w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Review
article
Serum
free
light
chain
assays
not
total
light
chain
assays
are
the
standard
of
care
to
assess
Monoclonal
Gammopathies
Vania
Tietsche
de
Moraes
Hungria
a,
Syreeta
Allen
b,
Petros
Kampanis
b,
Elyara
Maria
Soares
c,∗aFaculdadedeCiênciasMédicasdaSantaCasadeSãoPaulo(FCMSCSP),SãoPaulo,SP,Brazil bTheBindingSite,Birmingham,UnitedKingdom
cTheBindingSite,SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received8June2015 Accepted18November2015 Availableonline1February2016
Keywords:
Freelite®
Serumfreelightchainassay Totallightchainassay Multiplemyeloma
a
b
s
t
r
a
c
t
ThediagnosisofMultipleMyelomaisachallengetothephysicianduetothenon-specific symptoms(anemia,bonepainandrecurrentinfections)thatarecommonplaceintheelderly population.However,earlydiagnosisisassociatedwithlessseveredisease,includingfewer patientspresentingwithacuterenalinjury,pathologicalfracturesandsevereanemia.Since 2006,theserumfreelightchaintestFreelite®hasbeenincludedalongsidestandard
labora-torytests(serumandurineproteinelectrophoresis,andserumandurineimmunofixation) asanaidintheidentification ofmonoclonalproteins,whichareacornerstoneforthe diagnosisofMultipleMyeloma.Theserumfreelightchainassayrecognizesthelightchain componentoftheimmunoglobulininitsfreeformwithhighsensitivity.Otherassaysthat measurelightchainsinthefreeandintactimmunoglobulinformsaresensitive,but unfor-tunately,duetothenomenclatureused,theseassays(totallightchains)aresometimesused inplaceofthefreelightchainassay.Thispaperreviewstheavailableliteraturecomparing thetwoassaysandtriestoclarifyhypotheticallimitationsofthetotalassaytodetect Multi-pleMyeloma.Furthermore,weelaborateonourstudycomparingthetwoassaysusedin11 LightChainMultipleMyelomapatientsatpresentationand103patientstakenthroughthe courseoftheirdisease.Theaimofthisarticleistoprovideacleardiscriminationbetween thetwoassaysandtoprovideinformationtophysiciansandlaboratorytechnicianssothat theycanutilizetheInternationalMyelomaWorkingGroupguidelines.
©2015Associac¸ãoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.Allrightsreserved.
∗ Correspondingauthorat:TheBindingSite,Brasil,Av.Paulista,2444,Cj18/05,01310-300SãoPaulo,SP,Brazil. E-mailaddress:elyara.soares@bindingsite.com.br(E.M.Soares).
http://dx.doi.org/10.1016/j.bjhh.2015.11.003
Introduction
MonoclonalGammopathies(MGs)includepremalignant Mon-oclonal Gammopathies of Uncertain Significance (MGUS), Smoldering/IndolentMultipleMyelomaandmalignant [Soli-tary Plasmocytoma, Multiple Myeloma (MM), Light Chain AmyloidosisorWaldenstrom’sMacroglobulinemia(WM)] con-ditions.Thesedisordersarecommonlycharacterizedbythe productionofmonoclonalproteinswhichmaybeeitherintact immunoglobulins(M-Ig),serumfreelightchains(sFLC),a com-binationofboth,or rarely,freeheavychainsonly.1,2 Alow percentageofthesedisorderspresentwithouttheproduction ofanymonoclonalprotein.
Theasymptomaticdisordersareidentifiedthroughroutine laboratoryinvestigations,whilstthediagnosis ofthe symp-tomaticdisorderscanpresentconsiderabledifficultiestothe physician as the symptoms (anemia, recurrent infections, fatigueand bonepain) are common inelderlypopulations and are notspecific to the disease.3–5 However, there is a needfortimelydiagnosisasdelayscanleadtoanincreased severity of the disease, including acute renal failure and pathologicalfractures,whichcanresultinashorteroverall survival.6
Immunoglobulin
structure
and
sequence
variation
Immunoglobulinsarethesoluble,secretedformoftheB-cell receptorandarecomposedofrepeatingmirrorimages com-prisingtwoidenticalheavychains(gamma–␥,alpha–␣,mu–
,delta–␦orepsilon–)andtwoidenticallightchains(kappa–
orlambda–).Immunoglobulinheavyandlightchainseach haveconstantandvariableregions.Apairofheavyandlight chain variable regions together forms the antigen-binding site.Thevariableregionsexhibitenormousstructural diver-sity, particularly of antigen-binding contacts, allowing the recognitionofahugevarietyofantigens.
In humans, it is calculated that there are atleast 1011
possibleantibody structuralvariants, which allows forthe recognitionofavastnumberofdifferentantigens.7The diver-sityisgeneratedinfourmainways.
Firstly,differentcombinationsofgenesegmentsareused intherearrangementofheavyandlightchaingenesduring earlyB-celldevelopment.Kappalightchainsareconstructed fromoneofapproximately 40functional variable(V)gene
segments,oneof5joining (J)genesegments and asingle
constant(C)gene.Lambdalightchainsareconstructedfrom
oneofapproximately30variable(V)genesegments,andone
offour(ormore)pairsoffunctionaljoining(J)genesegments
andconstant(C)genes.7Theheavychainvariableregionis
formedfromoneofaround60variable(VH),oneof30diversity
(DH),andoneofsixjoining(JH)genesegments.7This
combina-tionaldiversityaccountsforasubstantialamountofvariable regiondiversity.Secondly,diversityarisesfromtheaddition orremovalofnucleotidesatthejunctionsbetweenV(D)andJ genesegmentsduringrecombination.Athirdsourceof diver-sityarisesfromthemanydifferentcombinationsofheavyand lightchains,and finally,somatichypermutation introduces
pointmutationsinthevariableregiongenesoflightandheavy chainsinmatureactivatedB-cells.7
Inlightchains,variationsarealsofoundinaregionofthe variabledomaincorrespondingtothefirst23aminoacidsof thefirstframeworkregion(aregionnotassociatedwith anti-genbinding).Usingmonoclonalantibodies,four(VI−V
IV)andsixsubgroups(VI−VVI)havebeenidentified.8
Suchdiversityisbestidentifiedusingpolyclonalantibodies thatcanrecognizeanextensiverangeofdifferentepitopes.
Introduction
to
Freelite
®Freelite® (The Binding Site, UK) is the only
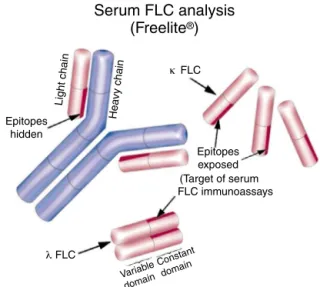
nephelo-metric/turbidimetric assay cleared by the Food and Drug Administration (FDA) of the United States of America for the measurement of serum FLC (sFLC). It uses polyclonal antibodiesproducedinsheepthatspecificallyrecognizeand quantifythekappa()andlambda()sFLCseparately,enabling calculationofthekappa/lambdasFLCratio(rFLC)whichcan beusedtodetermineclonality.9,10Theantibodiesspecifically recognizeepitopespresentintheconstantregionofthelight chains,whicharehiddenwhenjoinedtoaheavychain part-ner(i.e.in theformofthe intactimmunoglobulin) but are exposedwhenthelightchainsareintheirfreeform(Figure1). Thesensitivityofassayshasallowedquantificationofnormal circulating sFLCconcentrationsforthefirsttime[Reference intervals:–median7.3mg/L(95thpercentile:3.3–19.4mg/L);
– median 12.4mg/L (95thpercentile: 5.7–26.3mg/L); rFLC is 0.26–1.65].10,11 The majority of results of plasma cell dyscrasiasshowincreasedproductionofeithertheorsFLC. Individualswho haverFLCvalues>1.65 mayhavea mono-clonalsFLC andthosewithrFLCvalues<0.26may havea monoclonalFLC.12TheapplicabilityoftherFLCinthe clin-icalpracticehasbeenprovenbyanumberofscientific publi-cationswhichled toitsinclusion indifferentinternational guidelines.13
Serum FLC analysis
Light chain Hea
vy chain
(Freelite
®)
Epitopes hidden
Variable domain
Constant domain
Epitopes exposed (Target of serum FLC immunoassays
FLC
FLC
λ
κ
Current
techniques
used
for
the
detection
of
monoclonal
proteins
Serumproteinelectrophoresis(SPEP)isroutinelyusedto iden-tifyandquantifyintactM-Ig,withimmunofixation usedto classifyaccordingtotheheavychain(␥,␣,,␦and)andlight chain(or)isotypes.8Whilstthistechniqueisadequatefor most,grosslyelevatedintactM-Igs,sensitivitycanbelimited duetoco-migrationand atlowserum concentrations. Fur-thermore,SPEPpoorlyidentifiessFLC14meaningtheassayis inadequateforthedetectionandquantitationofparaproteins producedinlightchainMMorAmyloidosis.15Historically, 24-hoururinecollectionhasbeenrecommendedforthedetection ofimmunoglobulinfreelightchains,howeverthereisoften poorcompliance16–18andrenalfunctioncanheavilyinfluence theaccuracyoftheresults.19
Differences
between
free
light
chain
and
total
light
chain
assays
(hypothetical)
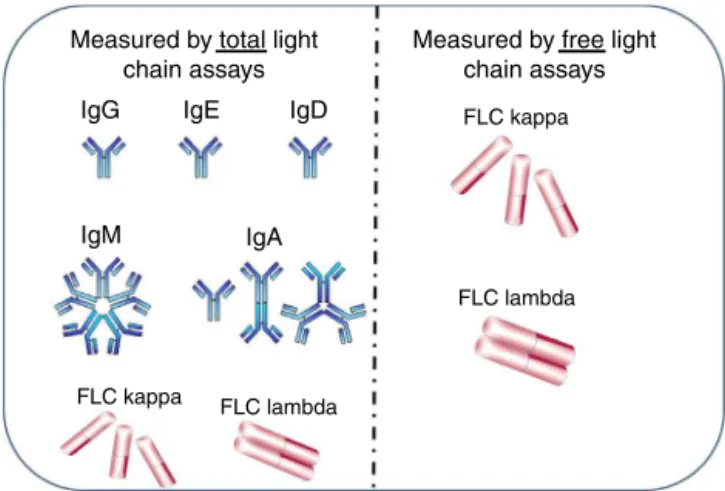
Theuseofthe Freelite assaysinthe diagnosis ofMGshas been well established.13 However there is often confusion betweenFreeliteandsimilarlynamedassayswhichdetermine thetotallightchainconcentrationinserum andurine.The totallightchainassaymeasurestheconcentrationofall anti-bodiesandfreelightchainsofaparticularlightchainclassi.e. IgG-+IgA-+IgM-+IgD-+IgE-+free.Freelite measures onlythefreeformofthelightchain(freeinourexample– Figure2).Due tothedifferenceinspecificity ofthe assays, totallightchainassays identifythelightchaincomponent ofintact immunoglobulins and freelight chains in serum whereas Freelite recognizes only the freelight chain com-ponent. Therefore, thereisa largedifference in sensitivity
Measured by total light chain assays
Measured by free light chain assays
IgG
IgM
FLC kappa FLC lambda
FLC lambda FLC kappa
IgA
IgE IgD
Figure2–Measurementofkappa()andlambda()light chainsinfreeandtotalassays.Totallightchainassays measurelightchainswhenboundtoheavychainsinintact immunoglobulinsplusfreelightchains(FLC).Thefreelight chainassaymeasuresonlyfreelightchains.
FigureadaptedfromBeckmanCoulterImmageSystems Chemistryplan,November2007.
(Table 1) betweenthe two assays. The presenceofa poly-clonalbackgroundpreventsthetotalimmunoglobulinassay frombeingabletodistinguishclonalityat<4g/L,whereasthe Freeliteassaycandetectclonalityatmg/Lconcentrations.
Differences
between
the
Freelite
and
total
light
chain
assays
(data)
Recently,Hungria et al.20 publishedastudy comparing the sensitivityofthesFLCassaystothetotallightchainassaysfor samplesobtainedfrom114lightchainMM(LCMM)patients taken through thecourseoftheir disease.In keepingwith previousreports15,19,21–34theFLCidentifiedclonalityin11/11 samplesatpresentationandidentifiedpersistentdiseasein 80/103samplestakenthroughoutthecourseofthedisease.In contrast,thetotallightchainassayidentifiedonly2/11 sam-plesatpresentationand25/103samplestakenthroughoutthe courseofthedisease.Somewhatconfusingly,thelightchain isotypewasmisreportedin11samplesastheoppositelight chain.
TheInternationalMyelomaWorkingGroup(IMWG) guide-lines forthe identificationofmonoclonalimmunoglobulins at presentationrecommend an algorithmof Freelite+SPEP (Table 2). Hungria et al. showed that, in this study, 11/11 patientswereidentifiedusingtheFreeliteassayandtherewas noneedforSPEP.Incontrast,totallightchain+SPEPidentified only8/11 samples,clearlyhighlightingthelackof sensitiv-ityofthisalgorithmrecommendedbytheIMWGguidelines (Figure3).
Thesensitivityoftotal/andsFLCassayswerecompared in a study byMarien et al.35 Sixteen serum samplesfrom LCMM patientswere investigated.Totaland concentra-tionsweremeasuredusingBeckman-Coulterreagentsonthe IMMAGE®nephelometerandsFLCconcentrationswere
mea-suredbyFreeliteassays(TheBindingSite).Allsampleswere abnormalbysFLCassayscomparedtoonlyfiveofthe16 sam-plesbytotalandassays.Inaddition, onepatientwas mistypedasbythetotallightchainassay.Otherstudieshave confirmedthattotallightchainassaysarelesssensitivethan sFLCanalysisforthediagnosisofLCMM,Non-secretoryMM andAmyloidosis.36–38
Summary
of
the
importance
of
the
serum
free
light
chain
assay
screening
TheIMWGconcludedthat,forthepurposeofscreeningforall MGs(withtheexceptionofAmyloidosis),Freelitecanreplace 24-hoururineassessments.13Furthermore,Katzmannetal.39 concludedthatFreelitecostsapproximatelyhalfasmuchas 24-hour urine assessmentbased upon Medicare, USA2006 reimbursementvalues.
RecentlytheIMWGupdatedthedefinitionofMMtoinclude additional, validated biomarkersalongsideCRAB (hypercal-cemia,renalfailure,anemia,andbonelesions)assessments. A rFLC ≥100, with an involved freelight chain
Table1–Referenceintervalsandlowerlimitsofsensitivityoffreelightchainassaysandtotallightchainassaysin serum.
Parameter Kappareferenceinterval (mg/L)
Kappasensitivity (mg/L)
Lambdareferenceinterval (mg/L)
Lambdasensitivity (mg/L)
Freelightchains(TheBindingSite) 3.3–19.4 0.3 5.7–26.3 0.4
Totallightchains(BeckmanCoulter) 6290–13500 111 3130–7230 300
Totallightchains(Roche) 1380–3750 300 930–2420 300
100 000
100.00
1000
100
10
100.00
1000
100
10 1
0.1
0.1 1 10 100
κFLC (mg/L)
κTLC (mg/dL) FLC Data
TLC Data
λ
FLC (mg/L)
λ
TLC (mg/dL)
1000 100.00 100 000
10 100 1000 100.00 100 000
Normals Kappa Lambda Presentation kappa Presentation lambda
Normals Kappa Lambda Presentation kappa Presentation lambda
Sensitivity
(%) Specificity
(%) PPV NPV (%) Accuracy
(%) (%)
FLC FLC +
80
94
94 81 87
SPE
Total light
83
92
92 83 88
chain
25
92
78 52 56
Total light chain + SPE
48
91
86 61 69
Figure3–ScatterchartsshowingthedifferencesinsensitivitybetweentheFreelite®andtotallightchainassaysforlight chainmyelomapatientstakenatpresentation(n=11)andthroughthecourseoftheirdisease(n=103)comparedtoa100
Table2–InternationalMyelomaWorkingGrouprecommendations.
MM:MultipleMyeloma;sIFE:serumimmunofixation;uIFE:urineimmunofixation;LCE:lightchainescape;sFLCs:Serumfreelightchains.
Hematological
response
InternationalguidelineshaveincludedFreelite assessments as the most effective monitoring tool in patients with Amyloidosis.41,42 Morerecently,newresponse criteriawere defined based upon changes in Freelite values during treatment.43TheresponsecriteriautilizedtherFLCandthe differencebetweeninvolvedanduninvolvedfreelightchains (dFLC);importantlythedepthoftheassignedresponse corre-latedtooverallsurvival.
TheIMWGrecommendFreeliteastheonlyavailableand reliablemethodforthedeterminationofresponseinpatients withNon-secretoryandOligosecretoryMM.13Morerecently inLCMM,comparisonsofresponseassessmentasdetermined by24-hoururineevaluationandFreelitehavesuggestedthat Freeliteisfarsuperiortothe24-hoururineexamasatoolto measurepatientresponse(Table2).44
In all MM patients, normalization of the Freelite ratio corresponds to superior outcome independently of overall response.45TheIMWGhaverefinedthedefinitionofcomplete response,(i) negativeimmunofixation in serum and urine, (ii)disappearanceofplasmacytomas,and (iii)bonemarrow infiltrationofplasmacellsbelow5%,toincludeanormal Freel-iteratio.46 Thenewresponsedefinition,stringentcomplete response (sCR) relies on a normalFreelite ratio and nega-tiveplasmacellevaluation,andcorrespondstoanimproved overallsurvival.47
Conclusions
EarlydetectionofpatientswithMMiskeytoareductionin comorbiditiesthat can impact the quality and duration of life.ThesFLC assay, but notthe total lightchainassay, is animportant part ofthe routine laboratory test algorithm thatcontributesto theidentification ofpatientswithMGs, including MM. To date, there are two pivotal studies that highlightthelimitedutilityofthetotallightchainassayin thedetectionofMGs.InthestudypresentedbyHungriaetal.,
theadditionoftotallightchaintoSPEPfailedtoidentifyall LCMMpatients,highlightinganimportantinsensitivitywhen utilizingthis assay.SimilarresultswerereportedbyMarien etal.Theassayscanbeeasilydistinguishedbasedupontheir normalrangesandcautionisurgedtoutilizethesFLCassay, butnotthetotallightchainassayforthescreening,diagnosis andhematologicalresponsesinMGs.
Conflicts
of
interest
Dr.VaniaTMHungriareceivesresearchgrantsandisaspeaker forTheBindingSite.Theothersauthorsdeclarenoconflicts ofinterest.
Acknowledgments
TheauthorsarethankfultoTheBindingSiteUK,Universityof Birmingham,UKandFundac¸ãoHemocentrodaSantaCasade SãoPaulo,SPfortheirsupport.
r
e
f
e
r
e
n
c
e
s
1.GertzMA.Immunoglobulinlightchainamyloidosis:2014 updateondiagnosis,prognosis,andtreatment.AmJ Hematol.2014;89(12):1133–40.
2.KyleRA,GertzMA,WitzigTE,LustJA,LacyMQ,DispenzieriA, etal.Reviewof1027patientswithnewlydiagnosedmultiple myeloma.MayoClinProc.2003;78(1):21–33.
3.BridgenML,WebberD.ClinicalPathologyRounds:thecaseof theanaplasticcarcinomathatwasnot–potentialproblems intheinterpretationofmonoclonalproteins.LabMed. 2000;31(12):661–5.
4.vanZaanenHC,DiderichPP,PegelsJG,RuizevelddeWinter JA.Renalinsufficiencyduetolightchainmultiplemyeloma. NedTijdschrGeneeskd.2000;144(45):2133–7.
6. TsakirisDJ,StelVS,FinneP,FraserE,HeafJ,deMeesterJ,etal. Incidenceandoutcomeofpatientsstartingrenalreplacement therapyforend-stagerenaldiseaseduetomultiplemyeloma orlight-chaindepositdisease:anERA-EDTARegistrystudy. NephrolDialTransplant.2010;25(4):1200–6.
7. JanewayCAJr,TraversP,WalportM.Thegenerationof diversityinimmunoglobulins.Immunobiology:theimmune systeminhealthanddisease.NewYork:GarlandScience; 2001.
8. SolomonA.Lightchainsofhumanimmunoglobulins. MethodsEnzymol.1985;116:101–21.
9. PalladiniG,RussoP,BosoniT,VergaL,SaraisG,LavatelliF, etal.Identificationofamyloidogeniclightchainsrequiresthe combinationofserum-freelightchainassaywith
immunofixationofserumandurine.ClinChem. 2009;55(3):499–504.
10.BakshiNA,GulbransonR,GarstkaD,BradwellAR,KerenDF. Serumfreelightchain(FLC)measurementcanaidcapillary zoneelectrophoresisindetectingsubtleFLC-producingM proteins.AmJClinPathol.2005;124(2):214–8.
11.KatzmannJA,ClarkRJ,AbrahamRS,BryantS,LympJF, BradwellAR,etal.Serumreferenceintervalsanddiagnostic rangesforfreekappaandfreelambdaimmunoglobulinlight chains:relativesensitivityfordetectionofmonoclonallight chains.ClinChem.2002;48(9):1437–44.
12.AbadieJM,BanksonDD.Assessmentofserumfreelightchain assaysforplasmacelldisorderscreeninginaVeteransAffairs population.AnnClinLabSci.2006;36(2):157–62.
13.DispenzieriA,KyleR,MerliniG,MiguelJS,LudwigH,HajekR, etal.InternationalMyelomaWorkingGroupguidelinesfor serum-freelightchainanalysisinmultiplemyelomaand relateddisorders.Leukemia.2009;23(2):215–24.
14.KatzmannJA,SnyderMR,RajkumarSV,KyleRA,Therneau TM,BensonJT,etal.Long-termbiologicvariationofserum proteinelectrophoresisM-spike,urineM-spike,and monoclonalserumfreelightchainquantification:
implicationsformonitoringmonoclonalgammopathies.Clin Chem.2011;57(12):1687–92.
15.AbrahamRS,ClarkRJ,BryantSC,LympJF,LarsonT,KyleRA, etal.Correlationofserumimmunoglobulinfreelightchain quantificationwithurinaryBenceJonesproteininlightchain myeloma.ClinChem.2002;48(4):655–7.
16.FidlerCJ,HusseinAK,GandhiN,KarurV,SharmaM,Klumpp TR,etal.Evaluatingtrendsindiagnosticandprognostic testingformultiplemyeloma.Blood.2011;118:2067. 17.RobsonEJ,TaylorJ,BeardsmoreC,BasuS,MeadG,LovattT.
Utilityofserumfreelightchainanalysiswhenscreeningfor lymphoproliferativedisorders.LabMed.2009;40(6):325–9. 18.BeethamR,WassellJ,WallageMJ,WhitewayAJ,JamesJA.Can
serumfreelightchainsreplaceurineelectrophoresisinthe detectionofmonoclonalgammopathies?AnnClinBiochem. 2007;44Pt6:516–22.
19.NowrousianMR,BrandhorstD,SammetC,KellertM,Daniels R,SchuettP,etal.Serumfreelightchainanalysisandurine immunofixationelectrophoresisinpatientswithmultiple myeloma.ClinCancerRes.2005;1124(Pt1):8706–14. 20.HungriaVT,KampanisP,DraysonMT,PlantT,CrusoeEQ,
PeresAL,etal.ComparisonofkappaandlambdaFreeliteto totalkappaandlambdaimmunoassaysforthedetectionof monoclonalgammopathies,bothasstandalonetestsand alongsideserumproteinelectrophoresis.Blood.
2014;124(21):5705.
21.BradwellAR,Carr-SmithHD,MeadGP,HarveyTC,Drayson MT.SerumtestforassessmentofpatientswithBenceJones myeloma.Lancet.2003;361(9356):489–91.
22.DraysonMT,MorganGJ,JacksonGH,DaviesFE,OwenRG, RossFM,etal.ProspectivestudyofserumFLCandother
M-proteinassays:whenandhowtomeasureresponse?Clin LymphomaMyeloma.2009;9Suppl.1:S56.
23.vanRheeF,BolejackV,HollmigK,Pineda-RomanM,Anaissie E,EpsteinJ,etal.Highserum-freelightchainlevelsandtheir rapidreductioninresponsetotherapydefineanaggressive multiplemyelomasubtypewithpoorprognosis.Blood. 2007;110(3):827–32.
24.KrajM,KrukB,PoglodR,SzczepinskiA.Correlationofserum freelightchainquantificationwithserumandurine immunofixationinmonoclonalgammopathies. Haematologica.2011;96:0861a.
25.AvetLoiseauH,MirbahaiL,YoungP,MathiotC,AttalM, MoreauP,etal.NephelometricmeasurementsofFLCand
FLCformonitoringlightchainmultiplemyelomapatients. LymphomaMyeloma.2011.
26.Avet-LoiseauH,YoungP,MathiotC,AttalM,HarousseauJ, BradwellAR,etal.NephelometricmeasurementsofFLCand
FLCformonitoringlightmyelomapatients.Haematologica. 2011;96:0853a.
27.KangSY,SuhJT,LeeHJ,YoonHJ,LeeWI.Clinicalusefulnessof freelightchainconcentrationasatumormarkerinmultiple myeloma.AnnHematol.2005;84(9):588–93.
28.SchneiderN,WynckelA,KolbB,SablonE,GilleryP,Maquart FX.Comparativeanalysisofimmunoglobulinfreelightchains quantificationbyFreelite(TheBindingSite)andNLatexFLC (Siemens)methods.AnnBiolClin(Paris).2013;71(1): 13–9.
29.HutchisonCA,PlantT,DraysonM,CockwellP,KountouriM, BasnayakeK,etal.Serumfreelightchainmeasurementaids thediagnosisofmyelomainpatientswithsevererenal failure.BMCNephrol.2008;9:11.
30.MösbauerU,AyukF,SchiederH,LioznovM,ZanderAR, KrogerN.Monitoringserumfreelightchainsinpatientswith multiplemyelomawhoachievednegativeimmunofixation afterallogeneicstemcelltransplantation.Haematologica. 2007;92(2):275–6.
31.PiehlerAP,GulbrandsenN,KierulfP,UrdalP.Quantitationof serumfreelightchainsincombinationwithprotein electrophoresisandclinicalinformationfordiagnosing multiplemyelomainageneralhospitalpopulation.Clin Chem.2008;54(11):1823–30.
32.HardingSJ,MeadGP,BradwellAR,BerardAM.Serumfreelight chainimmunoassayasanadjuncttoserumprotein
electrophoresisandimmunofixationelectrophoresisinthe detectionofmultiplemyelomaandotherB-cellmalignancies. ClinChemLabMed.2009;47(3):302–4.
33.GiarinMM,GiacconeL,CaraccioloD,BrunoB,FalcoP,Omedè P,etal.Serumfreelightchains(SFLC)assay:asuggestivenew criteriaforevaluatingdiseaseresponse,progressionand relapseinplasma-celldisorders(PD)andaprognosticfactor inmonoclonalgammopathyofundeterminedsignificance (MGUS).Haematologica.2006;91:PO151a.
34.WolffF,ThiryC,WillemsD.Assessmentoftheanalytical performanceandthesensitivityofserumfreelightchains immunoassayinpatientswithmonoclonalgammopathy. ClinBiochem.2007;40(5–6):351–4.
35.MarienG,OrisE,BradwellAR,BlanckaertN,BossuytX. Detectionofmonoclonalproteinsinserabycapillaryzone electrophoresisandfreelightchainmeasurements.Clin Chem.2002;48(9):1600–1.
36.CavalcantiE,BarchiesiV,CuomoM,DiPaolaF,MorabitoF, CavalcantiS.Aparticularcaseoflambdachainmultiple myeloma.BiochimClin.2013;37:428–30.
38.SmithLJ,MeadGP,BradwellAR.Comparativesensitivityof serumandurineassaysforfreelightchains.ClinChemLab Med.2003:41.
39.KatzmannJA,AbrahamRS,DispenzieriA,LustJA,KyleRA. Diagnosticperformanceofquantitativekappaandlambda freelightchainassaysinclinicalpractice.ClinChem. 2005;51(5):878–81.
40.RajkumarSV,DimopolousMA,PalumboA,BladeJ,MerliniG, MateosMV,etal.InternationalMyelomaWorkingGroup updatedcriteriaforthediagnosisofmultiplemyeloma. LancetOncol.2014;15(12):e538–48.
41.GertzMA,ComenzoR,FalkRH,FermandJP,HazenbergBP, HawkinsPN,etal.Definitionoforganinvolvementand treatmentresponseinimmunoglobulinlightchain amyloidosis(AL):aconsensusopinionfromthe10th InternationalSymposiumonAmyloidandAmyloidosis, Tours,France,18–22April2004.AmJHematol.
2005;79(4):319–28.
42.BirdJM,CavenaghJ,SamsonD,MehtaA,HawkinsP, LachmannH.Guidelinesonthediagnosisandmanagement ofALamyloidosis.BrJHaematol.2004;125(6):
681–700.
43.PalladiniG,DispenzieriA,GertzMA,KumarS,WechalekarA, HawkinsPN,etal.Newcriteriaforresponsetotreatmentin immunoglobulinlightchainamyloidosisbasedonfreelight chainmeasurementandcardiacbiomarkers:impacton survivaloutcomes.JClinOncol.2012;30(36):4541–9.
44.CorreJ,DejoieT,CaillonH,AttalM,Avet-LoiseauH,MoreauP. Serumfreelightchainsshouldbethetargetofresponse evaluationinlightchainmultiplemyelomaratherthan urines:resultsfromtheIFM/DFCI2009trial.Blood. 2014;124:180a.
45.MoustafaMA,RajkumarSV,DispenzieriA,GertzMA,Lacy MQ,BuadiFK,etal.Utilityofserumfreelightchain measurementsinmultiplemyelomapatientsnotachieving completeresponsetotherapy.Leukemia.2015;29(10):2033–8. 46.DurieBG,HarousseauJL,MiguelJS,BladeJ,BarlogieB,
AndersonK,etal.Internationaluniformresponsecriteriafor multiplemyeloma.Leukemia.2006;20:1467–73.